 Found 586 hits with Last Name = 'jeppesen' and Initial = 'l'
Found 586 hits with Last Name = 'jeppesen' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 4.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 8.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 8.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM85816

(NNC 11-1607)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1cccc(c1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.66,-.26,;4,.51,;5.33,-.26,;6.58,.64,;7.82,-.26,;7.35,-1.73,;5.81,-1.73,;4.72,-2.82,;4.72,-4.36,;3.38,-5.13,;2.05,-4.36,;2.05,-2.82,;3.38,-2.05,;4.15,-3.38,;2.67,-3.78,;1.33,.51,;-0,1.28,;-1.34,2.05,;-1.34,3.59,;-2.67,4.36,;-4,3.59,;-4,2.05,;-2.67,1.28,;-5.34,1.28,;-6.67,.51,;-8.01,-.26,;-9.34,.51,;-10.67,-.26,;-11.92,.64,;-13.16,-.26,;-12.69,-1.73,;-11.15,-1.73,;-10.06,-2.82,;-10.06,-4.36,;-8.73,-5.13,;-7.39,-4.36,;-7.39,-2.82,;-8.73,-2.05,;-9.5,-3.38,;-8.01,-3.78,)| Show InChI InChI=1S/C30H32N6O2S2/c1-4-21(6-2-16-37-29-27(31-39-33-29)25-19-35-12-8-23(25)9-13-35)18-22(5-1)7-3-17-38-30-28(32-40-34-30)26-20-36-14-10-24(26)11-15-36/h1,4-5,18,23-26H,8-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50072228
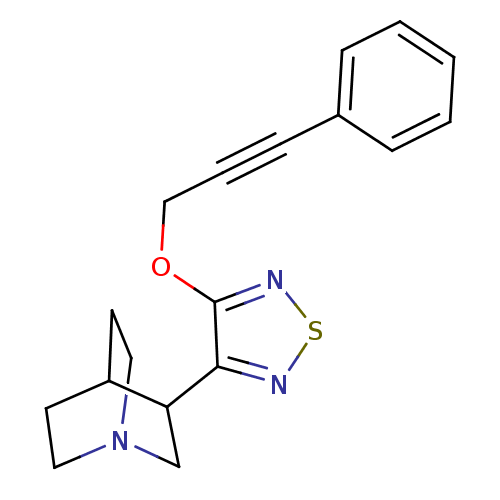
(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072228

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 41.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50072228

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072228

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 58.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50072228

