

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15131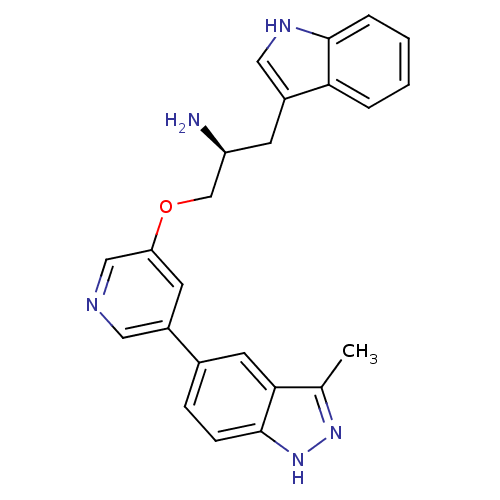 (5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Astex | Assay Description The purified PKB beta enzyme was assayed with a peptide substrate and test compound in the presence of 30 uM ATP/ [gamma-33P]ATP in 96-well plates. I... | J Mol Biol 367: 882-94 (2007) Article DOI: 10.1016/j.jmb.2007.01.004 BindingDB Entry DOI: 10.7270/Q29Z934H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM12588 ((2R,3S)-3-({[5-chloro-2-(1H-1,2,3,4-tetrazol-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Astex | Assay Description The cleavage of the substrate was followed by monitoring the change in fluorescence at 460 nm (excitation at 365 nm) for 25 min at room temperature o... | J Med Chem 49: 1346-55 (2006) Article DOI: 10.1021/jm050850v BindingDB Entry DOI: 10.7270/Q20863J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041945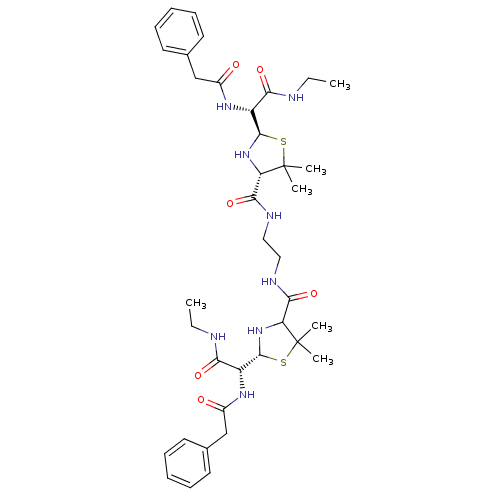 (1N-ethyl-2-benzylcarboxamido-2-[4-{2-[2-benzylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM12589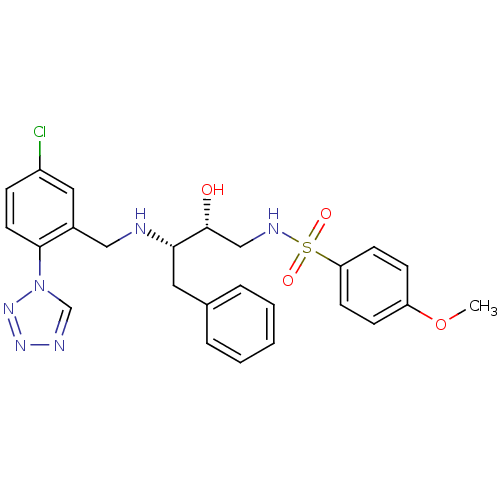 ((2R,3S)-3-({[5-chloro-2-(1H-1,2,3,4-tetrazol-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Astex | Assay Description The cleavage of the substrate was followed by monitoring the change in fluorescence at 460 nm (excitation at 365 nm) for 25 min at room temperature o... | J Med Chem 49: 1346-55 (2006) Article DOI: 10.1021/jm050850v BindingDB Entry DOI: 10.7270/Q20863J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041946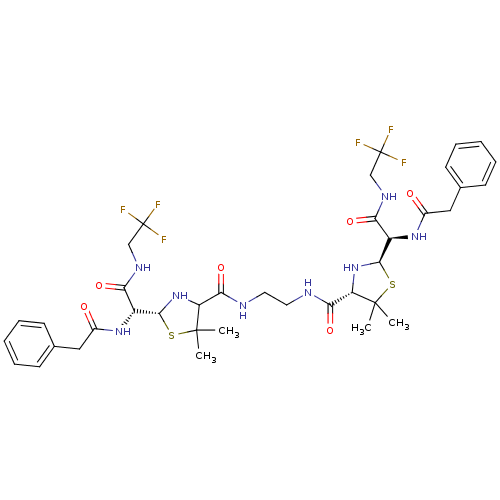 (1N-(2,2,2-trifluoroethyl)-2-benzylcarboxamido-2-[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041944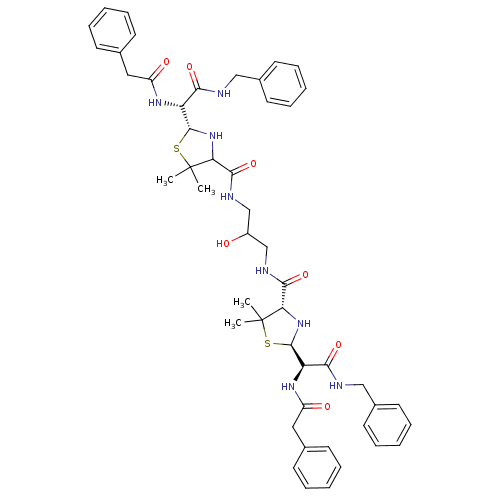 (1N-benzyl-2-[4-{3-[2-benzylcarbamoyl(benzylcarboxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041949 (4N-{2-[5-ethylcarbamoyl(5-methyl-3-phenyl-4-isoxaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041948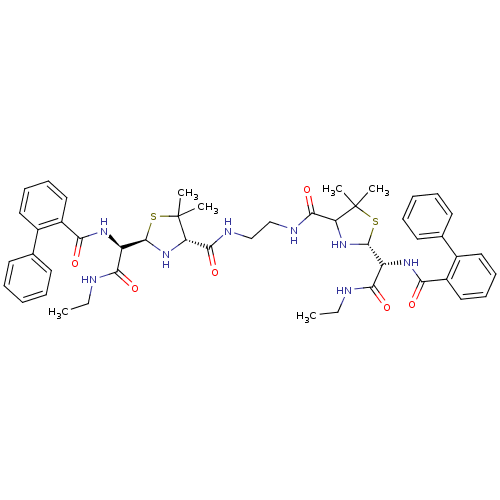 (4N-{2-[5-ethylcarbamoyl(2-phenylphenylcarboxamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078544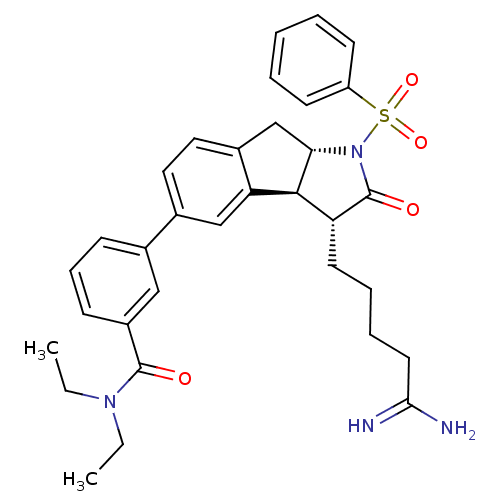 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM15131 (5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Astex | Assay Description The recombinant alpha catalytic subunit of bovine PKA was assayed with a peptide substrate and test compound in the presence of 40 uM ATP/ [gamma-33P... | J Mol Biol 367: 882-94 (2007) Article DOI: 10.1016/j.jmb.2007.01.004 BindingDB Entry DOI: 10.7270/Q29Z934H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078553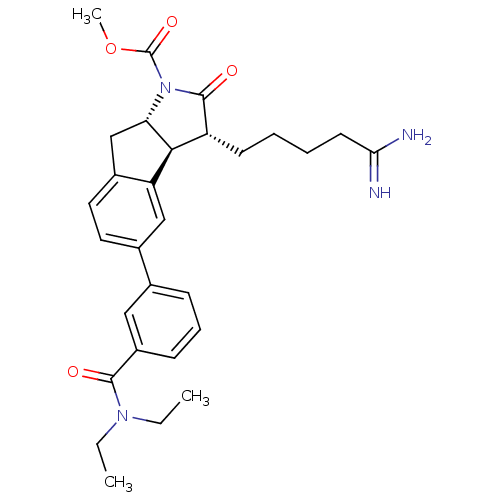 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13344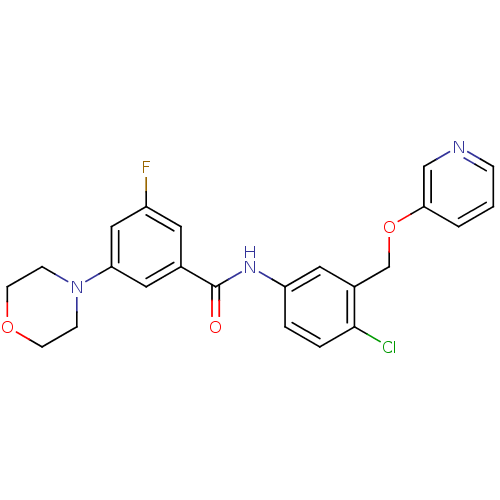 (N-[4-Chloro-3-(3-pyridinyloxymethyl)phenyl]-3-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astex | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 48: 414-26 (2005) Article DOI: 10.1021/jm049575n BindingDB Entry DOI: 10.7270/Q26M352S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078544 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078544 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1658-1692] (Hepatitis C virus) | BDBM92629 ((3S)-3-{[(1R)-1-(4-chloro-2-fluoro-3-phenoxyphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | 62 | n/a | n/a | n/a | n/a | 25 |
