

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50278436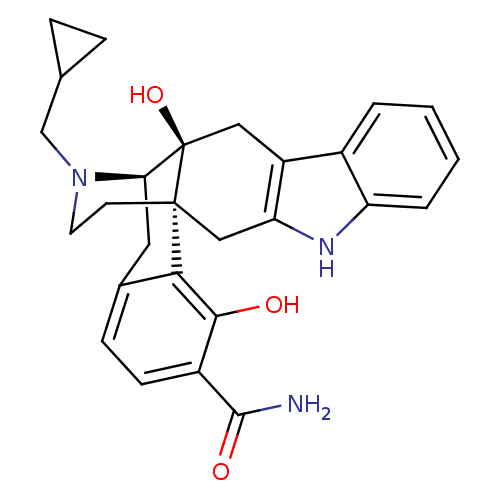 ((1R,13S,14R)-24-(cyclopropylmethyl)-13,20-dihydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412728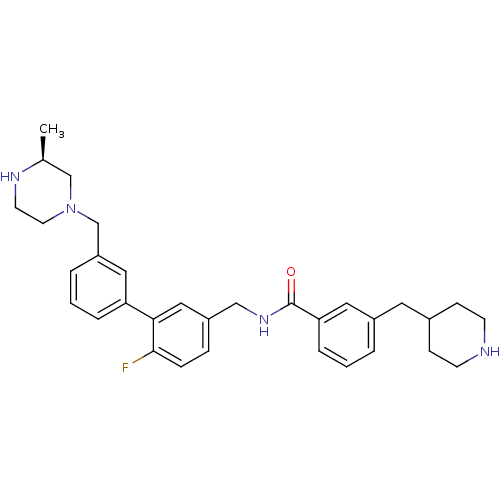 (CHEMBL521523) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 5915-8 (2008) Article DOI: 10.1021/jm800935u BindingDB Entry DOI: 10.7270/Q21G0NHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50278265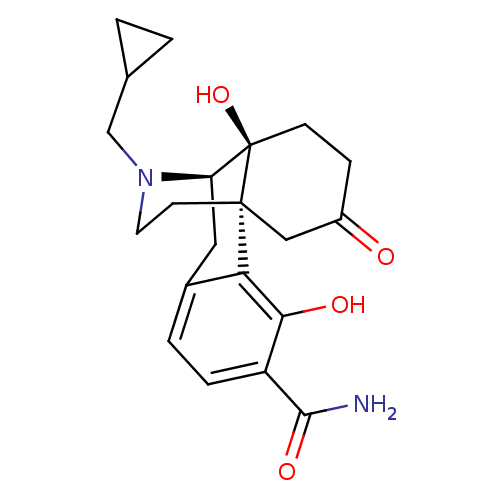 (CCDC 710249, HCl salt | CHEMBL471243) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50278263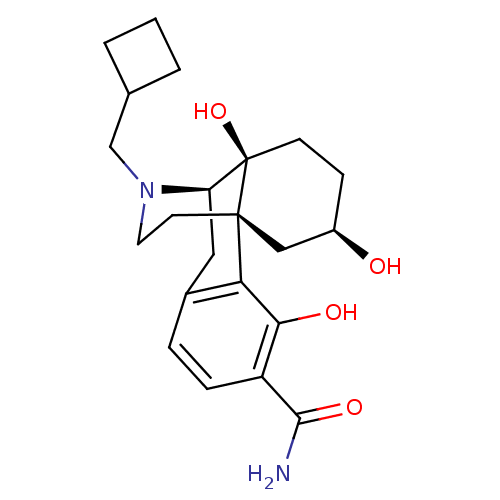 ((1R,9R,10S,13R)-17-(cyclobutylmethyl)-3,10,13-trih...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human DOT1L using oligo-nucleosome/[3H]-SAM as substrate preincubated for 30 mins followed by substrate addition measured after 120 min... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)8-OH-DPAT from human 5HT1A receptor expressed in human HeLa cells measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50278383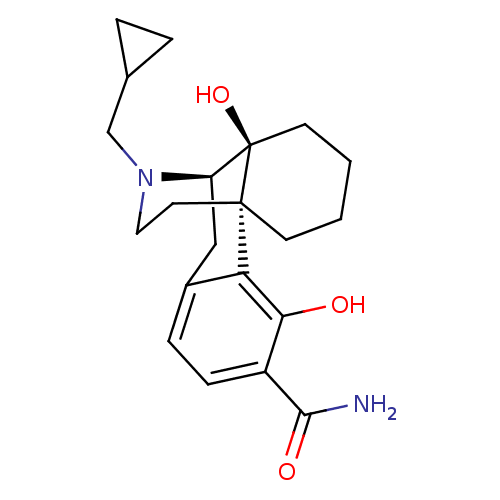 ((1S,9R,10S)-17-(cyclopropylmethyl)-3,10-dihydroxy-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5-HT2A receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127909 BindingDB Entry DOI: 10.7270/Q2R2154C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001043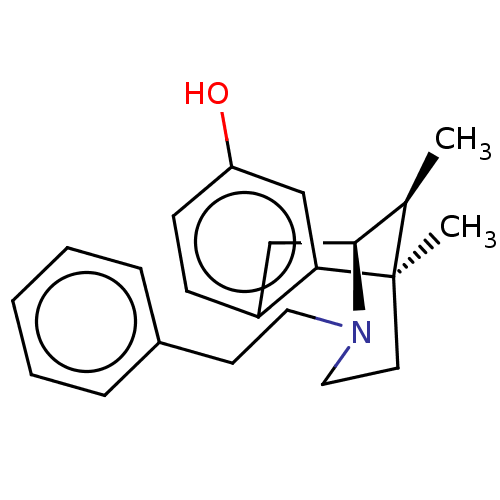 