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 77.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118750
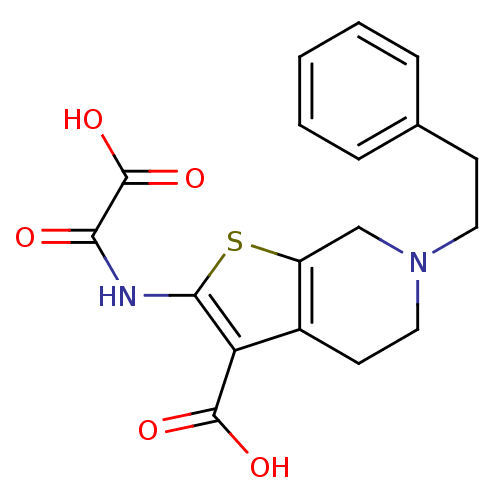
(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118792
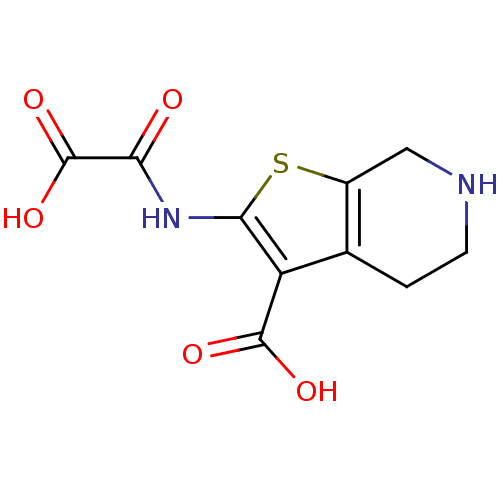
(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118786
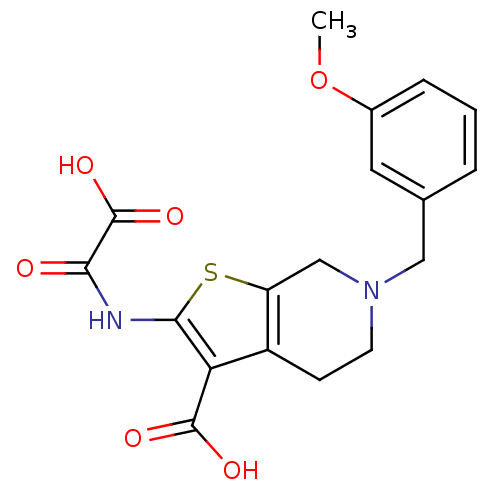
(6-(3-Methoxy-benzyl)-2-(oxalyl-amino)-4,5,6,7-tetr...)Show SMILES COc1cccc(CN2CCc3c(C2)sc(NC(=O)C(O)=O)c3C(O)=O)c1 Show InChI InChI=1S/C18H18N2O6S/c1-26-11-4-2-3-10(7-11)8-20-6-5-12-13(9-20)27-16(14(12)17(22)23)19-15(21)18(24)25/h2-4,7H,5-6,8-9H2,1H3,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118794
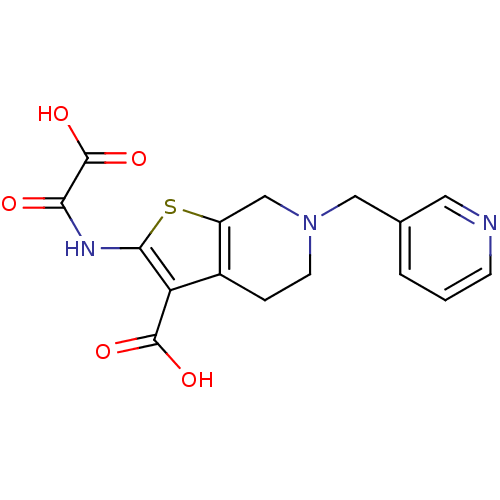
(2-(Oxalyl-amino)-6-pyridin-3-ylmethyl-4,5,6,7-tetr...)Show InChI InChI=1S/C16H15N3O5S/c20-13(16(23)24)18-14-12(15(21)22)10-3-5-19(8-11(10)25-14)7-9-2-1-4-17-6-9/h1-2,4,6H,3,5,7-8H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118793

(6-Methyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...)Show InChI InChI=1S/C11H12N2O5S/c1-13-3-2-5-6(4-13)19-9(7(5)10(15)16)12-8(14)11(17)18/h2-4H2,1H3,(H,12,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118774
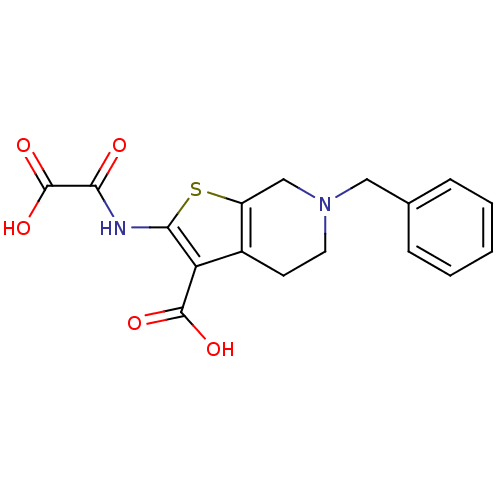
(6-Benzyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...)Show InChI InChI=1S/C17H16N2O5S/c20-14(17(23)24)18-15-13(16(21)22)11-6-7-19(9-12(11)25-15)8-10-4-2-1-3-5-10/h1-5H,6-9H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118742