Astex Pharmaceuticals | Assay Description The protease activity of the full-length NS3-NS4a and the protease domain were measured using a FRET-based assay using a peptide substrate derived fr... | Nat Chem Biol 8: 920-5 (2012) Article DOI: 10.1038/nchembio.1081 BindingDB Entry DOI: 10.7270/Q26Q1VVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078552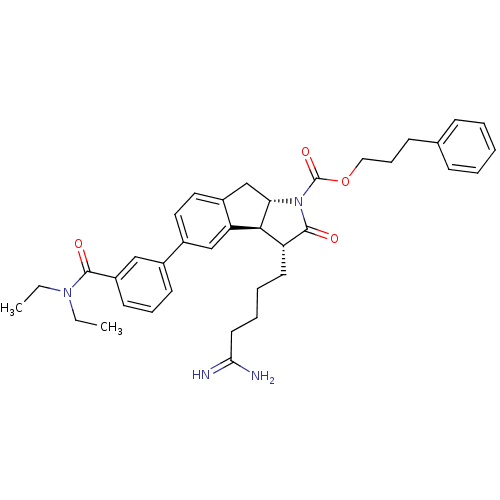 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM16222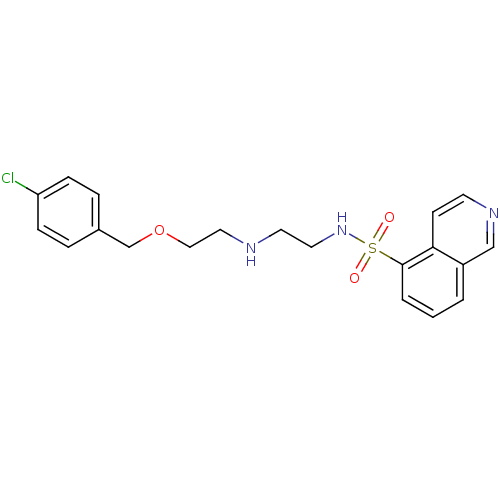 (CCT077373 | N-[2-({2-[(4-chlorobenzyl)oxy]ethyl}am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Astex | Assay Description The recombinant alpha catalytic subunit of bovine PKA was assayed with a peptide substrate and test compound in the presence of 40 uM ATP/ [gamma-33P... | J Mol Biol 367: 882-94 (2007) Article DOI: 10.1016/j.jmb.2007.01.004 BindingDB Entry DOI: 10.7270/Q29Z934H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13354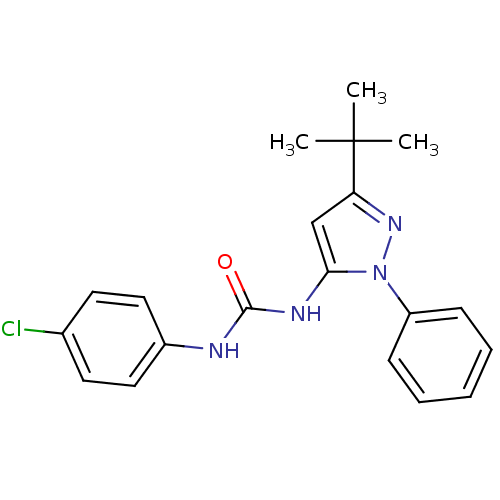 (3-(3-tert-butyl-1-phenyl-1H-pyrazol-5-yl)-1-(4-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astex | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 48: 414-26 (2005) Article DOI: 10.1021/jm049575n BindingDB Entry DOI: 10.7270/Q26M352S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM12587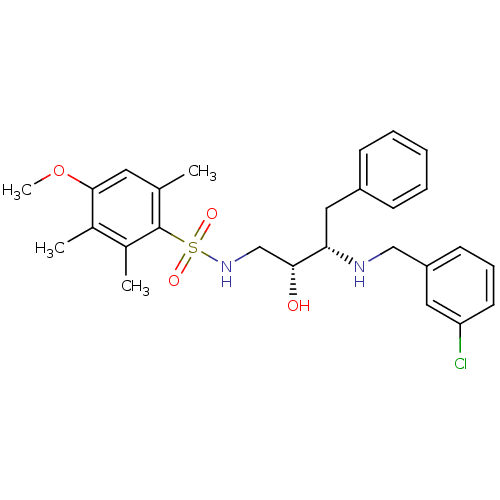 ((2R,3S)-3-{[(3-chlorophenyl)methyl]amino}-2-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Astex | Assay Description The cleavage of the substrate was followed by monitoring the change in fluorescence