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in guinea pig brain membrane incubated for 2.5 hrs by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112144 BindingDB Entry DOI: 10.7270/Q2WM1J54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50278265 (CCDC 710249, HCl salt | CHEMBL471243) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50278385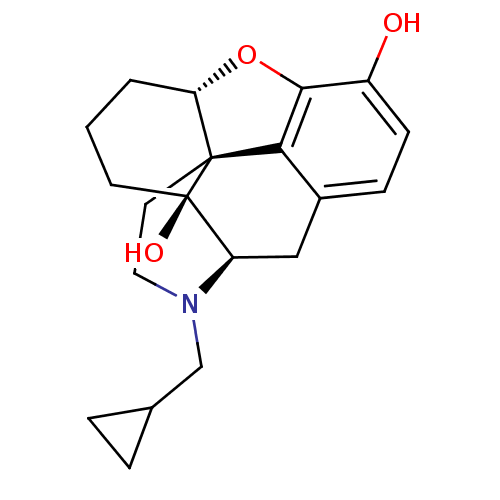 (6-desoxonaltrexone | CHEMBL511816) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50278385 (6-desoxonaltrexone | CHEMBL511816) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50241341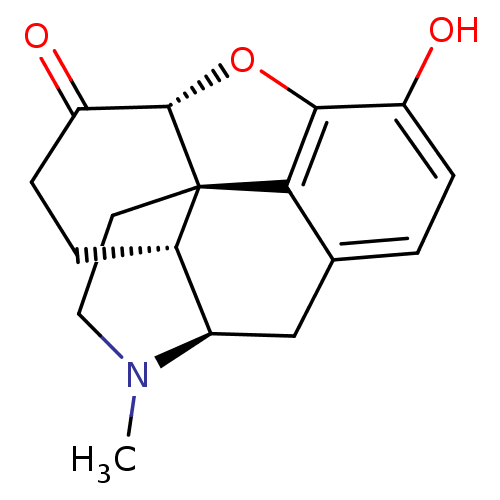 ((-)-(5R)-4,5-Epoxy-3-hydroxy-9alpha-methylmorphina...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50278383 ((1S,9R,10S)-17-(cyclopropylmethyl)-3,10-dihydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50208447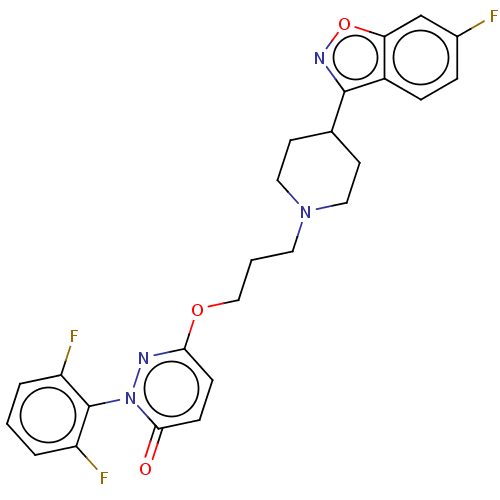 (CHEMBL3883955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5-HT2A receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127909 BindingDB Entry DOI: 10.7270/Q2R2154C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185696 (UNC10108017 | US9156822, 40) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50434823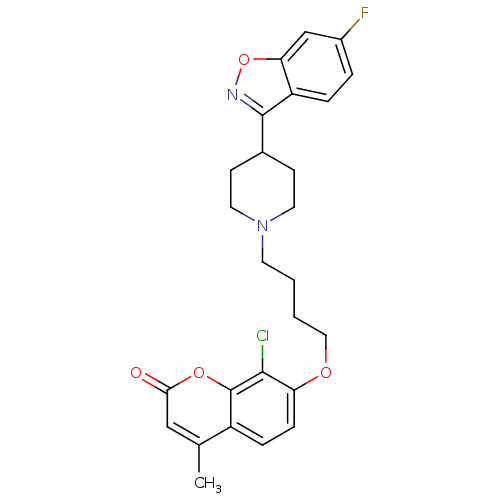 (CHEMBL2387229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5-HT2A receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127909 BindingDB Entry DOI: 10.7270/Q2R2154C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50349866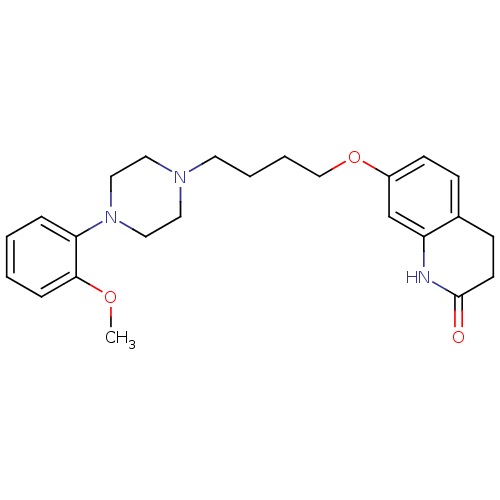 (CHEMBL160296 | CHEMBL1813590 | UNC10108016 | US915...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D2L receptor expressed in CHO cells after 1.5 hrs by microbeta counting method | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244020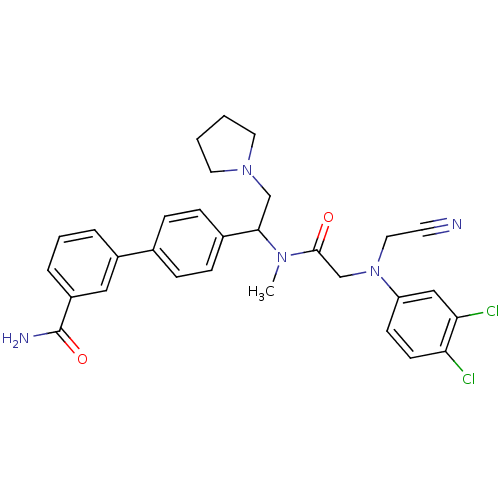 (4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50278386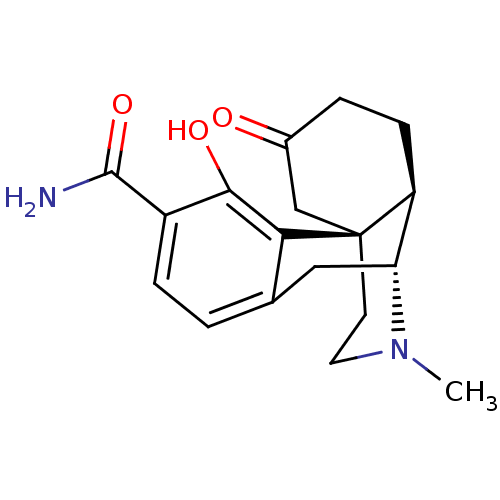 ((1S,9R,10R)-3-hydroxy-17-methyl-13-oxo-17-azatetra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | DrugBank Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]raclopride from human D2 long receptor expressed in CHO cells measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50278437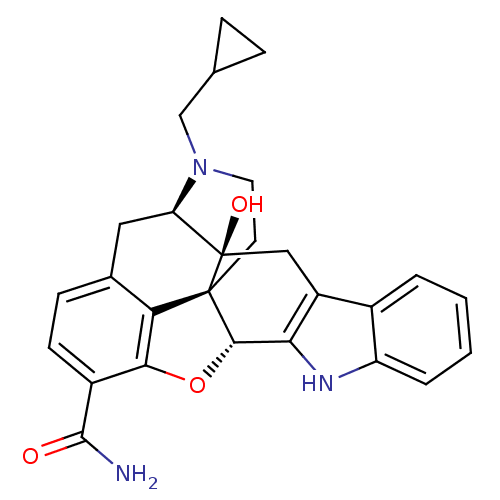 ((1S,2S,13R,21R)-22-(cyclopropylmethyl)-2-hydroxy-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396023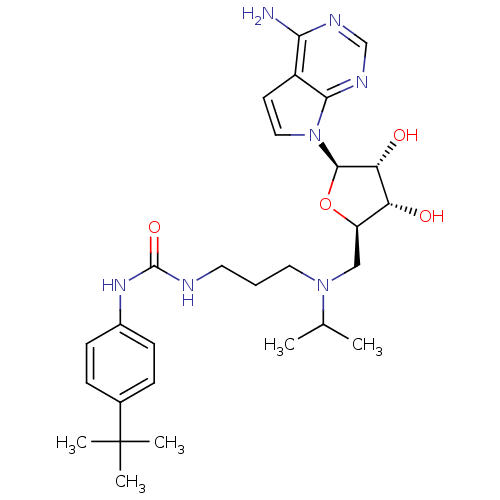 (CHEMBL2169919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Binding affinity to human DOT1L after 120 mins | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396023 (CHEMBL2169919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human DOT1L using oligo-nucleosome/[3H]-SAM as substrate preincubated for 30 mins followed by substrate addition measured after 120 min... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412340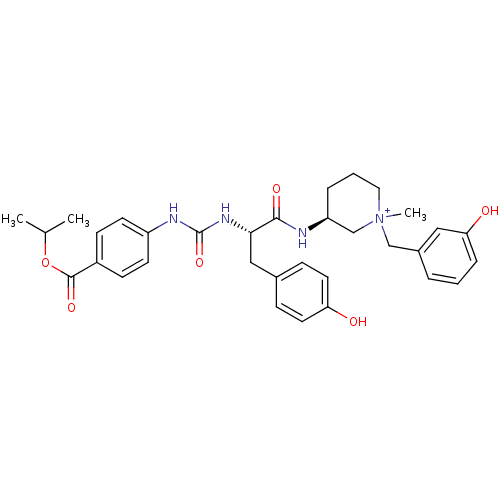 (CHEMBL540359) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M1 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50349866 (CHEMBL160296 | CHEMBL1813590 | UNC10108016 | US915...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50075073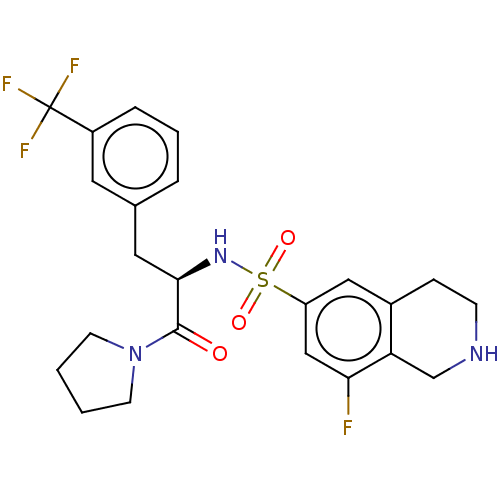 (CHEMBL3414622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of full-length human SETD7 expressed in Escherichia coli BL21 (DE3) using biotinylated histone H3 (1 to 25) as substrate after 1 hr by Fla... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50278263 ((1R,9R,10S,13R)-17-(cyclobutylmethyl)-3,10,13-trih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112144 BindingDB Entry DOI: 10.7270/Q2WM1J54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185698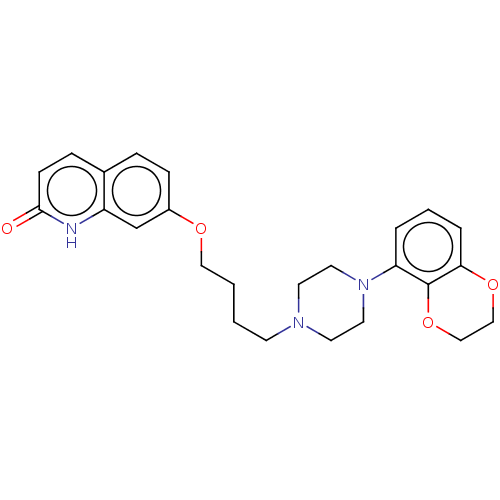 (UNC10108019 | US9156822, 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185697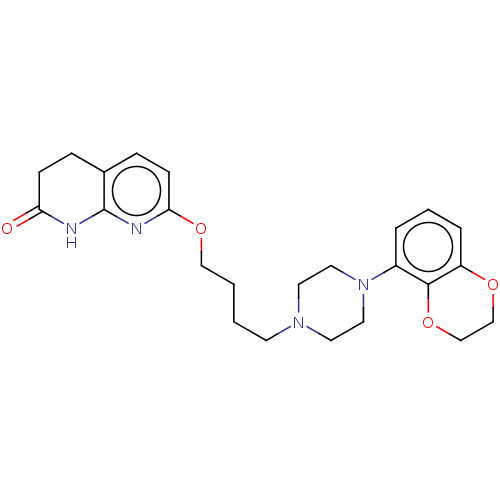 (UNC10108018 | US9156822, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50278212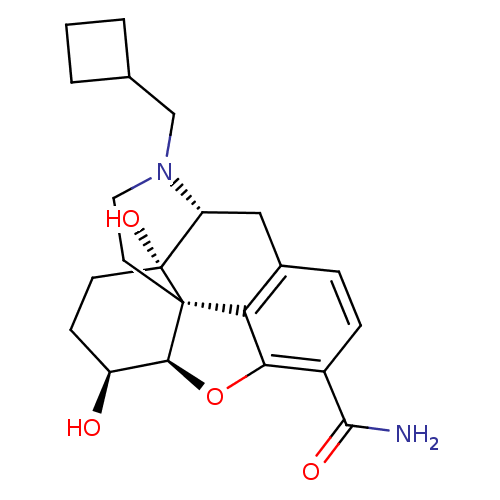 ((1S,5R,13R,14S,17S)-4-(cyclobutylmethyl)-14,17-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185695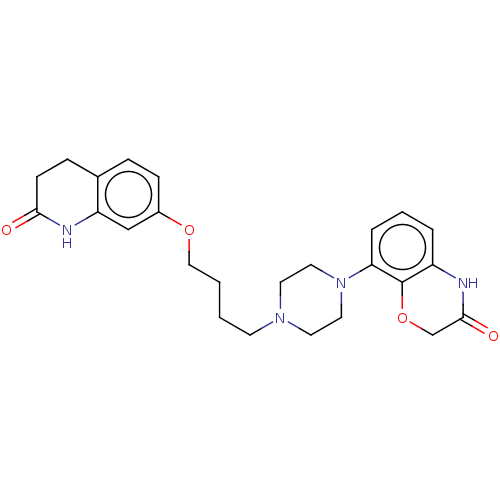 (UNC10108010 | US9156822, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO-K1 cells measured after 20 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641C mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641S mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641N mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641H mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641F mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 A677G mutant assessed as H3K27me1 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 A677G mutant assessed as H3K27me0 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human wild-type EZH2 assessed as H3K27me0 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208447 (CHEMBL3883955) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5-HT6 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127909 BindingDB Entry DOI: 10.7270/Q2R2154C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50347782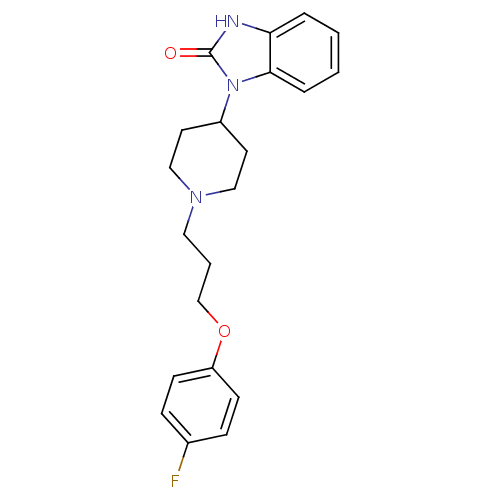 (CHEMBL1802360) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to dopamine D2 receptor | ACS Med Chem Lett 1: 244-248 (2010) Article DOI: 10.1021/ml100105x BindingDB Entry DOI: 10.7270/Q20R9QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50278436 ((1R,13S,14R)-24-(cyclopropylmethyl)-13,20-dihydrox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50278335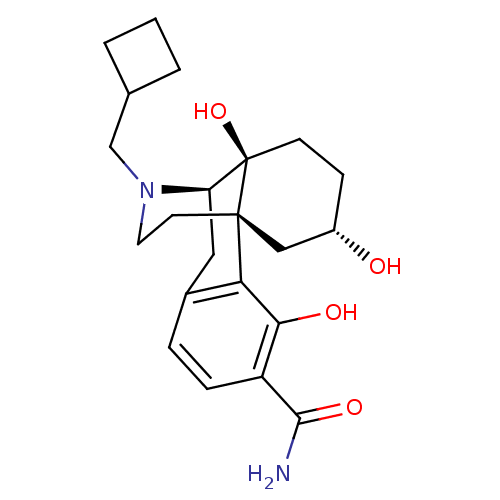 ((1R,9R,10S,13S)-17-(cyclobutylmethyl)-3,10,13-trih...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7185 total ) | Next | Last >> |