(2-(Oxalyl-amino)-6-pyridin-2-ylmethyl-4,5,6,7-tetr...)Show InChI InChI=1S/C16H15N3O5S/c20-13(16(23)24)18-14-12(15(21)22)10-4-6-19(8-11(10)25-14)7-9-3-1-2-5-17-9/h1-3,5H,4,6-8H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
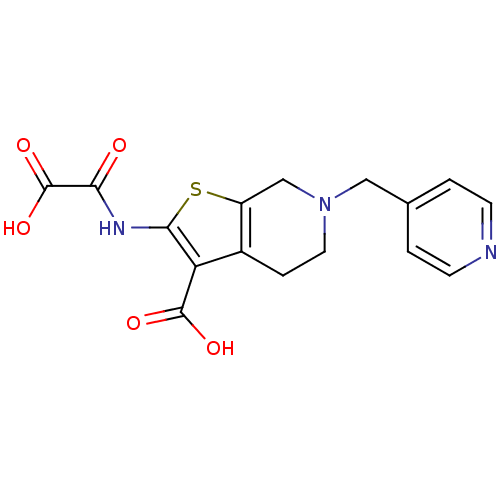
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118755
(2-(Oxalyl-amino)-6-pyridin-4-ylmethyl-4,5,6,7-tetr...)Show InChI InChI=1S/C16H15N3O5S/c20-13(16(23)24)18-14-12(15(21)22)10-3-6-19(8-11(10)25-14)7-9-1-4-17-5-2-9/h1-2,4-5H,3,6-8H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118772
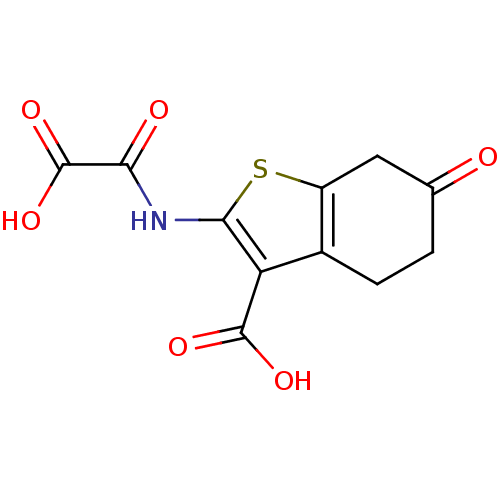
(2-(Oxalyl-amino)-6-oxo-4,5,6,7-tetrahydro-benzo[b]...)Show InChI InChI=1S/C11H9NO6S/c13-4-1-2-5-6(3-4)19-9(7(5)10(15)16)12-8(14)11(17)18/h1-3H2,(H,12,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118767
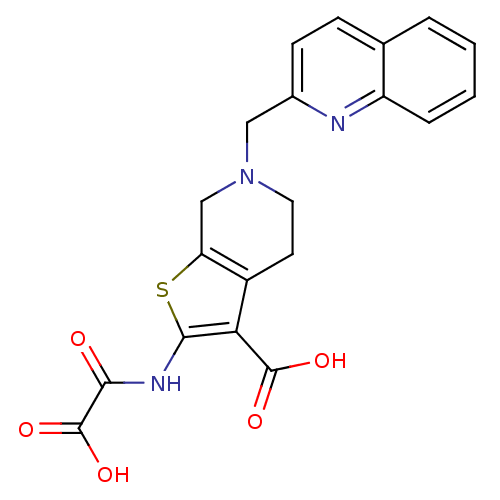
(2-(Oxalyl-amino)-6-quinolin-2-ylmethyl-4,5,6,7-tet...)Show SMILES OC(=O)C(=O)Nc1sc2CN(Cc3ccc4ccccc4n3)CCc2c1C(O)=O Show InChI InChI=1S/C20H17N3O5S/c24-17(20(27)28)22-18-16(19(25)26)13-7-8-23(10-15(13)29-18)9-12-6-5-11-3-1-2-4-14(11)21-12/h1-6H,7-10H2,(H,22,24)(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118771