at 460 nm (excitation at 365 nm) for 25 min at room temperature o... | J Med Chem 49: 1346-55 (2006) Article DOI: 10.1021/jm050850v BindingDB Entry DOI: 10.7270/Q20863J2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM16222 (CCT077373 | N-[2-({2-[(4-chlorobenzyl)oxy]ethyl}am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Astex | Assay Description The purified PKB beta enzyme was assayed with a peptide substrate and test compound in the presence of 30 uM ATP/ [gamma-33P]ATP in 96-well plates. I... | J Mol Biol 367: 882-94 (2007) Article DOI: 10.1016/j.jmb.2007.01.004 BindingDB Entry DOI: 10.7270/Q29Z934H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13336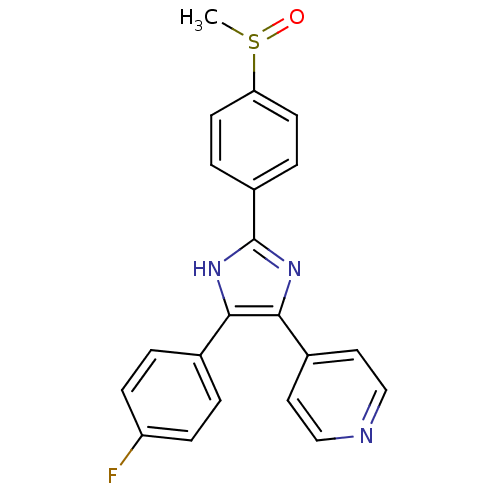 (4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astex | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 48: 414-26 (2005) Article DOI: 10.1021/jm049575n BindingDB Entry DOI: 10.7270/Q26M352S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13353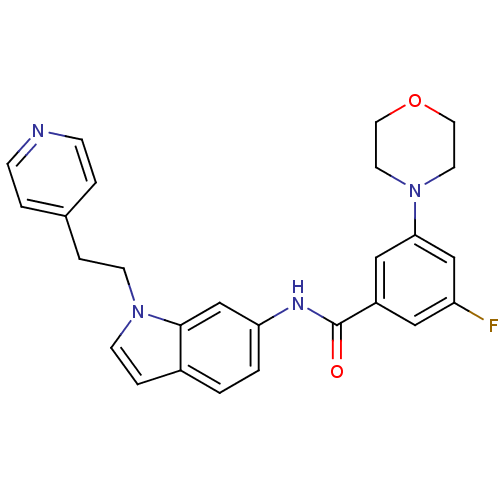 (3-fluoro-5-(morpholin-4-yl)-N-{1-[2-(pyridin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astex | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 48: 414-26 (2005) Article DOI: 10.1021/jm049575n BindingDB Entry DOI: 10.7270/Q26M352S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13345 (1-(5-tert-Butyl-2H-pyrazol-3-yl)-3-[4-chloro-3-(py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astex | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 48: 414-26 (2005) Article DOI: 10.1021/jm049575n BindingDB Entry DOI: 10.7270/Q26M352S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078556 (3-[(3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-2-oxo-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041947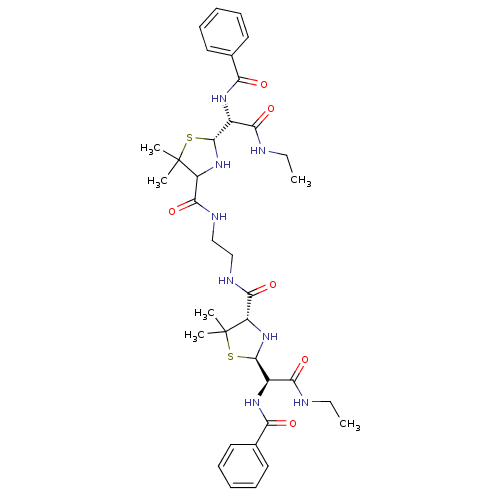 (4N-{2-[5-ethylcarbamoyl(phenylcarboxamido)methyl-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041950 (1N-benzyl-2-[4-{3-[2-benzylcarbamoyl(benzylcarboxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078557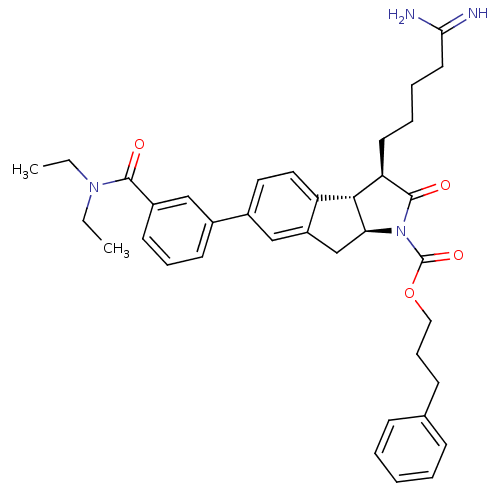 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13351 (3-fluoro-5-(morpholin-4-yl)-N-{3-[2-(pyridin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astex | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 48: 414-26 (2005) Article DOI: 10.1021/jm049575n BindingDB Entry DOI: 10.7270/Q26M352S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078557 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1658-1692] (Hepatitis C virus) | BDBM92628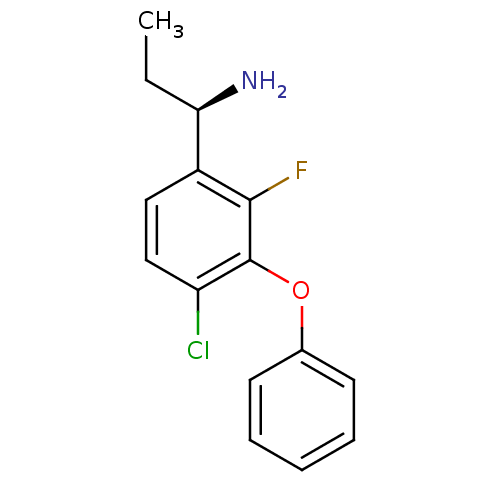 ((1R)-1-(2,4-difluoro-3-phenoxyphenyl)propan-1-amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Astex Pharmaceuticals | Assay Description The protease activity of the full-length NS3-NS4a and the protease domain were measured using a FRET-based assay using a peptide substrate derived fr... | Nat Chem Biol 8: 920-5 (2012) Article DOI: 10.1038/nchembio.1081 BindingDB Entry DOI: 10.7270/Q26Q1VVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13349 (2-({4-[2-(1H-indol-3-yl)ethyl]pyrimidin-2-yl}amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astex | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 48: 414-26 (2005) Article DOI: 10.1021/jm049575n BindingDB Entry DOI: 