 Found 521 hits with Last Name = 'johnson' and Initial = 'ta'
Found 521 hits with Last Name = 'johnson' and Initial = 'ta' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483375

(CHEMBL1236446)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](CC(=O)NO)CO[C@H](CO)[C@H]1O |r| Show InChI InChI=1S/C22H41NO7/c1-2-3-4-5-6-7-8-9-10-11-12-13-20(26)30-22-17(14-19(25)23-28)16-29-18(15-24)21(22)27/h17-18,21-22,24,27-28H,2-16H2,1H3,(H,23,25)/t17-,18+,21+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50221405
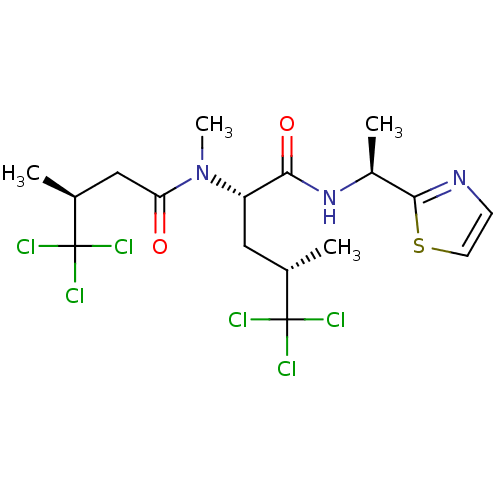
(CHEMBL391079 | dysidenin)Show SMILES C[C@@H](C[C@H](N(C)C(=O)C[C@H](C)C(Cl)(Cl)Cl)C(=O)N[C@@H](C)c1nccs1)C(Cl)(Cl)Cl Show InChI InChI=1S/C17H23Cl6N3O2S/c1-9(16(18,19)20)7-12(14(28)25-11(3)15-24-5-6-29-15)26(4)13(27)8-10(2)17(21,22)23/h5-6,9-12H,7-8H2,1-4H3,(H,25,28)/t9-,10-,11-,12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human 15-hLO1 |
Bioorg Med Chem 15: 6900-8 (2007)
Article DOI: 10.1016/j.bmc.2007.08.015
BindingDB Entry DOI: 10.7270/Q2Z89D81 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50221405

(CHEMBL391079 | dysidenin)Show SMILES C[C@@H](C[C@H](N(C)C(=O)C[C@H](C)C(Cl)(Cl)Cl)C(=O)N[C@@H](C)c1nccs1)C(Cl)(Cl)Cl Show InChI InChI=1S/C17H23Cl6N3O2S/c1-9(16(18,19)20)7-12(14(28)25-11(3)15-24-5-6-29-15)26(4)13(27)8-10(2)17(21,22)23/h5-6,9-12H,7-8H2,1-4H3,(H,25,28)/t9-,10-,11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human 12-hLO |
Bioorg Med Chem 15: 6900-8 (2007)
Article DOI: 10.1016/j.bmc.2007.08.015
BindingDB Entry DOI: 10.7270/Q2Z89D81 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50221404
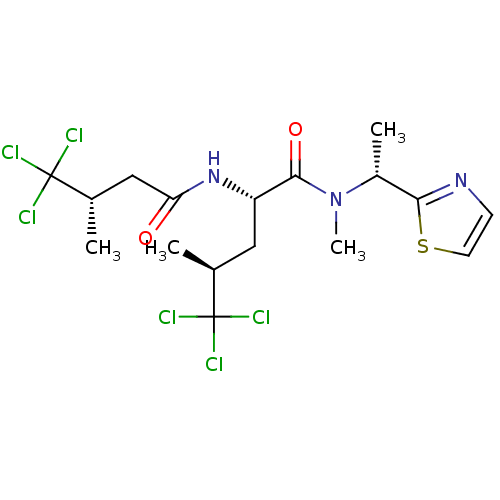
(CHEMBL392270 | neodysidenin)Show SMILES C[C@@H](C[C@H](NC(=O)C[C@H](C)C(Cl)(Cl)Cl)C(=O)N(C)[C@H](C)c1nccs1)C(Cl)(Cl)Cl Show InChI InChI=1S/C17H23Cl6N3O2S/c1-9(16(18,19)20)7-12(25-13(27)8-10(2)17(21,22)23)15(28)26(4)11(3)14-24-5-6-29-14/h5-6,9-12H,7-8H2,1-4H3,(H,25,27)/t9-,10-,11+,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human 12-hLO |
Bioorg Med Chem 15: 6900-8 (2007)
Article DOI: 10.1016/j.bmc.2007.08.015
BindingDB Entry DOI: 10.7270/Q2Z89D81 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50221404

(CHEMBL392270 | neodysidenin)Show SMILES C[C@@H](C[C@H](NC(=O)C[C@H](C)C(Cl)(Cl)Cl)C(=O)N(C)[C@H](C)c1nccs1)C(Cl)(Cl)Cl Show InChI InChI=1S/C17H23Cl6N3O2S/c1-9(16(18,19)20)7-12(25-13(27)8-10(2)17(21,22)23)15(28)26(4)11(3)14-24-5-6-29-14/h5-6,9-12H,7-8H2,1-4H3,(H,25,27)/t9-,10-,11+,12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human 15-hLO1 |
Bioorg Med Chem 15: 6900-8 (2007)
Article DOI: 10.1016/j.bmc.2007.08.015
BindingDB Entry DOI: 10.7270/Q2Z89D81 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484911

(CHEMBL2012204)Show SMILES Cc1cc(no1)[C@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O5/c1-14-12-18(23-28-14)19(24)21(2,20(25)22-26)27-13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-12,19,24,26H,13H2,1-2H3,(H,22,25)/t19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.376 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484910

(CHEMBL2012200)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ccn[nH]1)C(=O)NO |r| Show InChI InChI=1S/C20H21N3O4/c1-20(19(25)23-26,18(24)17-11-12-21-22-17)27-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-12,18,24,26H,13H2,1H3,(H,21,22)(H,23,25)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484904

