 Found 27 hits with Last Name = 'jiang' and Initial = 'hl'
Found 27 hits with Last Name = 'jiang' and Initial = 'hl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441

((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...)Show SMILES C\C=C1/C2Cc3[nH]c(=O)ccc3C1(N)CC(C)=C2 |c:18,TLB:10:11:2:17.15.14,THB:1:2:4.5.11:17.15.14| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 175 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... |
Biochemistry 41: 10810-8 (2002)
Article DOI: 10.1021/bi020151+
BindingDB Entry DOI: 10.7270/Q2HQ3X52 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10632
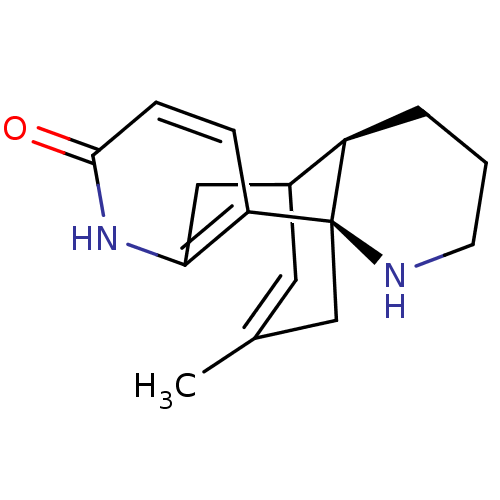
((-)-Huperzine B | (1R,10R)-16-methyl-6,14-diazatet...)Show SMILES CC1=CC2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,TLB:17:18:4.5.11:2.1.13,THB:10:11:18:2.1.13| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11?,12-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 334 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... |
Biochemistry 41: 10810-8 (2002)
Article DOI: 10.1021/bi020151+
BindingDB Entry DOI: 10.7270/Q2HQ3X52 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441

((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...)Show SMILES C\C=C1/C2Cc3[nH]c(=O)ccc3C1(N)CC(C)=C2 |c:18,TLB:10:11:2:17.15.14,THB:1:2:4.5.11:17.15.14| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.30E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... |
Biochemistry 41: 10810-8 (2002)
Article DOI: 10.1021/bi020151+
BindingDB Entry DOI: 10.7270/Q2HQ3X52 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50421411
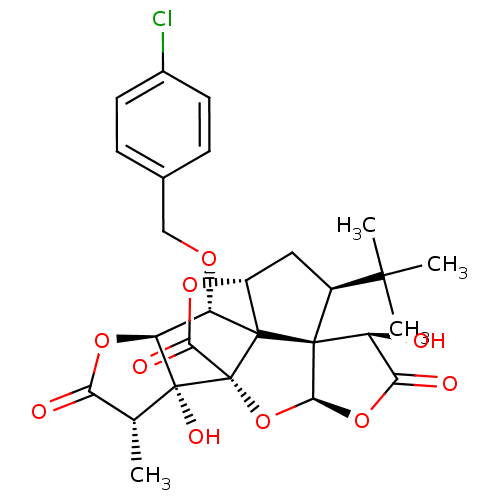
(CHEMBL2304168)Show SMILES C[C@@H]1C(=O)O[C@H]2[C@H](OCc3ccc(Cl)cc3)C34[C@H]5C[C@@H](C(C)(C)C)[C@@]33[C@@H](O)C(=O)O[C@H]3O[C@@]4(C(=O)O5)[C@@]12O Show InChI InChI=1S/C27H29ClO10/c1-11-19(30)36-18-17(34-10-12-5-7-13(28)8-6-12)25-15-9-14(23(2,3)4)24(25)16(29)20(31)37-22(24)38-27(25,21(32)35-15)26(11,18)33/h5-8,11,14-18,22,29,33H,9-10H2,1-4H3/t11-,14+,15-,16+,17+,18+,22+,24+,25?,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Compound was evaluated for anti-platelet activating factor potency |
Bioorg Med Chem Lett 8: 1291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5PB6 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50421412