10.7270/Q26M352S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078551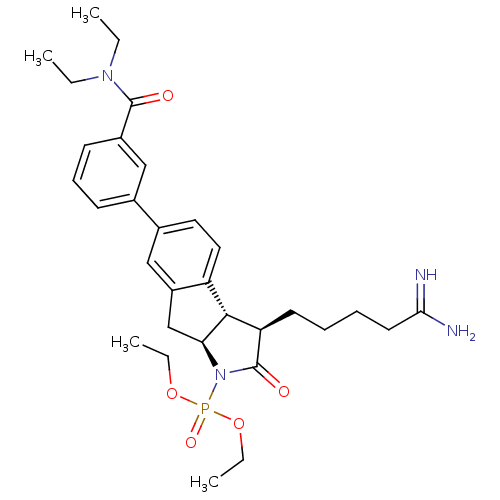 (CHEMBL46365 | [(3R,3aS,8aS)-3-(4-Carbamimidoyl-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078549 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078553 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078553 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078552 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078546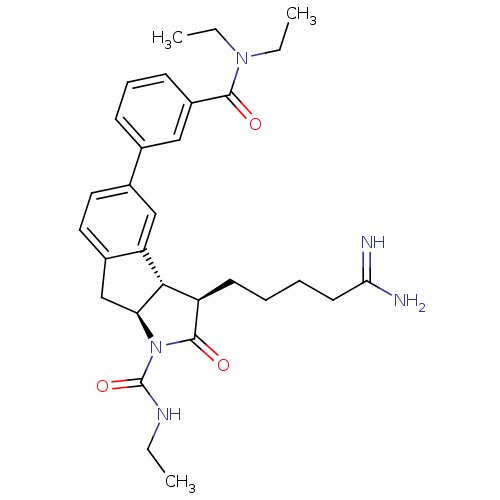 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078551 (CHEMBL46365 | [(3R,3aS,8aS)-3-(4-Carbamimidoyl-but...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078557 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078552 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078560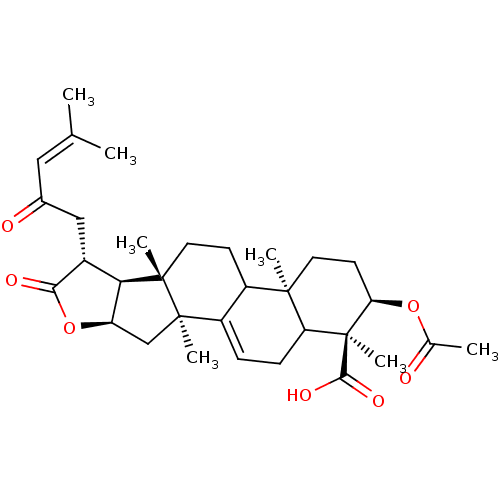 ((1S,2R,4aR,6aS,6bS,7R,9aR,10aS)-2-Acetoxy-1,4a,6a,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078548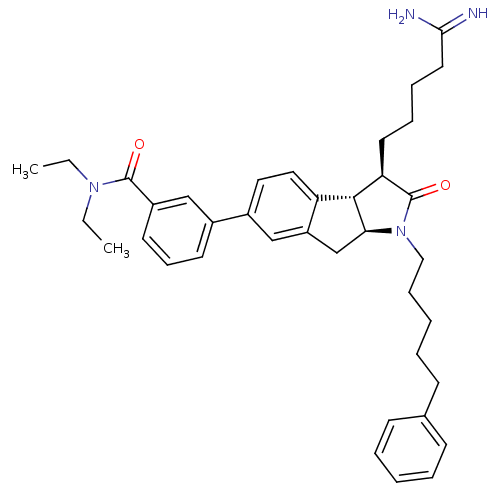 (3-[(3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-2-oxo-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078547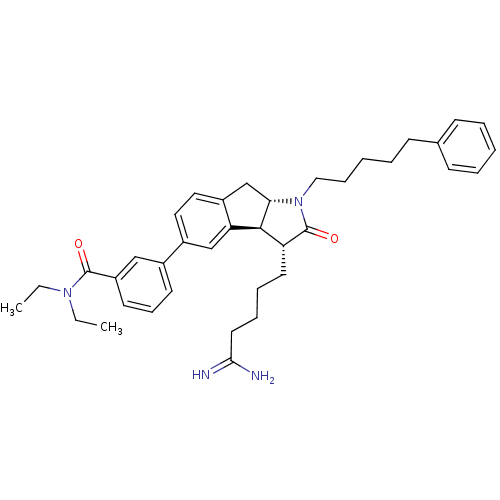 (3-[(3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-2-oxo-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078549 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078549 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078556 (3-[(3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-2-oxo-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078548 (3-[(3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-2-oxo-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |