 Found 1369 hits with Last Name = 'jiaang' and Initial = 'wt'
Found 1369 hits with Last Name = 'jiaang' and Initial = 'wt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50326392

((2S)-1-({(2S,4S)-4-[2-(1,3-Dihydro-2H-isoindol-2-y...)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)[C@@H]1C[C@@H](CC(=O)N2Cc3ccccc3C2)C(=O)N1 |r| Show InChI InChI=1S/C20H20F2N4O3/c21-20(22)7-15(8-23)26(11-20)19(29)16-5-14(18(28)24-16)6-17(27)25-9-12-3-1-2-4-13(12)10-25/h1-4,14-16H,5-7,9-11H2,(H,24,28)/t14-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysis |
J Med Chem 53: 6572-83 (2010)
Article DOI: 10.1021/jm1002556
BindingDB Entry DOI: 10.7270/Q23F4QM9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50326373
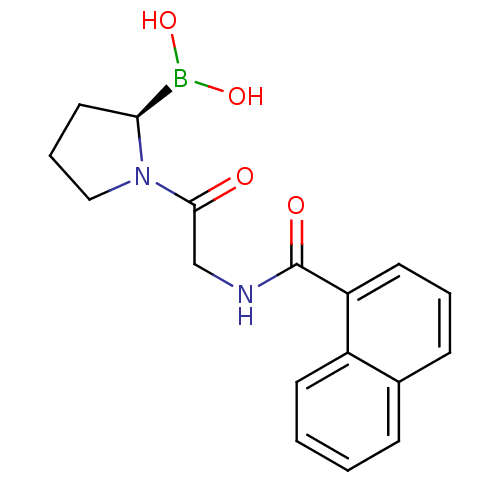
((R)-1-(2-(1-naphthamido)acetyl)pyrrolidin-2-ylboro...)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C17H19BN2O4/c21-16(20-10-4-9-15(20)18(23)24)11-19-17(22)14-8-3-6-12-5-1-2-7-13(12)14/h1-3,5-8,15,23-24H,4,9-11H2,(H,19,22)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysis |
J Med Chem 53: 6572-83 (2010)
Article DOI: 10.1021/jm1002556
BindingDB Entry DOI: 10.7270/Q23F4QM9 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT A loop exon 17 D820Y single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 phosphorylation at 0.1 to 1000 nM after 1 hr by... |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 ITD mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 ITD phosphorylation at 0.1 to 1000 n... |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 D835Y mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 D835Y phosphorylation at 0.1 to 10... |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT A loop exon 17 Y823D single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT A loop exon 11/17 V560G/N822K double mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot k... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT A loop exon 17 D816H single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT A loop exon 17 D820E single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT A loop exon 18 A829P single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT JM domain exon 11 V560G single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389234
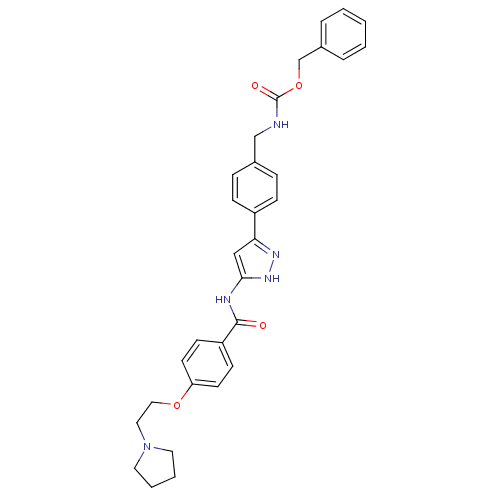