(CHEMBL2304167)Show SMILES CC1C(=O)O[C@H]2[C@H](O)C34[C@H]5C[C@@H](C(C)(C)C)[C@]33[C@@H](OC(=O)[C@@H]3OCOCc3ccccc3)O[C@@]4(C(=O)O5)[C@@]12O Show InChI InChI=1S/C28H32O11/c1-13-20(30)37-18-17(29)26-16-10-15(24(2,3)4)25(26)19(35-12-34-11-14-8-6-5-7-9-14)21(31)38-23(25)39-28(26,22(32)36-16)27(13,18)33/h5-9,13,15-19,23,29,33H,10-12H2,1-4H3/t13?,15-,16+,17-,18-,19-,23-,25-,26?,27+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Compound was evaluated for anti-platelet activating factor potency |
Bioorg Med Chem Lett 8: 1291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5PB6 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50421410
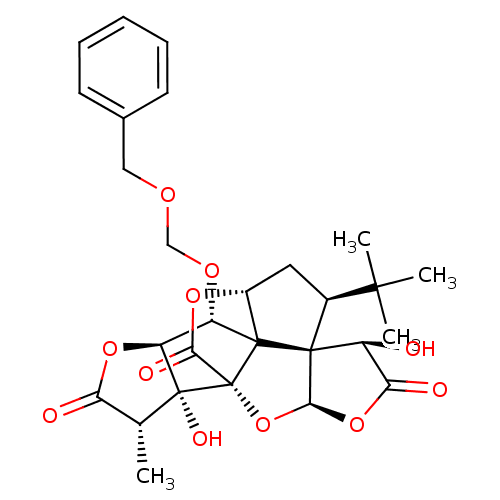
(CHEMBL2304166)Show SMILES C[C@@H]1C(=O)O[C@H]2[C@H](OCOCc3ccccc3)C34[C@H]5C[C@@H](C(C)(C)C)[C@@]33[C@@H](O)C(=O)O[C@H]3O[C@@]4(C(=O)O5)[C@@]12O Show InChI InChI=1S/C28H32O11/c1-13-20(30)37-19-18(35-12-34-11-14-8-6-5-7-9-14)26-16-10-15(24(2,3)4)25(26)17(29)21(31)38-23(25)39-28(26,22(32)36-16)27(13,19)33/h5-9,13,15-19,23,29,33H,10-12H2,1-4H3/t13-,15+,16-,17+,18+,19+,23+,25+,26?,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Compound was evaluated for anti-platelet activating factor potency |
Bioorg Med Chem Lett 8: 1291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5PB6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346088

((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@]1(CC[C@@H](CC1)C(O)=O)C#N |r,wU:17.22,14.25,(4.79,2.97,;3.46,3.74,;2.12,2.97,;.79,3.74,;-.54,2.97,;-.54,1.43,;.79,.66,;2.12,1.43,;3.46,.66,;3.46,-.88,;4.7,-1.79,;4.23,-3.25,;2.69,-3.25,;2.21,-1.79,;-1.88,.66,;-3.42,.71,;-4.23,-.6,;-3.51,-1.96,;-1.97,-2.01,;-1.16,-.7,;-4.33,-3.26,;-3.6,-4.62,;-5.87,-3.21,;-1.77,2.19,;-1.66,3.73,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23)/t14-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50251276
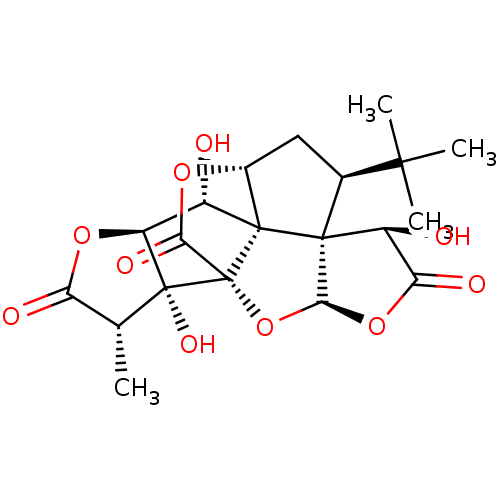
(BN 52021 | CHEMBL514432 | GINKOLIDE B | Gingkolide...)Show SMILES C[C@@H]1C(=O)O[C@H]2[C@H](O)[C@@]34[C@H]5C[C@@H](C(C)(C)C)[C@@]33[C@@H](O)C(=O)O[C@H]3O[C@@]4(C(=O)O5)[C@@]12O |r| Show InChI InChI=1S/C20H24O10/c1-6-12(23)28-11-9(21)18-8-5-7(16(2,3)4)17(18)10(22)13(24)29-15(17)30-20(18,14(25)27-8)19(6,11)26/h6-11,15,21-22,26H,5H2,1-4H3/t6-,7+,8-,9+,10+,11+,15+,17+,18+,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Compound was evaluated for anti-platelet activating factor potency |
Bioorg Med Chem Lett 8: 1291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5PB6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50365061

(CHEMBL1951070)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCCCNCC(O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |t:11| Show InChI InChI=1S/C31H42N4O6/c1-40-28-14-12-22(18-29(28)41-2)30-23-9-5-6-10-24(23)31(39)35(34-30)16-8-4-3-7-15-32-19-27(38)21-11-13-26(37)25(17-21)33-20-36/h11-14,17-18,20,23-24,27,32,37-38H,3-10,15-16,19H2,1-2H3,(H,33,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50365060
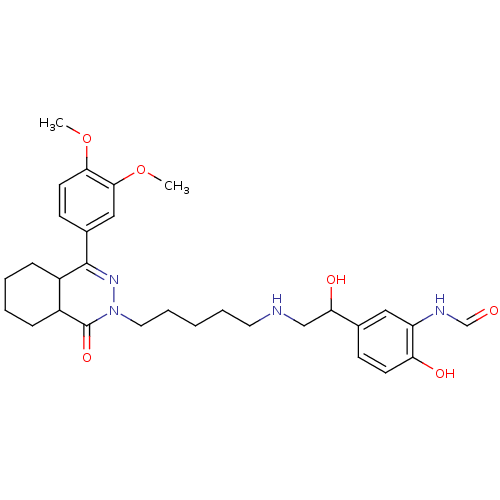
(CHEMBL1951069)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCCNCC(O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |t:11| Show InChI InChI=1S/C30H40N4O6/c1-39-27-13-11-21(17-28(27)40-2)29-22-8-4-5-9-23(22)30(38)34(33-29)15-7-3-6-14-31-18-26(37)20-10-12-25(36)24(16-20)32-19-35/h10-13,16-17,19,22-23,26,31,36-37H,3-9,14-15,18H2,1-2H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50365058

(CHEMBL1951067)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCNC[C@H](O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |r,t:11| Show InChI InChI=1S/C29H38N4O6/c1-38-26-12-10-20(16-27(26)39-2)28-21-7-3-4-8-22(21)29(37)33(32-28)14-6-5-13-30-17-25(36)19-9-11-24(35)23(15-19)31-18-34/h9-12,15-16,18,21-22,25,30,35-36H,3-8,13-14,17H2,1-2H3,(H,31,34)/t21?,22?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50365057
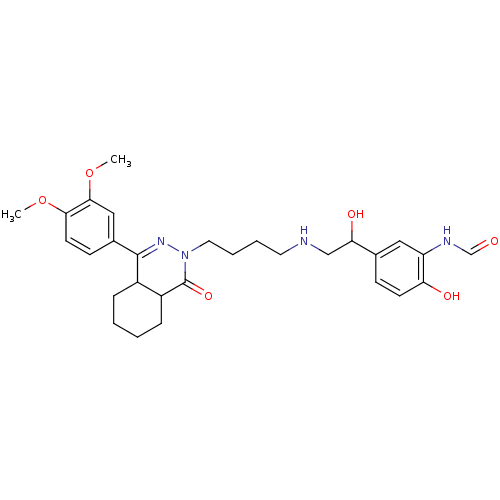
(CHEMBL1951066)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCNCC(O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |t:11| Show InChI InChI=1S/C29H38N4O6/c1-38-26-12-10-20(16-27(26)39-2)28-21-7-3-4-8-22(21)29(37)33(32-28)14-6-5-13-30-17-25(36)19-9-11-24(35)23(15-19)31-18-34/h9-12,15-16,18,21-22,25,30,35-36H,3-8,13-14,17H2,1-2H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50365056

(CHEMBL1951065)Show SMILES COc1ccc(cc1OC)C1=NN(CCNCC(O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |t:11| Show InChI InChI=1S/C27H34N4O6/c1-36-24-10-8-18(14-25(24)37-2)26-19-5-3-4-6-20(19)27(35)31(30-26)12-11-28-15-23(34)17-7-9-22(33)21(13-17)29-16-32/h7-10,13-14,16,19-20,23,28,33-34H,3-6,11-12,15H2,1-2H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50365059
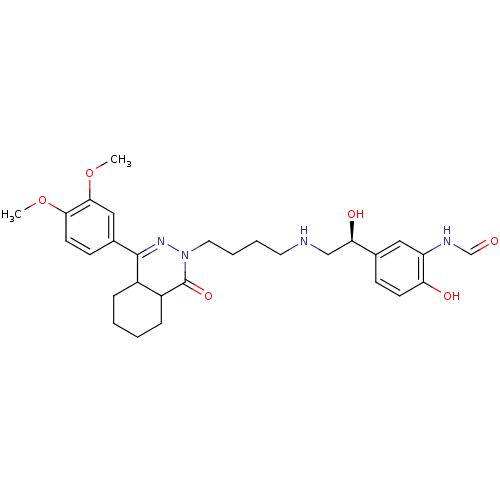
(CHEMBL1951068)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCNC[C@@H](O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |r,t:11| Show InChI InChI=1S/C29H38N4O6/c1-38-26-12-10-20(16-27(26)39-2)28-21-7-3-4-8-22(21)29(37)33(32-28)14-6-5-13-30-17-25(36)19-9-11-24(35)23(15-19)31-18-34/h9-12,15-16,18,21-22,25,30,35-36H,3-8,13-14,17H2,1-2H3,(H,31,34)/t21?,22?,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50042058

((-)-rolipram | (4R)-4-[3-(cyclopentyloxy)-4-methox...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50365055
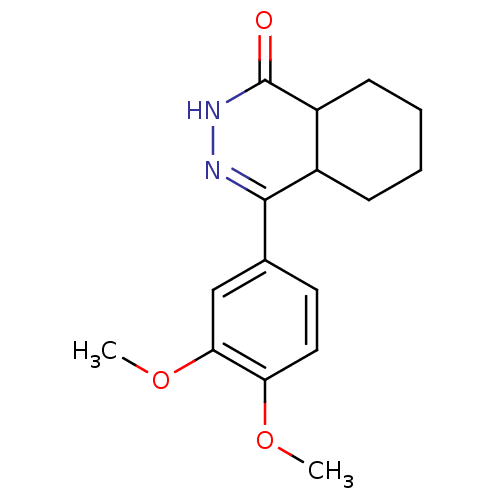
(CHEMBL82318)Show InChI InChI=1S/C16H20N2O3/c1-20-13-8-7-10(9-14(13)21-2)15-11-5-3-4-6-12(11)16(19)18-17-15/h7-9,11-12H,3-6H2,1-2H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50106185
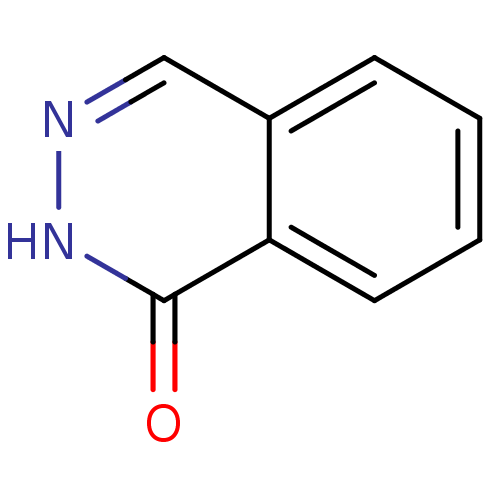
(2H-Phthalazin-1-one | CHEMBL124706)Show InChI InChI=1S/C8H6N2O/c11-8-7-4-2-1-3-6(7)5-9-10-8/h1-5H,(H,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B2-mediated cAMP hydrolysis for 30 mins by colorimetric assay |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50365057

(CHEMBL1951066)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCNCC(O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |t:11| Show InChI InChI=1S/C29H38N4O6/c1-38-26-12-10-20(16-27(26)39-2)28-21-7-3-4-8-22(21)29(37)33(32-28)14-6-5-13-30-17-25(36)19-9-11-24(35)23(15-19)31-18-34/h9-12,15-16,18,21-22,25,30,35-36H,3-8,13-14,17H2,1-2H3,(H,31,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenoceptor in guinea pig assessed as relaxation of histamine-induced tracheal ring contraction |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50365059

(CHEMBL1951068)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCNC[C@@H](O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |r,t:11| Show InChI InChI=1S/C29H38N4O6/c1-38-26-12-10-20(16-27(26)39-2)28-21-7-3-4-8-22(21)29(37)33(32-28)14-6-5-13-30-17-25(36)19-9-11-24(35)23(15-19)31-18-34/h9-12,15-16,18,21-22,25,30,35-36H,3-8,13-14,17H2,1-2H3,(H,31,34)/t21?,22?,25-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenoceptor in guinea pig assessed as relaxation of histamine-induced tracheal ring contraction |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50365060

(CHEMBL1951069)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCCNCC(O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |t:11| Show InChI InChI=1S/C30H40N4O6/c1-39-27-13-11-21(17-28(27)40-2)29-22-8-4-5-9-23(22)30(38)34(33-29)15-7-3-6-14-31-18-26(37)20-10-12-25(36)24(16-20)32-19-35/h10-13,16-17,19,22-23,26,31,36-37H,3-9,14-15,18H2,1-2H3,(H,32,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenoceptor in guinea pig assessed as relaxation of histamine-induced tracheal ring contraction |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50365058

(CHEMBL1951067)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCNC[C@H](O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |r,t:11| Show InChI InChI=1S/C29H38N4O6/c1-38-26-12-10-20(16-27(26)39-2)28-21-7-3-4-8-22(21)29(37)33(32-28)14-6-5-13-30-17-25(36)19-9-11-24(35)23(15-19)31-18-34/h9-12,15-16,18,21-22,25,30,35-36H,3-8,13-14,17H2,1-2H3,(H,31,34)/t21?,22?,25-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Competitive antagonist activity at beta2-adrenoreceptor in guinea pig tracheal ring in the presence of N-(5-((1R)-2-(4-(4-(3,4-dimethoxyphenol)-1-oxo... |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50365056

(CHEMBL1951065)Show SMILES COc1ccc(cc1OC)C1=NN(CCNCC(O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |t:11| Show InChI InChI=1S/C27H34N4O6/c1-36-24-10-8-18(14-25(24)37-2)26-19-5-3-4-6-20(19)27(35)31(30-26)12-11-28-15-23(34)17-7-9-22(33)21(13-17)29-16-32/h7-10,13-14,16,19-20,23,28,33-34H,3-6,11-12,15H2,1-2H3,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenoceptor in guinea pig assessed as relaxation of histamine-induced tracheal ring contraction |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM25392

(4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...)Show InChI InChI=1S/C11H17NO3/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8/h3-5,7,11-15H,6H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenoceptor in guinea pig assessed as relaxation of histamine-induced tracheal ring contraction |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50151720
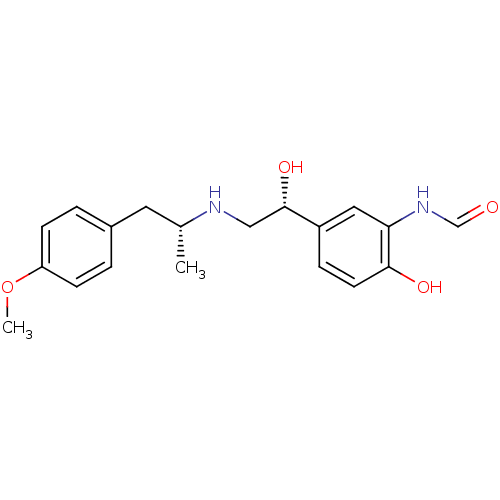
(ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c(NC=O)c2)cc1 |r| Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22)/t13-,19+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenoceptor in guinea pig assessed as relaxation of histamine-induced tracheal ring contraction |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50365061

(CHEMBL1951070)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCCCNCC(O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |t:11| Show InChI InChI=1S/C31H42N4O6/c1-40-28-14-12-22(18-29(28)41-2)30-23-9-5-6-10-24(23)31(39)35(34-30)16-8-4-3-7-15-32-19-27(38)21-11-13-26(37)25(17-21)33-20-36/h11-14,17-18,20,23-24,27,32,37-38H,3-10,15-16,19H2,1-2H3,(H,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenoceptor in guinea pig assessed as relaxation of histamine-induced tracheal ring contraction |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50365058

(CHEMBL1951067)Show SMILES COc1ccc(cc1OC)C1=NN(CCCCNC[C@H](O)c2ccc(O)c(NC=O)c2)C(=O)C2CCCCC12 |r,t:11| Show InChI InChI=1S/C29H38N4O6/c1-38-26-12-10-20(16-27(26)39-2)28-21-7-3-4-8-22(21)29(37)33(32-28)14-6-5-13-30-17-25(36)19-9-11-24(35)23(15-19)31-18-34/h9-12,15-16,18,21-22,25,30,35-36H,3-8,13-14,17H2,1-2H3,(H,31,34)/t21?,22?,25-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenoceptor in guinea pig assessed as relaxation of histamine-induced tracheal ring contraction |
Bioorg Med Chem Lett 22: 1523-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.013
BindingDB Entry DOI: 10.7270/Q2794548 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data