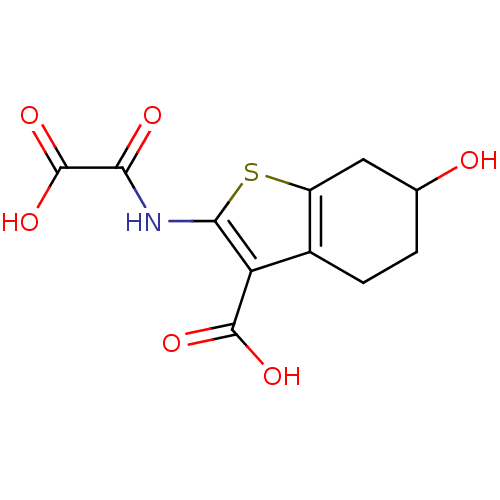
(2-(carboxyformamido)-6-hydroxy-4,5,6,7-tetrahydrob...)Show InChI InChI=1S/C11H11NO6S/c13-4-1-2-5-6(3-4)19-9(7(5)10(15)16)12-8(14)11(17)18/h4,13H,1-3H2,(H,12,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118741
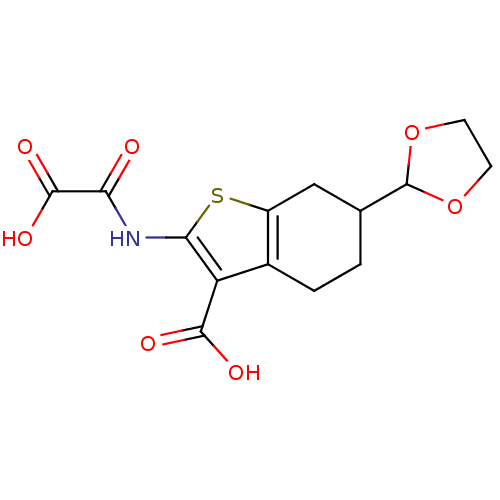
(6-[1,3]Dioxolan-2-yl-2-(oxalyl-amino)-4,5,6,7-tetr...)Show InChI InChI=1S/C14H15NO7S/c16-10(13(19)20)15-11-9(12(17)18)7-2-1-6(5-8(7)23-11)14-21-3-4-22-14/h6,14H,1-5H2,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118765
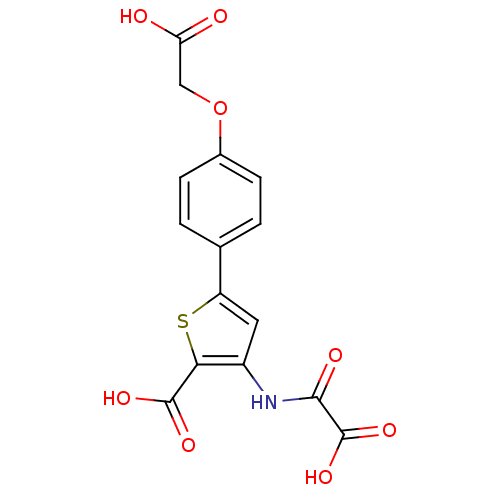
(3-(carboxyformamido)-5-(4-(carboxymethoxy)phenyl)t...)Show SMILES OC(=O)COc1ccc(cc1)-c1cc(NC(=O)C(O)=O)c(s1)C(O)=O Show InChI InChI=1S/C15H11NO8S/c17-11(18)6-24-8-3-1-7(2-4-8)10-5-9(12(25-10)14(20)21)16-13(19)15(22)23/h1-5H,6H2,(H,16,19)(H,17,18)(H,20,21)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118750