(CHEMBL2063324)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C31H33N5O4/c37-30(26-12-14-27(15-13-26)39-19-18-36-16-4-5-17-36)33-29-20-28(34-35-29)25-10-8-23(9-11-25)21-32-31(38)40-22-24-6-2-1-3-7-24/h1-3,6-15,20H,4-5,16-19,21-22H2,(H,32,38)(H2,33,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human MV4-11 cells after 2 hrs by Western blot analysis |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT ATP binding domain exon 13 K642E single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspo... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT ATP binding domain exon 14 T670I single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspo... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12178
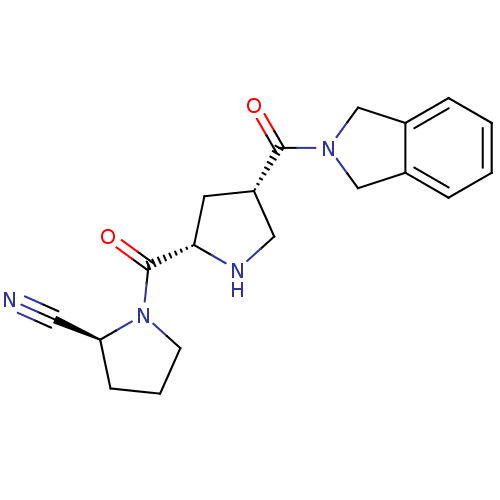
((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...)Show SMILES O=C([C@@H]1CN[C@@H](C1)C(=O)N1CCC[C@H]1C#N)N1Cc2ccccc2C1 |r| Show InChI InChI=1S/C19H22N4O2/c20-9-16-6-3-7-23(16)19(25)17-8-15(10-21-17)18(24)22-11-13-4-1-2-5-14(13)12-22/h1-2,4-5,15-17,21H,3,6-8,10-12H2/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes
| Assay Description
The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... |
Bioorg Med Chem Lett 16: 3268-72 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.037
BindingDB Entry DOI: 10.7270/Q22805V8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50257317

((S)-1-((S)-2-amino-5-(isoindolin-2-yl)-5-oxopentan...)Show SMILES N[C@@H](CCC(=O)N1Cc2ccccc2C1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H22N4O2/c19-10-15-6-3-9-22(15)18(24)16(20)7-8-17(23)21-11-13-4-1-2-5-14(13)12-21/h1-2,4-5,15-16H,3,6-9,11-12,20H2/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50434621
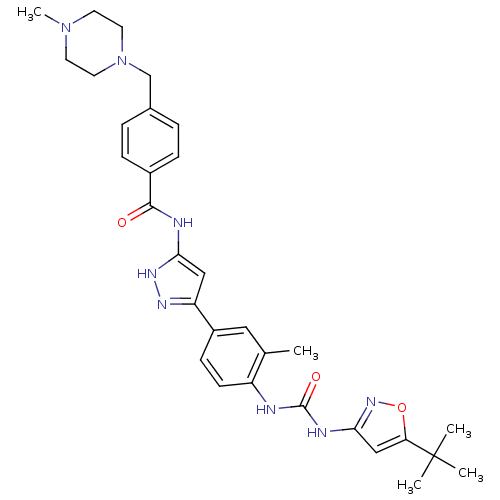
(CHEMBL2386796)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cc(n[nH]2)-c2ccc(NC(=O)Nc3cc(on3)C(C)(C)C)c(C)c2)CC1 Show InChI InChI=1S/C31H38N8O3/c1-20-16-23(10-11-24(20)32-30(41)34-28-18-26(42-37-28)31(2,3)4)25-17-27(36-35-25)33-29(40)22-8-6-21(7-9-22)19-39-14-12-38(5)13-15-39/h6-11,16-18H,12-15,19H2,1-5H3,(H2,32,34,37,41)(H2,33,35,36,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST tagged FLT3 kinase (567 to 993) (unknown origin) transfected in insect sf9 cells after 4 hrs by wallac counting analysis |
Bioorg Med Chem 21: 2856-67 (2013)
Article DOI: 10.1016/j.bmc.2013.03.083
BindingDB Entry DOI: 10.7270/Q22N53NF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50265158

(CHEMBL497992 | N-((trans)-4-((S)-1-amino-2-oxo-2-(...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(NS(=O)(=O)CC(F)(F)F)cc1)C(=O)N1CCCC1 |r,wU:5.8,wD:2.1,1.0,(13.27,5.92,;14.6,5.15,;14.6,3.61,;13.27,2.83,;13.27,1.28,;14.6,.51,;15.95,1.28,;15.95,2.83,;14.6,-1.03,;13.27,-1.79,;14.03,-3.13,;12.5,-.46,;11.93,-2.55,;10.6,-1.76,;9.26,-2.51,;9.25,-4.05,;7.91,-4.81,;7.89,-6.35,;6.35,-6.34,;9.43,-6.37,;7.88,-7.89,;6.54,-8.65,;5.2,-9.4,;7.3,-9.99,;5.78,-7.31,;10.58,-4.84,;11.91,-4.08,;15.93,5.92,;15.93,7.46,;17.27,5.15,;17.43,3.63,;18.93,3.31,;19.7,4.64,;18.67,5.79,)| Show InChI InChI=1S/C20H29F3N4O5S2/c21-20(22,23)13-33(29,30)25-15-7-9-17(10-8-15)34(31,32)26-16-5-3-14(4-6-16)18(24)19(28)27-11-1-2-12-27/h7-10,14,16,18,25-26H,1-6,11-13,24H2/t14-,16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus system |
Eur J Med Chem 43: 1603-11 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.014
BindingDB Entry DOI: 10.7270/Q2V69JCD |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged VEGFR2 expressed in Sf9 insect cells after 120 mins by Kinase-Glo assay |
Bioorg Med Chem 19: 4173-82 (2011)
Article DOI: 10.1016/j.bmc.2011.06.016
BindingDB Entry DOI: 10.7270/Q2CR5TQV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human C-src using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50225074

((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |TLB:1:12:15:20.18.17,THB:13:14:17:22.12.21,13:12:15.14.20:17,21:12:15:20.18.17,21:18:15:22.13.12,19:18:15:22.13.12| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3596-600 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.124
BindingDB Entry DOI: 10.7270/Q29Z953W |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389222
(CHEMBL2063336)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3cccnc3)cc2)nc(C)n1 Show InChI InChI=1S/C27H31N9O2/c1-19-30-24(15-26(31-19)36-12-10-35(2)11-13-36)32-25-14-23(33-34-25)22-7-5-20(6-8-22)17-29-27(37)38-18-21-4-3-9-28-16-21/h3-9,14-16H,10-13,17-18H2,1-2H3,(H,29,37)(H2,30,31,32,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50434614
(CHEMBL2386803)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cc(n[nH]2)-c2ccc(CNC(=O)Nc3cc(on3)C(C)(C)C)cc2)CC1 Show InChI InChI=1S/C31H38N8O3/c1-31(2,3)26-18-28(37-42-26)34-30(41)32-19-21-5-9-23(10-6-21)25-17-27(36-35-25)33-29(40)24-11-7-22(8-12-24)20-39-15-13-38(4)14-16-39/h5-12,17-18H,13-16,19-20H2,1-4H3,(H2,32,34,37,41)(H2,33,35,36,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST tagged FLT3 kinase (567 to 993) (unknown origin) transfected in insect sf9 cells after 4 hrs by wallac counting analysis |
Bioorg Med Chem 21: 2856-67 (2013)
Article DOI: 10.1016/j.bmc.2013.03.083
BindingDB Entry DOI: 10.7270/Q22N53NF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50521626

(CHEMBL4448433)Show SMILES CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccc(NC(=O)Nc3cc(CC)on3)cc2)ncn1 Show InChI InChI=1S/C25H29N9O2S/c1-3-19-13-22(32-36-19)30-24(35)29-18-7-5-17(6-8-18)20-15-26-25(37-20)31-21-14-23(28-16-27-21)34-11-9-33(4-2)10-12-34/h5-8,13-16H,3-4,9-12H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human c-KIT ATP binding domain exon 13 V654A single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspo... |
J Med Chem 62: 3940-3957 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01845
BindingDB Entry DOI: 10.7270/Q2TB1B92 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50519725

(CHEMBL4513768)Show SMILES Cl.CCN1CCN(CC1)c1cc(Nc2ncc(s2)-c2ccncc2)nc(C)n1 Show InChI InChI=1S/C19H23N7S/c1-3-25-8-10-26(11-9-25)18-12-17(22-14(2)23-18)24-19-21-13-16(27-19)15-4-6-20-7-5-15/h4-7,12-13H,3,8-11H2,1-2H3,(H,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma ATP by hotspot kinase assay |
J Med Chem 62: 11135-11150 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01229
BindingDB Entry DOI: 10.7270/Q2BV7M1W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50257374

((S)-1-((S)-2-cyanopyrrolidin-1-yl)-5-(3,4-dihydroi...)Show SMILES [NH3+][C@@H](CCC(=O)N1CCc2ccccc2C1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C19H24N4O2/c20-12-16-6-3-10-23(16)19(25)17(21)7-8-18(24)22-11-9-14-4-1-2-5-15(14)13-22/h1-2,4-5,16-17H,3,6-11,13,21H2/p+1/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50257435

((S)-1-((S)-2-cyanopyrrolidin-1-yl)-1,5-dioxo-5-(3-...)Show SMILES [NH3+][C@@H](CCC(=O)N1CCn2c(C1)nnc2C(F)(F)F)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H20F3N7O2/c17-16(18,19)15-23-22-12-9-24(6-7-26(12)15)13(27)4-3-11(21)14(28)25-5-1-2-10(25)8-20/h10-11H,1-7,9,21H2/p+1/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389223
(CHEMBL2063337)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3cc(C)on3)cc2)nc(C)n1 Show InChI InChI=1S/C26H31N9O3/c1-17-12-21(33-38-17)16-37-26(36)27-15-19-4-6-20(7-5-19)22-13-24(32-31-22)30-23-14-25(29-18(2)28-23)35-10-8-34(3)9-11-35/h4-7,12-14H,8-11,15-16H2,1-3H3,(H,27,36)(H2,28,29,30,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21079
(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged VEGFR2 expressed in Sf9 insect cells after 120 mins by Kinase-Glo assay |
Bioorg Med Chem 19: 4173-82 (2011)
Article DOI: 10.1016/j.bmc.2011.06.016
BindingDB Entry DOI: 10.7270/Q2CR5TQV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50146971

(2-(S)-Amino-4-(2-chloro-benzylamino)-1-piperidin-1...)Show InChI InChI=1S/C16H24ClN3O/c17-14-7-3-2-6-13(14)12-19-9-8-15(18)16(21)20-10-4-1-5-11-20/h2-3,6-7,15,19H,1,4-5,8-12,18H2/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged DPP2 expressed in insect cells |
J Med Chem 53: 6572-83 (2010)
Article DOI: 10.1021/jm1002556
BindingDB Entry DOI: 10.7270/Q23F4QM9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320120
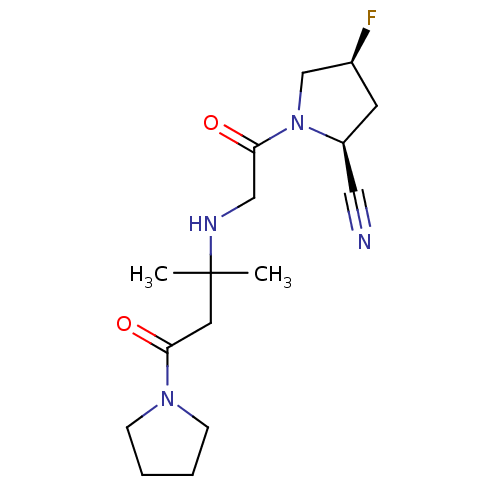
((2S,4S)-4-fluoro-1-(2-(2-methyl-4-oxo-4-(pyrrolidi...)Show SMILES CC(C)(CC(=O)N1CCCC1)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C16H25FN4O2/c1-16(2,8-14(22)20-5-3-4-6-20)19-10-15(23)21-11-12(17)7-13(21)9-18/h12-13,19H,3-8,10-11H2,1-2H3/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in presence of 50% human serum |
Bioorg Med Chem Lett 20: 3596-600 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.124
BindingDB Entry DOI: 10.7270/Q29Z953W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50257433
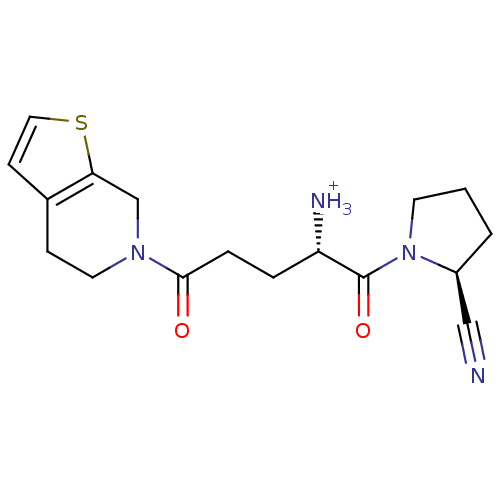
((S)-1-((S)-2-cyanopyrrolidin-1-yl)-5-(4,5-dihydrot...)Show SMILES [NH3+][C@@H](CCC(=O)N1CCc2ccsc2C1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H22N4O2S/c18-10-13-2-1-7-21(13)17(23)14(19)3-4-16(22)20-8-5-12-6-9-24-15(12)11-20/h6,9,13-14H,1-5,7-8,11,19H2/p+1/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50256766

((S)-1-((S)-2-cyanopyrrolidin-1-yl)-1,5-dioxo-5-(2-...)Show SMILES [NH3+][C@@H](CCC(=O)N1CCn2cc(nc2C1)C(F)(F)F)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H21F3N6O2/c18-17(19,20)13-9-24-6-7-25(10-14(24)23-13)15(27)4-3-12(22)16(28)26-5-1-2-11(26)8-21/h9,11-12H,1-7,10,22H2/p+1/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50434629

(CHEMBL2386788)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(NC(=O)Nc2cc(C)on2)cc1 Show InChI InChI=1S/C26H28N8O3/c1-17-15-24(32-37-17)29-26(36)27-20-7-3-18(4-8-20)22-16-23(31-30-22)28-25(35)19-5-9-21(10-6-19)34-13-11-33(2)12-14-34/h3-10,15-16H,11-14H2,1-2H3,(H2,27,29,32,36)(H2,28,30,31,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST tagged FLT3 kinase (567 to 993) (unknown origin) transfected in insect sf9 cells after 4 hrs by wallac counting analysis |
Bioorg Med Chem 21: 2856-67 (2013)
Article DOI: 10.1016/j.bmc.2013.03.083
BindingDB Entry DOI: 10.7270/Q22N53NF |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50349015
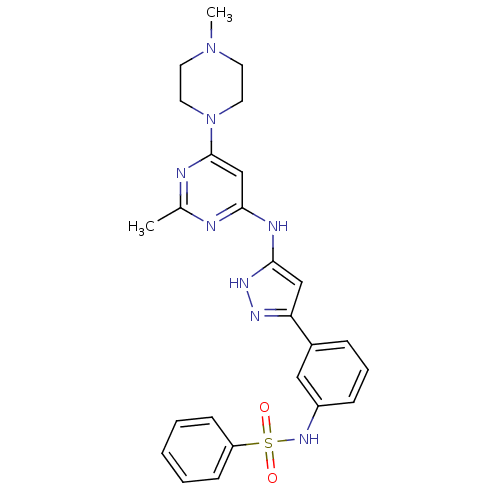
(CHEMBL1807483)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(n[nH]2)-c2cccc(NS(=O)(=O)c3ccccc3)c2)nc(C)n1 Show InChI InChI=1S/C25H28N8O2S/c1-18-26-23(17-25(27-18)33-13-11-32(2)12-14-33)28-24-16-22(29-30-24)19-7-6-8-20(15-19)31-36(34,35)21-9-4-3-5-10-21/h3-10,15-17,31H,11-14H2,1-2H3,(H2,26,27,28,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 expressing wild type kinase domain expressed in Sf9 insect cells after 4 hrs by Kinase-Glo assay |
Bioorg Med Chem 19: 4173-82 (2011)
Article DOI: 10.1016/j.bmc.2011.06.016
BindingDB Entry DOI: 10.7270/Q2CR5TQV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389233
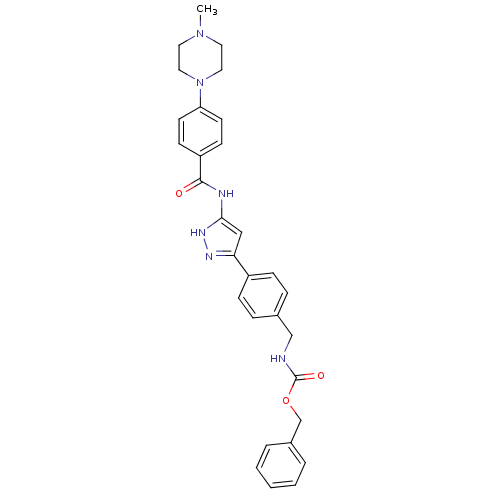
(CHEMBL2063323)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C30H32N6O3/c1-35-15-17-36(18-16-35)26-13-11-25(12-14-26)29(37)32-28-19-27(33-34-28)24-9-7-22(8-10-24)20-31-30(38)39-21-23-5-3-2-4-6-23/h2-14,19H,15-18,20-21H2,1H3,(H,31,38)(H2,32,33,34,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50257434
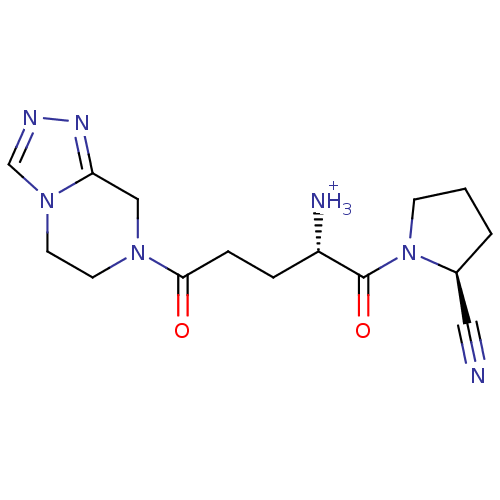
((S)-1-((S)-2-cyanopyrrolidin-1-yl)-5-(5,6-dihydro-...)Show SMILES [NH3+][C@@H](CCC(=O)N1CCn2cnnc2C1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C15H21N7O2/c16-8-11-2-1-5-22(11)15(24)12(17)3-4-14(23)20-6-7-21-10-18-19-13(21)9-20/h10-12H,1-7,9,17H2/p+1/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50256767
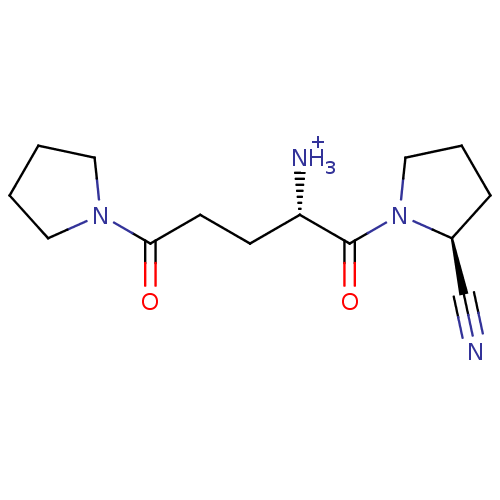
((S)-1-((S)-2-cyanopyrrolidin-1-yl)-1,5-dioxo-5-(py...)Show SMILES [NH3+][C@@H](CCC(=O)N1CCCC1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C14H22N4O2/c15-10-11-4-3-9-18(11)14(20)12(16)5-6-13(19)17-7-1-2-8-17/h11-12H,1-9,16H2/p+1/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50256830
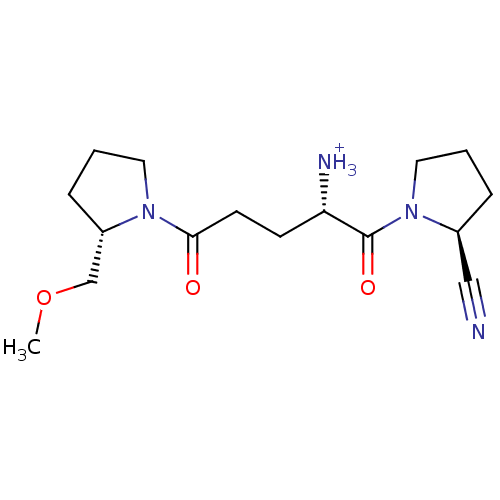
((S)-1-((S)-2-cyanopyrrolidin-1-yl)-5-((S)-2-(metho...)Show SMILES COC[C@@H]1CCCN1C(=O)CC[C@H]([NH3+])C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H26N4O3/c1-23-11-13-5-3-8-19(13)15(21)7-6-14(18)16(22)20-9-2-4-12(20)10-17/h12-14H,2-9,11,18H2,1H3/p+1/t12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50096120
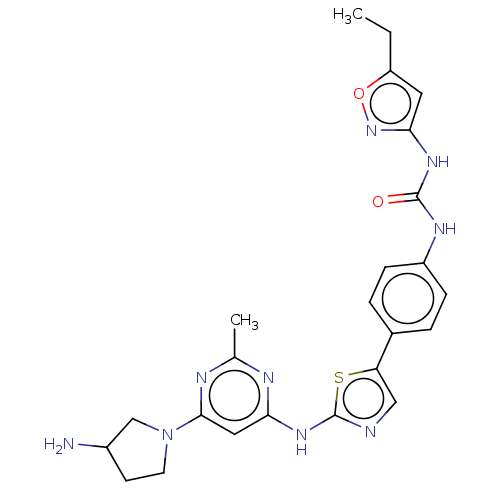
(CHEMBL3593291)Show SMILES CCc1cc(NC(=O)Nc2ccc(cc2)-c2cnc(Nc3cc(nc(C)n3)N3CCC(N)C3)s2)no1 Show InChI InChI=1S/C24H27N9O2S/c1-3-18-10-21(32-35-18)30-23(34)29-17-6-4-15(5-7-17)19-12-26-24(36-19)31-20-11-22(28-14(2)27-20)33-9-8-16(25)13-33/h4-7,10-12,16H,3,8-9,13,25H2,1-2H3,(H,26,27,28,31)(H2,29,30,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged VEGFR2 kinase domain (V789 to V1356) (unknown origin) expressed in insect Sf9 cells using polyGlu4:Tyr peptide a... |
Eur J Med Chem 100: 151-61 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.008
BindingDB Entry DOI: 10.7270/Q25H7J12 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50349013
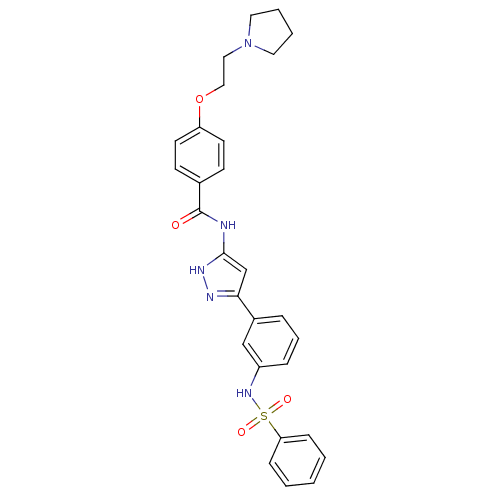
(CHEMBL1807478)Show SMILES O=C(Nc1cc(n[nH]1)-c1cccc(NS(=O)(=O)c2ccccc2)c1)c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C28H29N5O4S/c34-28(21-11-13-24(14-12-21)37-18-17-33-15-4-5-16-33)29-27-20-26(30-31-27)22-7-6-8-23(19-22)32-38(35,36)25-9-2-1-3-10-25/h1-3,6-14,19-20,32H,4-5,15-18H2,(H2,29,30,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 expressing wild type kinase domain expressed in Sf9 insect cells after 4 hrs by Kinase-Glo assay |
Bioorg Med Chem 19: 4173-82 (2011)
Article DOI: 10.1016/j.bmc.2011.06.016
BindingDB Entry DOI: 10.7270/Q2CR5TQV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389228

(CHEMBL2063319)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C29H30N6O3/c1-34-15-17-35(18-16-34)25-13-9-23(10-14-25)28(36)31-27-19-26(32-33-27)22-7-11-24(12-8-22)30-29(37)38-20-21-5-3-2-4-6-21/h2-14,19H,15-18,20H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389235
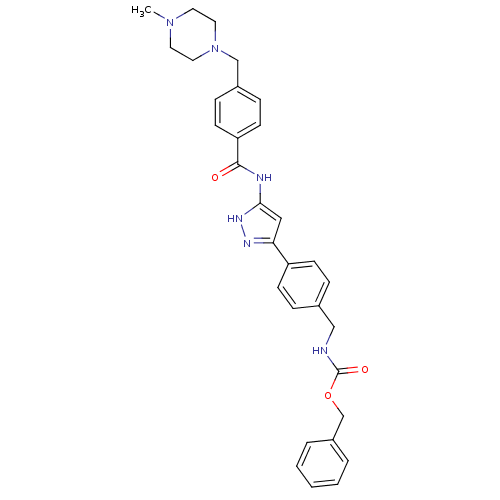
(CHEMBL2063325)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-36-15-17-37(18-16-36)21-24-9-13-27(14-10-24)30(38)33-29-19-28(34-35-29)26-11-7-23(8-12-26)20-32-31(39)40-22-25-5-3-2-4-6-25/h2-14,19H,15-18,20-22H2,1H3,(H,32,39)(H2,33,34,35,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50349004
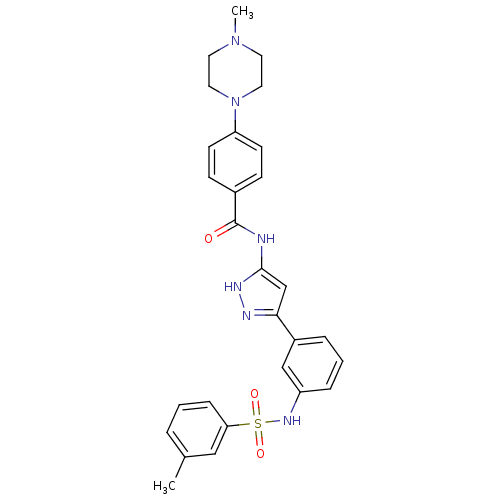
(CHEMBL1807469)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1cccc(NS(=O)(=O)c2cccc(C)c2)c1 Show InChI InChI=1S/C28H30N6O3S/c1-20-5-3-8-25(17-20)38(36,37)32-23-7-4-6-22(18-23)26-19-27(31-30-26)29-28(35)21-9-11-24(12-10-21)34-15-13-33(2)14-16-34/h3-12,17-19,32H,13-16H2,1-2H3,(H2,29,30,31,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 expressing wild type kinase domain expressed in Sf9 insect cells after 4 hrs by Kinase-Glo assay |
Bioorg Med Chem 19: 4173-82 (2011)
Article DOI: 10.1016/j.bmc.2011.06.016
BindingDB Entry DOI: 10.7270/Q2CR5TQV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50256935
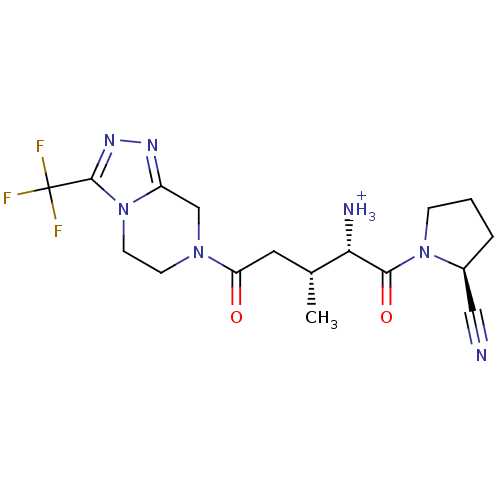
((2S,3R)-1-((S)-2-cyanopyrrolidin-1-yl)-3-methyl-1,...)Show SMILES C[C@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)[C@H]([NH3+])C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H22F3N7O2/c1-10(14(22)15(29)26-4-2-3-11(26)8-21)7-13(28)25-5-6-27-12(9-25)23-24-16(27)17(18,19)20/h10-11,14H,2-7,9,22H2,1H3/p+1/t10-,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50256877
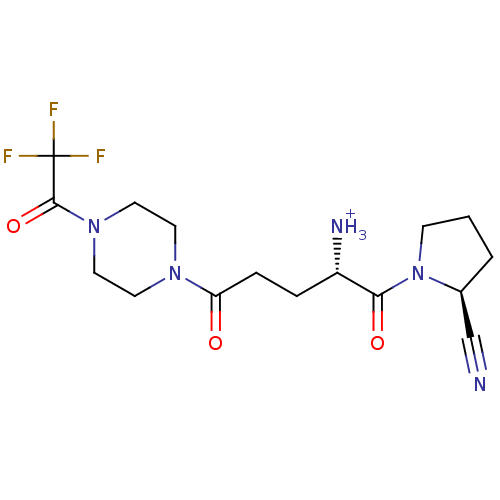
((S)-1-((S)-2-cyanopyrrolidin-1-yl)-1,5-dioxo-5-(4-...)Show SMILES [NH3+][C@@H](CCC(=O)N1CCN(CC1)C(=O)C(F)(F)F)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H22F3N5O3/c17-16(18,19)15(27)23-8-6-22(7-9-23)13(25)4-3-12(21)14(26)24-5-1-2-11(24)10-20/h11-12H,1-9,21H2/p+1/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50434623
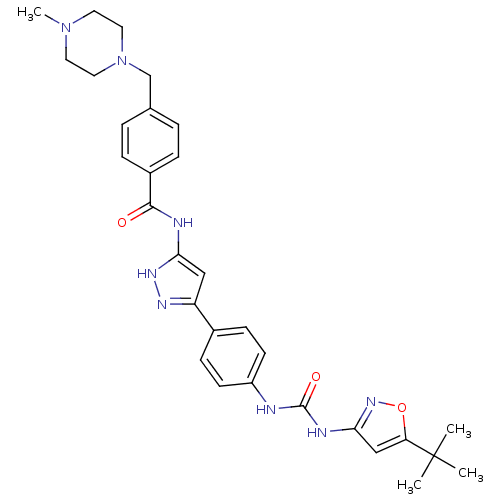
(CHEMBL2386794)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cc(n[nH]2)-c2ccc(NC(=O)Nc3cc(on3)C(C)(C)C)cc2)CC1 Show InChI InChI=1S/C30H36N8O3/c1-30(2,3)25-18-27(36-41-25)33-29(40)31-23-11-9-21(10-12-23)24-17-26(35-34-24)32-28(39)22-7-5-20(6-8-22)19-38-15-13-37(4)14-16-38/h5-12,17-18H,13-16,19H2,1-4H3,(H2,31,33,36,40)(H2,32,34,35,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST tagged FLT3 kinase (567 to 993) (unknown origin) transfected in insect sf9 cells after 4 hrs by wallac counting analysis |
Bioorg Med Chem 21: 2856-67 (2013)
Article DOI: 10.1016/j.bmc.2013.03.083
BindingDB Entry DOI: 10.7270/Q22N53NF |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50349002

(CHEMBL1807467)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1cccc(NS(=O)(=O)c2cccc(Cl)c2)c1 Show InChI InChI=1S/C27H27ClN6O3S/c1-33-12-14-34(15-13-33)23-10-8-19(9-11-23)27(35)29-26-18-25(30-31-26)20-4-2-6-22(16-20)32-38(36,37)24-7-3-5-21(28)17-24/h2-11,16-18,32H,12-15H2,1H3,(H2,29,30,31,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FLT3 expressing wild type kinase domain expressed in Sf9 insect cells after 4 hrs by Kinase-Glo assay |
Bioorg Med Chem 19: 4173-82 (2011)
Article DOI: 10.1016/j.bmc.2011.06.016
BindingDB Entry DOI: 10.7270/Q2CR5TQV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data