(CHEMBL2012203)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ccon1)C(=O)NO |r| Show InChI InChI=1S/C20H20N2O5/c1-20(19(24)21-25,18(23)17-11-12-27-22-17)26-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-12,18,23,25H,13H2,1H3,(H,21,24)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483411
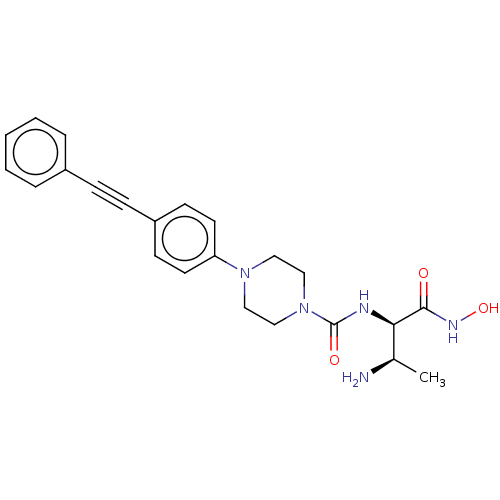
(CHEMBL1668464)Show SMILES C[C@@H](N)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C23H27N5O3/c1-17(24)21(22(29)26-31)25-23(30)28-15-13-27(14-16-28)20-11-9-19(10-12-20)8-7-18-5-3-2-4-6-18/h2-6,9-12,17,21,31H,13-16,24H2,1H3,(H,25,30)(H,26,29)/t17-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484896

(CHEMBL2012205)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1cc(CO)on1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O6/c1-21(20(26)22-27,19(25)18-11-17(12-24)29-23-18)28-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-11,19,24-25,27H,12-13H2,1H3,(H,22,26)/t19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484916

(CHEMBL2012199)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1c[nH]cn1)C(=O)NO |r| Show InChI InChI=1S/C20H21N3O4/c1-20(19(25)23-26,18(24)17-11-21-13-22-17)27-12-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-11,13,18,24,26H,12H2,1H3,(H,21,22)(H,23,25)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484895
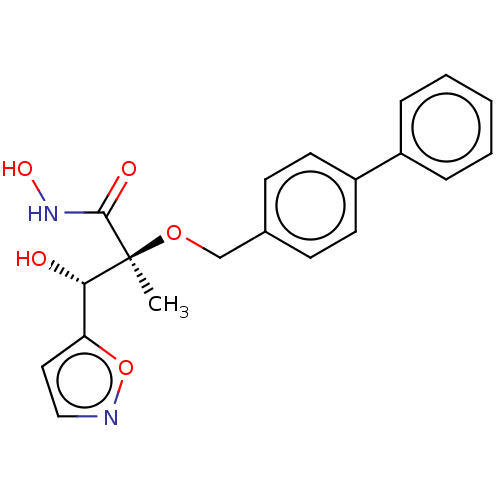
(CHEMBL2012202)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ccno1)C(=O)NO |r| Show InChI InChI=1S/C20H20N2O5/c1-20(19(24)22-25,18(23)17-11-12-21-27-17)26-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-12,18,23,25H,13H2,1H3,(H,22,24)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159770

(US10966980, Example 21 | US9035074, 21)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)N1CCC(CC1)c1cc[nH]n1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-7.52,1.48,;-6.19,.71,;-4.86,1.48,;-4.46,2.97,;-2.97,2.57,;-3.37,1.09,;-1.64,3.34,;-.3,2.57,;-1.07,1.24,;.47,3.91,;1.03,1.8,;1.03,.26,;2.36,-.51,;3.7,.26,;3.7,1.8,;2.36,2.57,;5.03,-.51,;6.28,.4,;7.52,-.51,;7.05,-1.97,;5.51,-1.97,;-6.19,-.83,;-7.52,-1.6,;-7.52,-3.14,;-6.19,-3.91,;-4.86,-3.14,;-3.39,-3.61,;-2.49,-2.37,;-3.39,-1.12,;-4.86,-1.6,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159756

(PF-02384554 | US10966980, Example 8 | US9035074, 8)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cc(ccn1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.61,1.61,;-4.28,.84,;-2.94,1.61,;-2.55,3.09,;-1.06,2.69,;-1.46,1.21,;.28,3.47,;1.61,2.69,;.52,1.61,;2.7,3.78,;2.94,1.93,;2.94,.38,;4.28,-.38,;5.61,.38,;5.61,1.93,;4.28,2.69,;4.28,-1.92,;4.28,-3.46,;-4.28,-.7,;-5.61,-1.47,;-5.61,-3.01,;-4.28,-3.78,;-2.94,-3.01,;-1.48,-3.49,;-.57,-2.24,;-1.48,-1,;-2.94,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484917
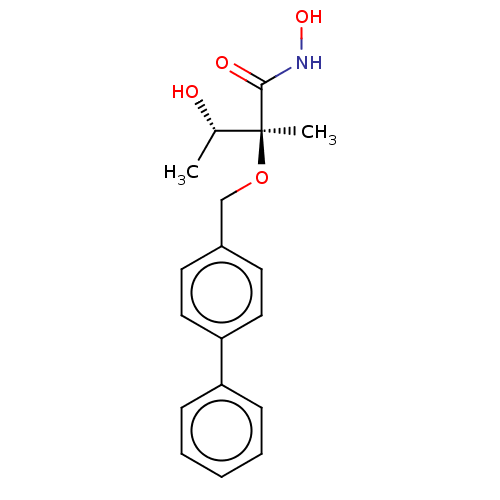
(CHEMBL2012187)Show SMILES C[C@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C18H21NO4/c1-13(20)18(2,17(21)19-22)23-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,13,20,22H,12H2,1-2H3,(H,19,21)/t13-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243852
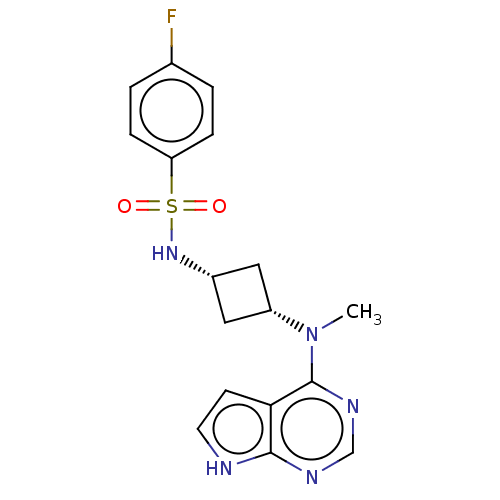
(CHEMBL4103698)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1ccc(F)cc1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(6.1,-10.71,;7.44,-11.48,;8.77,-10.7,;9.16,-9.21,;10.65,-9.6,;10.25,-11.09,;11.98,-8.83,;13.3,-9.59,;14.06,-10.92,;12.53,-10.91,;14.64,-8.82,;15.97,-9.6,;17.31,-8.83,;17.31,-7.29,;18.64,-6.52,;15.97,-6.52,;14.64,-7.29,;7.44,-13.01,;6.12,-13.78,;6.11,-15.33,;7.45,-16.1,;8.79,-15.32,;10.25,-15.78,;11.15,-14.53,;10.24,-13.29,;8.78,-13.78,)| Show InChI InChI=1S/C17H18FN5O2S/c1-23(17-15-6-7-19-16(15)20-10-21-17)13-8-12(9-13)22-26(24,25)14-4-2-11(18)3-5-14/h2-7,10,12-13,22H,8-9H2,1H3,(H,19,20,21)/t12-,13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484894
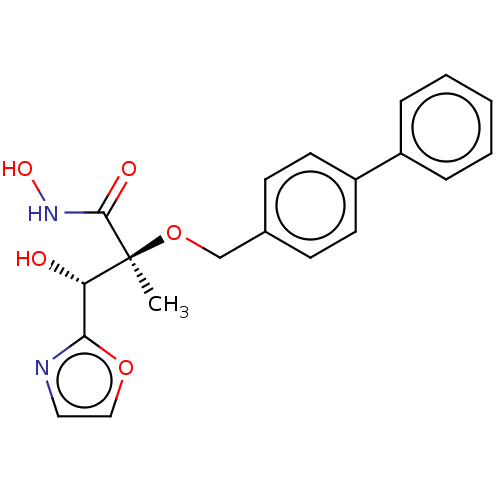
(CHEMBL2012201)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ncco1)C(=O)NO |r| Show InChI InChI=1S/C20H20N2O5/c1-20(19(24)22-25,17(23)18-21-11-12-26-18)27-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-12,17,23,25H,13H2,1H3,(H,22,24)/t17-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484899

(CHEMBL2012186)Show InChI InChI=1S/C18H21NO3/c1-18(13-20,17(21)19-22)12-11-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-10,20,22H,11-13H2,1H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159769

(US10966980, Example 20 | US9035074, 20)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)N1CC(C1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-6.55,1.61,;-5.22,.84,;-3.89,1.61,;-3.49,3.09,;-2,2.69,;-2.4,1.21,;-.67,3.47,;.67,2.69,;-.42,1.61,;1.76,3.78,;2,1.93,;2.4,.44,;3.89,.84,;3.49,2.32,;5.22,.07,;6.55,-.7,;-5.22,-.7,;-6.55,-1.47,;-6.55,-3.01,;-5.22,-3.78,;-3.89,-3.01,;-2.42,-3.49,;-1.52,-2.24,;-2.42,-1,;-3.89,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159786
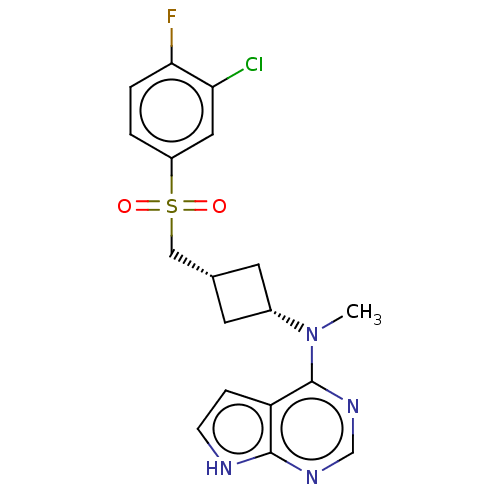
(US10966980, Example 36 | US9035074, 36)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)c2ccc(F)c(Cl)c2)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.4,(-5.98,.23,;-4.65,-.54,;-3.32,.23,;-3.32,1.77,;-1.78,1.77,;-.69,2.85,;.65,2.08,;-.12,.75,;1.74,1,;1.98,2.85,;1.98,4.39,;3.32,5.16,;4.65,4.39,;5.98,5.16,;4.65,2.85,;5.98,2.08,;3.32,2.08,;-1.78,.23,;-4.65,-2.08,;-5.98,-2.85,;-5.98,-4.39,;-4.65,-5.16,;-3.32,-4.39,;-1.85,-4.87,;-.95,-3.62,;-1.85,-2.38,;-3.32,-2.85,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484900
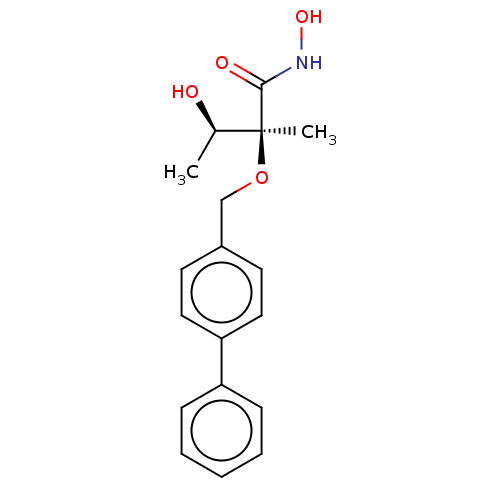
(CHEMBL2012188)Show SMILES C[C@@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C18H21NO4/c1-13(20)18(2,17(21)19-22)23-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,13,20,22H,12H2,1-2H3,(H,19,21)/t13-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50021656
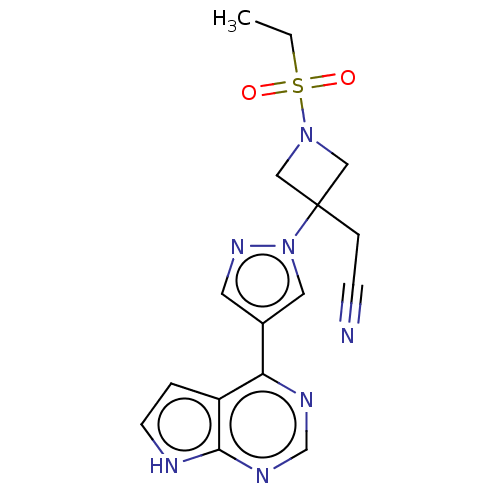
(BARICITINIB | INCB-028050 | LY-3009104 | US1011290...)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C16H17N7O2S/c1-2-26(24,25)22-9-16(10-22,4-5-17)23-8-12(7-21-23)14-13-3-6-18-15(13)20-11-19-14/h3,6-8,11H,2,4,9-10H2,1H3,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159788
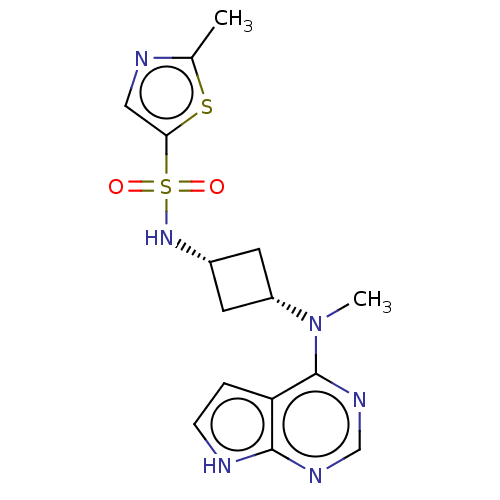
(US10966980, Example 38 | US9035074, 38)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cnc(C)s1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.46,.09,;-4.13,-.68,;-2.79,.09,;-2.4,1.58,;-.91,1.18,;-1.31,-.31,;.43,1.95,;1.76,1.18,;.99,-.15,;2.53,-.15,;3.09,1.95,;4.56,1.47,;5.46,2.72,;4.56,3.97,;5.33,5.3,;3.09,3.49,;-4.13,-2.22,;-5.46,-2.99,;-5.46,-4.53,;-4.13,-5.3,;-2.79,-4.53,;-1.33,-5.01,;-.43,-3.76,;-1.33,-2.51,;-2.79,-2.99,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243810
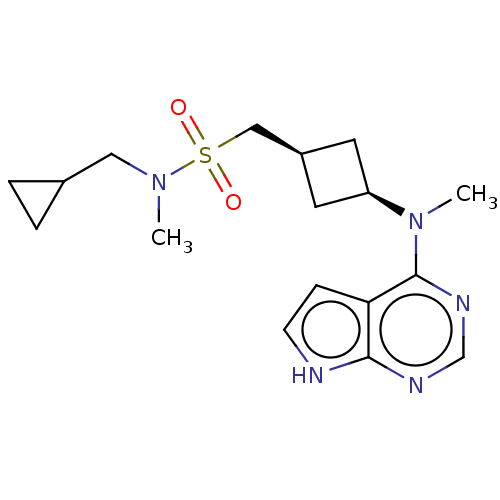
(CHEMBL4093955)Show SMILES CN(CC1CC1)S(=O)(=O)C[C@H]1C[C@H](C1)N(C)c1ncnc2[nH]ccc12 |r,wD:12.15,10.10,(14.93,-10.76,;14.93,-12.3,;16.27,-13.08,;17.6,-12.32,;19.14,-12.32,;18.38,-10.98,;13.59,-13.07,;14.35,-14.4,;12.82,-14.39,;12.26,-12.31,;10.93,-13.08,;9.44,-12.69,;9.05,-14.18,;10.53,-14.57,;7.72,-14.96,;6.38,-14.19,;7.72,-16.5,;6.39,-17.27,;6.39,-18.81,;7.72,-19.58,;9.06,-18.8,;10.53,-19.27,;11.43,-18.02,;10.52,-16.78,;9.06,-17.26,)| Show InChI InChI=1S/C17H25N5O2S/c1-21(9-12-3-4-12)25(23,24)10-13-7-14(8-13)22(2)17-15-5-6-18-16(15)19-11-20-17/h5-6,11-14H,3-4,7-10H2,1-2H3,(H,18,19,20)/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human AchE (Acetylcholinesterase) |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159761
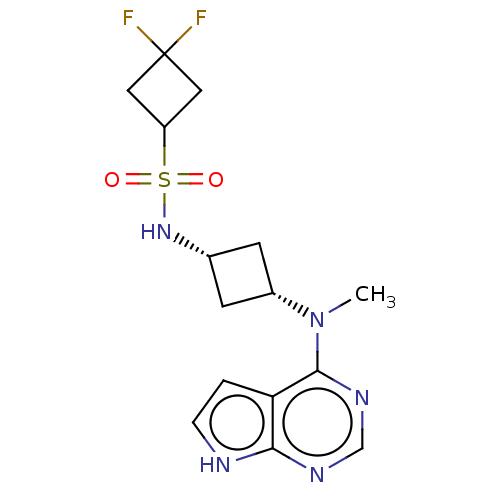
(US10966980, Example 13 | US9035074, 13)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)C1CC(F)(F)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.69,.29,;-4.36,-.48,;-3.03,.29,;-2.63,1.78,;-1.14,1.38,;-1.54,-.11,;.19,2.15,;1.53,1.38,;.44,.29,;2.61,.29,;2.61,2.47,;4.15,2.47,;4.15,4.01,;5.69,4.01,;5.24,5.1,;2.61,4.01,;-4.36,-2.02,;-5.69,-2.79,;-5.69,-4.33,;-4.36,-5.1,;-3.03,-4.33,;-1.56,-4.8,;-.66,-3.56,;-1.56,-2.31,;-3.03,-2.79,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159782
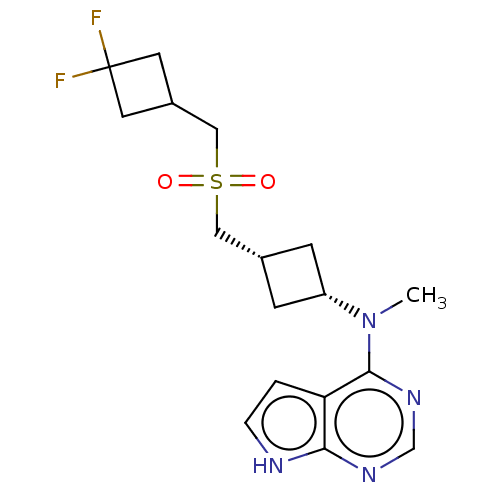
(US10966980, Example 33 | US9035074, 33)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)CC2CC(F)(F)C2)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.4,(-6.34,1.38,;-5,.61,;-3.67,1.38,;-3.67,2.92,;-2.13,2.92,;-1.04,4.01,;.29,3.24,;-.48,1.91,;1.38,2.15,;1.63,4.01,;2.96,3.24,;3.36,1.75,;4.85,2.15,;6.34,2.55,;5.62,.82,;4.45,3.64,;-2.13,1.38,;-5,-.93,;-6.34,-1.7,;-6.34,-3.24,;-5,-4.01,;-3.67,-3.24,;-2.2,-3.72,;-1.3,-2.47,;-2.2,-1.22,;-3.67,-1.7,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159777
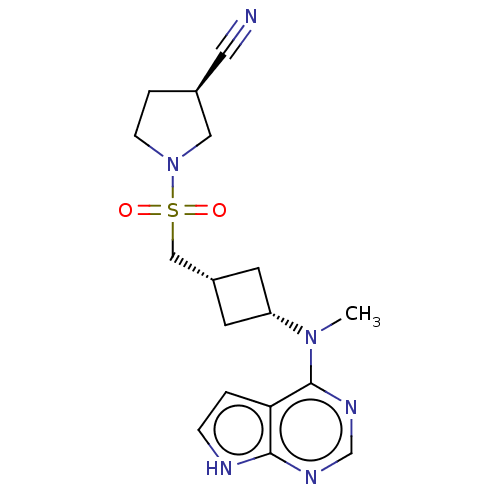
(US10966980, Example 28 | US9035074, 28)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)N2CC[C@H](C2)C#N)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.4,12.14,(-7.01,1.61,;-5.68,.84,;-4.34,1.61,;-3.94,3.09,;-2.46,2.69,;-1.12,3.47,;.21,2.69,;-.88,1.61,;1.3,3.78,;1.54,1.93,;1.56,.38,;3.03,-.1,;3.93,1.15,;3.03,2.4,;5.47,1.15,;7.01,1.15,;-2.86,1.21,;-5.68,-.7,;-7.01,-1.47,;-7.01,-3.01,;-5.68,-3.78,;-4.34,-3.01,;-2.88,-3.49,;-1.97,-2.24,;-2.88,-1,;-4.34,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159765
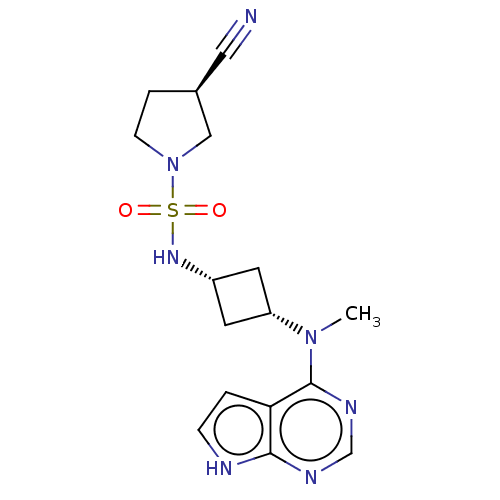
(US10966980, Example 17A | US9035074, 17A)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)N1CC[C@H](C1)C#N)c1ncnc2[nH]ccc12 |r,wU:13.16,wD:2.1,4.6,(-5.47,1.61,;-4.14,.84,;-2.8,1.61,;-2.4,3.09,;-.92,2.69,;-1.32,1.21,;.42,3.47,;1.75,2.69,;.66,1.61,;2.84,3.78,;3.08,1.93,;4.57,2.4,;5.47,1.15,;4.57,-.1,;3.1,.38,;4.96,-1.58,;5.36,-3.07,;-4.14,-.7,;-5.47,-1.47,;-5.47,-3.01,;-4.14,-3.78,;-2.8,-3.01,;-1.34,-3.49,;-.43,-2.24,;-1.34,-1,;-2.8,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159750

(US10966980, Example 4B | US9035074, 4B)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)[C@H]1C[C@H](CC#N)C1)c1ncnc2[nH]ccc12 |r,wU:10.10,wD:2.1,4.6,12.13,(-5.36,3.28,;-4.28,2.19,;-2.79,2.59,;-2.02,3.92,;-.68,3.15,;-1.45,1.82,;.8,3.55,;1.89,2.46,;3.38,2.86,;1.49,.97,;2.98,1.37,;4.52,1.37,;4.52,-.17,;5.61,-1.26,;4.84,-2.59,;4.07,-3.92,;2.98,-.17,;-4.28,.65,;-5.61,-.12,;-5.61,-1.66,;-4.28,-2.43,;-2.94,-1.66,;-1.48,-2.14,;-.57,-.89,;-1.48,.36,;-2.94,-.12,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159752
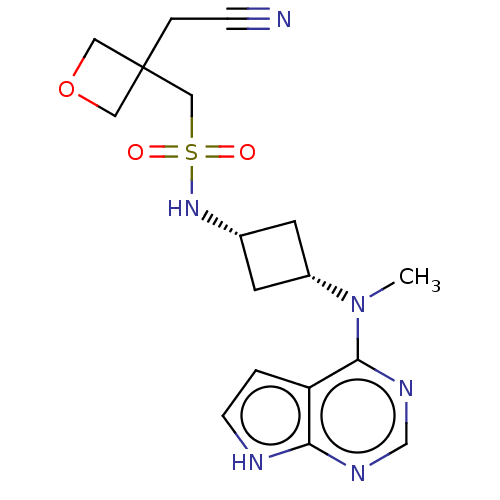
(US10966980, Example 5 | US9035074, 5)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)CC1(CC#N)COC1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-6.26,.84,;-4.93,.07,;-3.59,.84,;-3.59,2.38,;-2.05,2.38,;-2.05,.84,;-.96,3.47,;.52,3.07,;.92,4.55,;.13,1.58,;2.01,2.67,;2.41,1.18,;3.18,2.51,;4.72,2.51,;6.26,2.51,;1.32,.09,;2.41,-1,;3.5,.09,;-4.93,-1.47,;-6.26,-2.24,;-6.26,-3.78,;-4.93,-4.55,;-3.59,-3.78,;-2.13,-4.26,;-1.22,-3.01,;-2.13,-1.77,;-3.59,-2.24,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159760
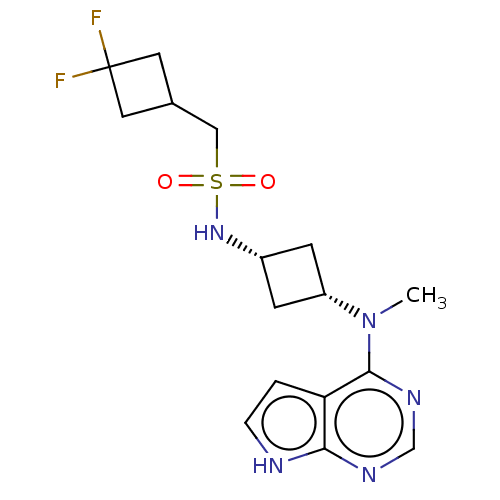
(US10966980, Example 12 | US9035074, 12)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)CC1CC(F)(F)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.73,.84,;-4.4,.07,;-3.06,.84,;-3.06,2.38,;-1.52,2.38,;-1.52,.84,;-.44,3.47,;1.05,3.07,;1.45,4.55,;.65,1.58,;2.54,2.67,;3.31,1.33,;2.91,-.15,;4.4,-.55,;5.73,-1.32,;3.63,-1.89,;4.8,.94,;-4.4,-1.47,;-5.73,-2.24,;-5.73,-3.78,;-4.4,-4.55,;-3.06,-3.78,;-1.6,-4.26,;-.69,-3.01,;-1.6,-1.77,;-3.06,-2.24,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159763
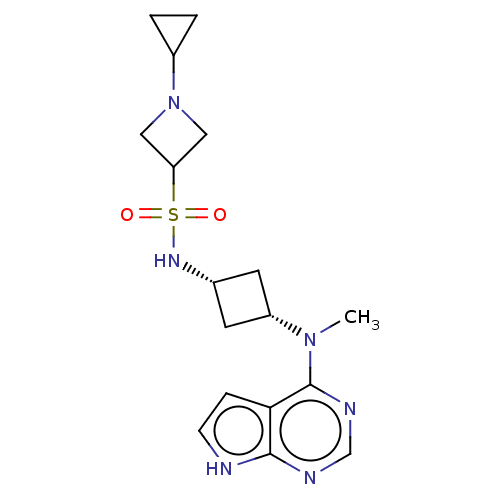
(US10966980, Example 15 | US9035074, 15)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)C1CN(C1)C1CC1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-6.66,1.77,;-5.32,1,;-3.99,1.77,;-3.59,3.25,;-2.1,2.85,;-2.5,1.37,;-.77,3.62,;.56,2.85,;.56,1.31,;1.9,3.62,;1.9,2.08,;2.3,.6,;3.78,1,;3.38,2.48,;5.12,.23,;5.89,-1.11,;6.66,.23,;-5.32,-.54,;-6.66,-1.31,;-6.66,-2.85,;-5.32,-3.62,;-3.99,-2.85,;-2.53,-3.33,;-1.62,-2.08,;-2.53,-.84,;-3.99,-1.31,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243869
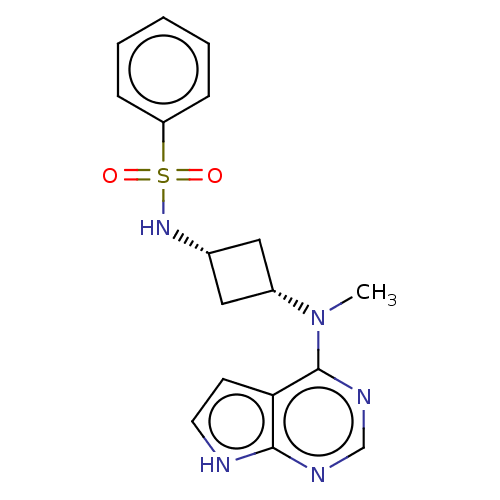
(CHEMBL4101374)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1ccccc1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(4.65,-10.97,;5.99,-11.74,;7.32,-10.96,;7.71,-9.46,;9.2,-9.86,;8.81,-11.35,;10.53,-9.08,;11.86,-9.85,;12.62,-11.18,;11.09,-11.17,;13.2,-9.08,;14.53,-9.85,;15.86,-9.08,;15.86,-7.53,;14.51,-6.77,;13.19,-7.55,;5.99,-13.28,;4.66,-14.05,;4.66,-15.59,;6,-16.36,;7.34,-15.58,;8.81,-16.05,;9.71,-14.8,;8.79,-13.55,;7.33,-14.04,)| Show InChI InChI=1S/C17H19N5O2S/c1-22(17-15-7-8-18-16(15)19-11-20-17)13-9-12(10-13)21-25(23,24)14-5-3-2-4-6-14/h2-8,11-13,21H,9-10H2,1H3,(H,18,19,20)/t12-,13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159787

(US10966980, Example 37 | US9035074, 37)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)c2cc(ccn2)C#N)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.4,(-6.94,.61,;-5.61,-.16,;-4.28,.61,;-3.88,2.1,;-2.39,1.7,;-1.06,2.47,;.28,1.7,;-.49,.37,;1.05,.37,;1.61,2.47,;2.94,1.7,;4.28,2.47,;4.28,4.01,;2.94,4.78,;1.61,4.01,;5.61,1.7,;6.94,.93,;-2.79,.21,;-5.61,-1.7,;-6.94,-2.47,;-6.94,-4.01,;-5.61,-4.78,;-4.28,-4.01,;-2.81,-4.49,;-1.91,-3.24,;-2.81,-1.99,;-4.28,-2.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM109086

(US10793535, Cmpd ID 727 | US8604016, 670 | US99382...)Show SMILES FC(F)(F)Oc1cccc(CC(=O)Nc2ccc(CCCCc3nnc(NC(=O)Cc4ccccn4)s3)nn2)c1 Show InChI InChI=1S/C26H24F3N7O3S/c27-26(28,29)39-20-9-5-6-17(14-20)15-22(37)31-21-12-11-18(33-34-21)7-1-2-10-24-35-36-25(40-24)32-23(38)16-19-8-3-4-13-30-19/h3-6,8-9,11-14H,1-2,7,10,15-16H2,(H,31,34,37)(H,32,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01398
BindingDB Entry DOI: 10.7270/Q25D8WGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM404825
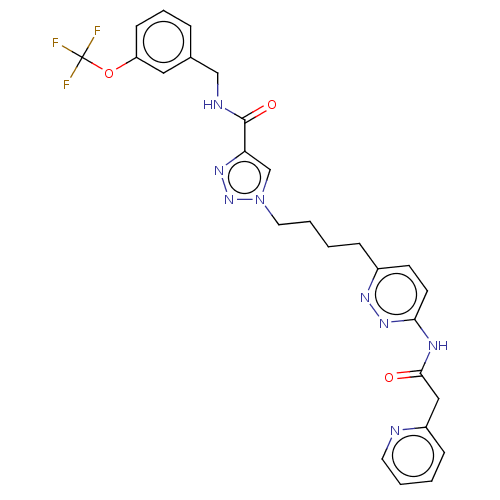
(1-(4-{6-[2-(pyridin-2- yl)acetamido]pyridazin-3-yl...)Show SMILES FC(F)(F)Oc1cccc(CNC(=O)c2cn(CCCCc3ccc(NC(=O)Cc4ccccn4)nn3)nn2)c1 Show InChI InChI=1S/C26H25F3N8O3/c27-26(28,29)40-21-9-5-6-18(14-21)16-31-25(39)22-17-37(36-34-22)13-4-2-7-19-10-11-23(35-33-19)32-24(38)15-20-8-1-3-12-30-20/h1,3,5-6,8-12,14,17H,2,4,7,13,15-16H2,(H,31,39)(H,32,35,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01398
BindingDB Entry DOI: 10.7270/Q25D8WGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159776

(US10966980, Example 26 | US9035074, 26)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)N2CCC(O)(CC2)C(F)(F)F)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.4,(-6.8,-.33,;-5.47,-1.1,;-4.14,-.33,;-4.14,1.21,;-2.6,1.21,;-1.51,2.3,;-.17,1.53,;-.94,.19,;.92,.44,;1.16,2.3,;2.49,1.53,;3.83,2.3,;3.83,3.84,;3.83,5.38,;2.49,4.61,;1.16,3.84,;5.32,4.23,;6.4,3.15,;5.71,5.72,;6.8,4.63,;-2.6,-.33,;-5.47,-2.64,;-6.8,-3.41,;-6.8,-4.95,;-5.47,-5.72,;-4.14,-4.95,;-2.67,-5.43,;-1.77,-4.18,;-2.67,-2.94,;-4.14,-3.41,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50021656

(BARICITINIB | INCB-028050 | LY-3009104 | US1011290...)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C16H17N7O2S/c1-2-26(24,25)22-9-16(10-22,4-5-17)23-8-12(7-21-23)14-13-3-6-18-15(13)20-11-19-14/h3,6-8,11H,2,4,9-10H2,1H3,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK2 using FITC-KGGEEEEYFELVKK as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50243837
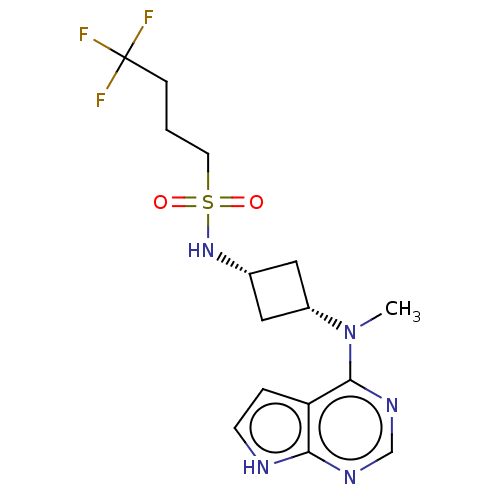
(CHEMBL4079179)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)CCCC(F)(F)F)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(6.04,-12.03,;7.37,-12.79,;8.7,-12.02,;9.1,-10.52,;10.58,-10.92,;10.19,-12.41,;11.92,-10.14,;13.24,-10.9,;14.01,-12.23,;12.47,-12.23,;14.58,-10.13,;15.92,-10.91,;17.25,-10.14,;18.58,-10.91,;19.92,-10.14,;18.58,-12.45,;19.91,-11.68,;7.38,-14.33,;6.05,-15.1,;6.05,-16.65,;7.38,-17.42,;8.72,-16.64,;10.19,-17.11,;11.09,-15.85,;10.18,-14.61,;8.71,-15.1,)| Show InChI InChI=1S/C15H20F3N5O2S/c1-23(14-12-3-5-19-13(12)20-9-21-14)11-7-10(8-11)22-26(24,25)6-2-4-15(16,17)18/h3,5,9-11,22H,2,4,6-8H2,1H3,(H,19,20,21)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484907
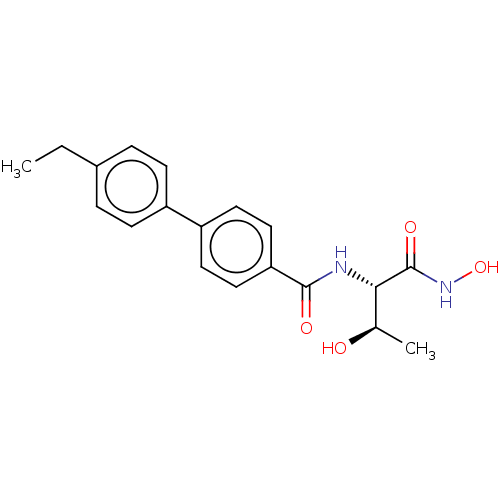
(CHEMBL2012182)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C19H22N2O4/c1-3-13-4-6-14(7-5-13)15-8-10-16(11-9-15)18(23)20-17(12(2)22)19(24)21-25/h4-12,17,22,25H,3H2,1-2H3,(H,20,23)(H,21,24)/t12-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484901
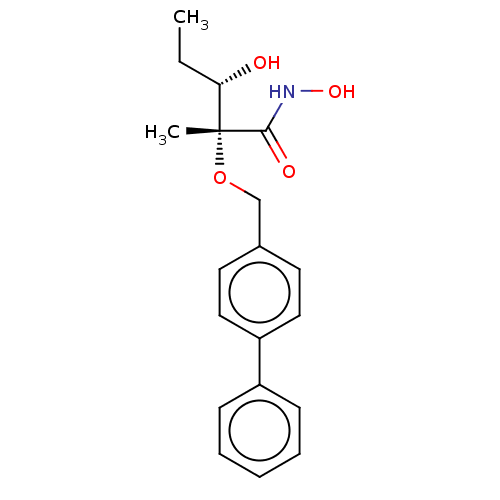
(CHEMBL2012189)Show SMILES CC[C@H](O)[C@@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C19H23NO4/c1-3-17(21)19(2,18(22)20-23)24-13-14-9-11-16(12-10-14)15-7-5-4-6-8-15/h4-12,17,21,23H,3,13H2,1-2H3,(H,20,22)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159772

(US10966980, Example 23 | US9035074, 23)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)N1C[C@H]2C[C@]2(C1)C#N)c1ncnc2[nH]ccc12 |r,wU:14.18,wD:2.1,4.6,12.13,(-6.58,1.61,;-5.25,.84,;-3.92,1.61,;-3.52,3.09,;-2.03,2.69,;-2.43,1.21,;-.7,3.47,;.64,2.69,;-.45,1.61,;1.72,3.78,;1.97,1.93,;2.13,.39,;3.64,.07,;5.18,.07,;4.41,1.41,;3.38,2.55,;5.5,2.5,;6.58,3.58,;-5.25,-.7,;-6.58,-1.47,;-6.58,-3.01,;-5.25,-3.78,;-3.92,-3.01,;-2.45,-3.49,;-1.55,-2.24,;-2.45,-1,;-3.92,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK2 using FITC-KGGEEEEYFELVKK as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159775
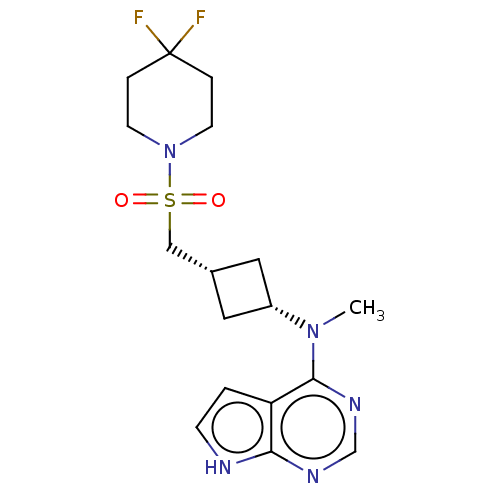
(US10966980, Example 25 | US9035074, 25)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)N2CCC(F)(F)CC2)C1)c1ncnc2[nH]ccc12 |r,wD:2.1,4.4,(-5.79,-.25,;-4.45,-1.02,;-3.12,-.25,;-2.72,1.23,;-1.23,.84,;.1,1.61,;1.43,.84,;.34,-.25,;2.52,-.25,;2.52,1.93,;4.01,1.53,;5.1,2.62,;4.7,4.1,;5.79,5.19,;4.7,5.64,;3.21,4.5,;2.12,3.41,;-1.63,-.65,;-4.45,-2.56,;-5.79,-3.33,;-5.79,-4.87,;-4.45,-5.64,;-3.12,-4.87,;-1.66,-5.35,;-.75,-4.1,;-1.66,-2.86,;-3.12,-3.33,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159779
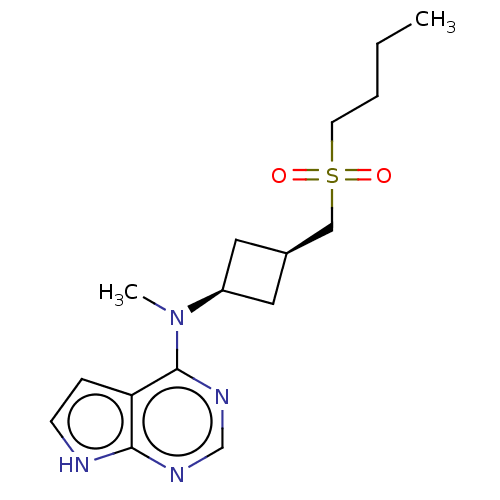
(US10966980, Example 30 | US9035074, 30)Show SMILES CCCCS(=O)(=O)C[C@H]1C[C@H](C1)N(C)c1ncnc2[nH]ccc12 |r,wD:10.12,8.7,(5.98,3.24,;4.65,4.01,;3.32,3.24,;1.98,4.01,;.65,3.24,;-.12,1.91,;1.74,2.15,;-.69,4.01,;-1.78,2.92,;-3.32,2.92,;-3.32,1.38,;-1.78,1.38,;-4.65,.61,;-5.98,1.38,;-4.65,-.93,;-5.98,-1.7,;-5.98,-3.24,;-4.65,-4.01,;-3.32,-3.24,;-1.85,-3.72,;-.95,-2.47,;-1.85,-1.22,;-3.32,-1.7,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay |
J Med Chem 61: 1130-1152 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01598
BindingDB Entry DOI: 10.7270/Q2D79DTW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159783
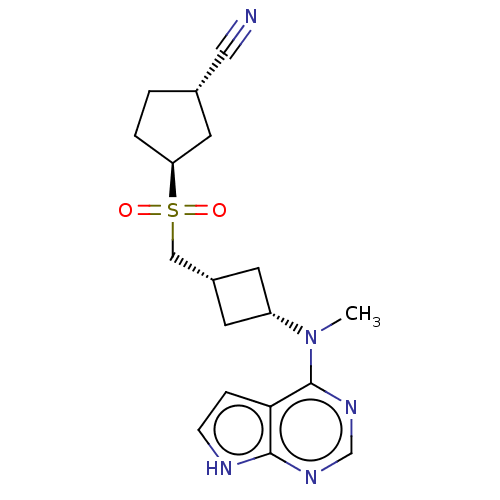
(US10966980, Example 34B | US9035074, 34B)Show SMILES CN([C@@H]1C[C@H](CS(=O)(=O)[C@H]2CC[C@@H](C2)C#N)C1)c1ncnc2[nH]ccc12 |r,wU:12.14,wD:2.1,4.4,9.8,(-7.01,1.61,;-5.68,.84,;-4.34,1.61,;-3.94,3.09,;-2.46,2.69,;-1.12,3.47,;.21,2.69,;-.88,1.61,;1.3,3.78,;1.54,1.93,;1.56,.38,;3.03,-.1,;3.93,1.15,;3.03,2.4,;5.47,1.15,;7.01,1.15,;-2.86,1.21,;-5.68,-.7,;-7.01,-1.47,;-7.01,-3.01,;-5.68,-3.78,;-4.34,-3.01,;-2.88,-3.49,;-1.97,-2.24,;-2.88,-1,;-4.34,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... |
US Patent US9035074 (2015)
BindingDB Entry DOI: 10.7270/Q26H4G56 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data