(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118750

(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118782
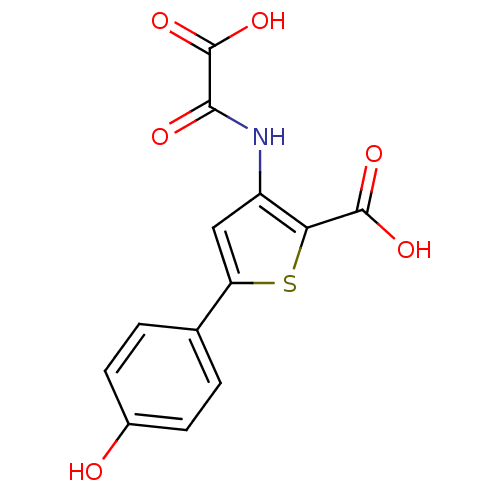
(3-(carboxyformamido)-5-(4-hydroxyphenyl)thiophene-...)Show InChI InChI=1S/C13H9NO6S/c15-7-3-1-6(2-4-7)9-5-8(10(21-9)12(17)18)14-11(16)13(19)20/h1-5,15H,(H,14,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118780
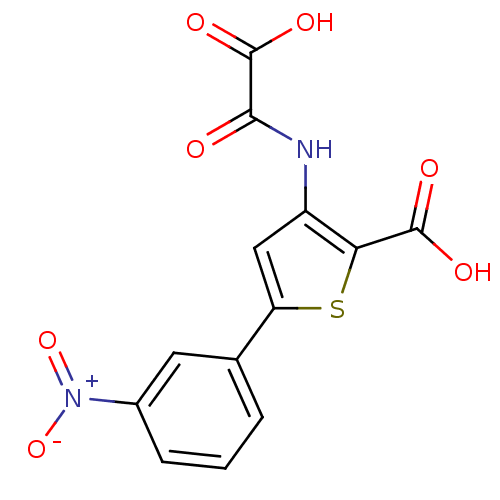
(3-(carboxyformamido)-5-(3-nitrophenyl)thiophene-2-...)Show SMILES OC(=O)C(=O)Nc1cc(sc1C(O)=O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C13H8N2O7S/c16-11(13(19)20)14-8-5-9(23-10(8)12(17)18)6-2-1-3-7(4-6)15(21)22/h1-5H,(H,14,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118792

(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118783

(2-(Oxalyl-amino)-6-oxo-4,5,6,7-tetrahydro-6lambda*...)Show InChI InChI=1S/C10H9NO6S2/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-19(17)3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50118752

(6-(3,3-Diphenyl-propyl)-2-(oxalyl-amino)-4,5,6,7-t...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCC(c3ccccc3)c3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C25H24N2O5S/c28-22(25(31)32)26-23-21(24(29)30)19-12-14-27(15-20(19)33-23)13-11-18(16-7-3-1-4-8-16)17-9-5-2-6-10-17/h1-10,18H,11-15H2,(H,26,28)(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against T cell protein tyrosine phosphatase (TC-PTP) using p-nitrophenyl phosphate as substrate at pH 7.0 |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118752

(6-(3,3-Diphenyl-propyl)-2-(oxalyl-amino)-4,5,6,7-t...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCC(c3ccccc3)c3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C25H24N2O5S/c28-22(25(31)32)26-23-21(24(29)30)19-12-14-27(15-20(19)33-23)13-11-18(16-7-3-1-4-8-16)17-9-5-2-6-10-17/h1-10,18H,11-15H2,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118758
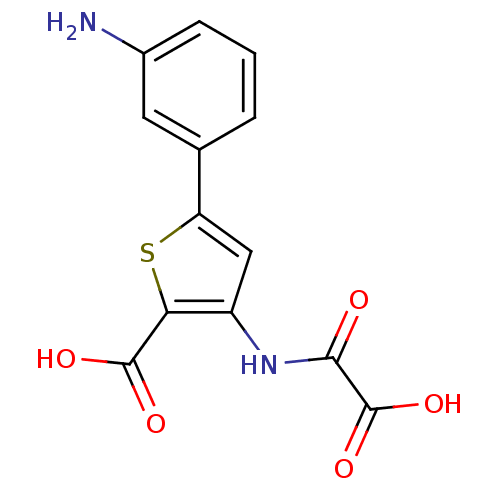
(5-(3-Amino-phenyl)-3-(oxalyl-amino)-thiophene-2-ca...)Show InChI InChI=1S/C13H10N2O5S/c14-7-3-1-2-6(4-7)9-5-8(10(21-9)12(17)18)15-11(16)13(19)20/h1-5H,14H2,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118763

(2-(Oxalyl-amino)-6,6-dioxo-4,5,6,7-tetrahydro-6lam...)Show InChI InChI=1S/C10H9NO7S2/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-20(17,18)3-5(4)19-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118790

(2-(Oxalyl-amino)-6-(3-phenyl-propyl)-4,5,6,7-tetra...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C19H20N2O5S/c22-16(19(25)26)20-17-15(18(23)24)13-8-10-21(11-14(13)27-17)9-4-7-12-5-2-1-3-6-12/h1-3,5-6H,4,7-11H2,(H,20,22)(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118756

(3-(carboxyformamido)-5-(3,5-dimethoxyphenyl)thioph...)Show SMILES COc1cc(OC)cc(c1)-c1cc(NC(=O)C(O)=O)c(s1)C(O)=O Show InChI InChI=1S/C15H13NO7S/c1-22-8-3-7(4-9(5-8)23-2)11-6-10(12(24-11)14(18)19)16-13(17)15(20)21/h3-6H,1-2H3,(H,16,17)(H,18,19)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118754
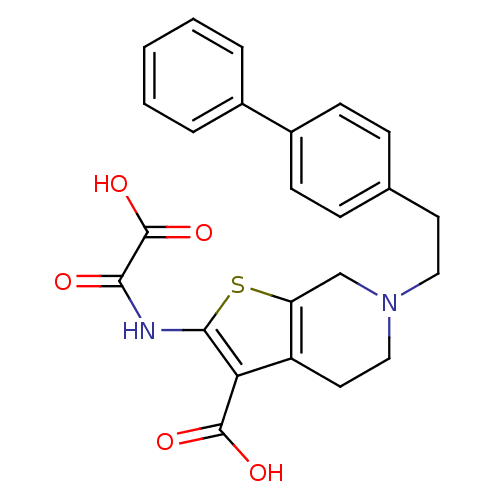
(6-(2-Biphenyl-4-yl-ethyl)-2-(oxalyl-amino)-4,5,6,7...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccc(cc3)-c3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C24H22N2O5S/c27-21(24(30)31)25-22-20(23(28)29)18-11-13-26(14-19(18)32-22)12-10-15-6-8-17(9-7-15)16-4-2-1-3-5-16/h1-9H,10-14H2,(H,25,27)(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118740

(3-(carboxyformamido)-5-(4-fluorophenyl)thiophene-2...)Show InChI InChI=1S/C13H8FNO5S/c14-7-3-1-6(2-4-7)9-5-8(10(21-9)12(17)18)15-11(16)13(19)20/h1-5H,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118762
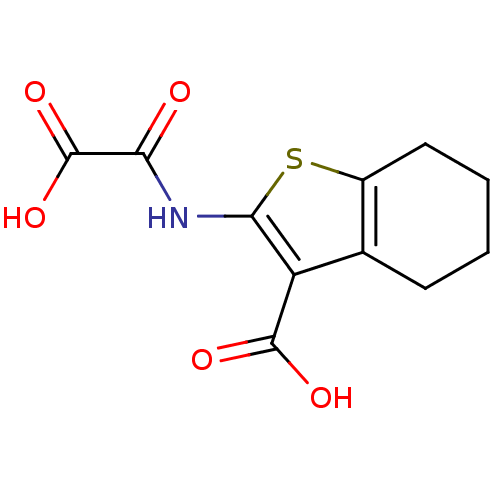
(2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...)Show InChI InChI=1S/C11H11NO5S/c13-8(11(16)17)12-9-7(10(14)15)5-3-1-2-4-6(5)18-9/h1-4H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118787
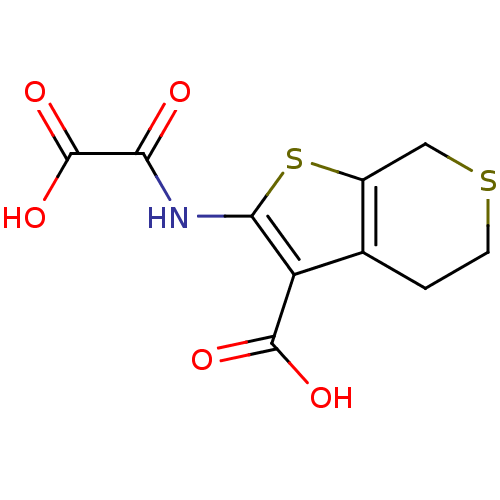
(2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]thiop...)Show InChI InChI=1S/C10H9NO5S2/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-17-3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118786

(6-(3-Methoxy-benzyl)-2-(oxalyl-amino)-4,5,6,7-tetr...)Show SMILES COc1cccc(CN2CCc3c(C2)sc(NC(=O)C(O)=O)c3C(O)=O)c1 Show InChI InChI=1S/C18H18N2O6S/c1-26-11-4-2-3-10(7-11)8-20-6-5-12-13(9-20)27-16(14(12)17(22)23)19-15(21)18(24)25/h2-4,7H,5-6,8-9H2,1H3,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118748
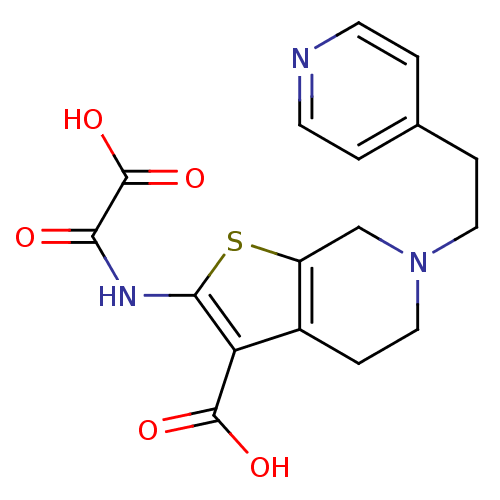
(2-(Oxalyl-amino)-6-(2-pyridin-4-yl-ethyl)-4,5,6,7-...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccncc3)CCc2c1C(O)=O Show InChI InChI=1S/C17H17N3O5S/c21-14(17(24)25)19-15-13(16(22)23)11-4-8-20(9-12(11)26-15)7-3-10-1-5-18-6-2-10/h1-2,5-6H,3-4,7-9H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118770

(2-(Oxalyl-amino)-6-(2-thiophen-3-yl-ethyl)-4,5,6,7...)Show InChI InChI=1S/C16H16N2O5S2/c19-13(16(22)23)17-14-12(15(20)21)10-2-5-18(7-11(10)25-14)4-1-9-3-6-24-8-9/h3,6,8H,1-2,4-5,7H2,(H,17,19)(H,20,21)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118759
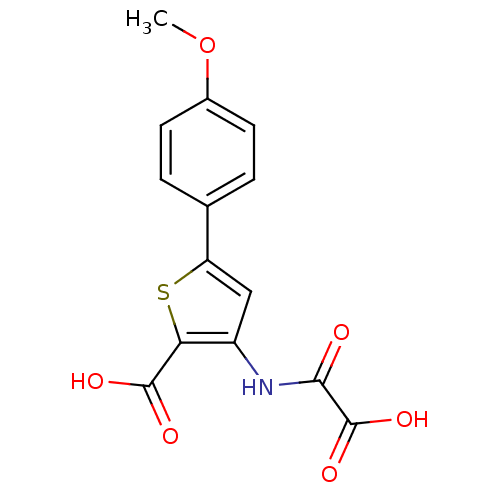
(3-(carboxyformamido)-5-(4-methoxyphenyl)thiophene-...)Show InChI InChI=1S/C14H11NO6S/c1-21-8-4-2-7(3-5-8)10-6-9(11(22-10)13(17)18)15-12(16)14(19)20/h2-6H,1H3,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data