

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291573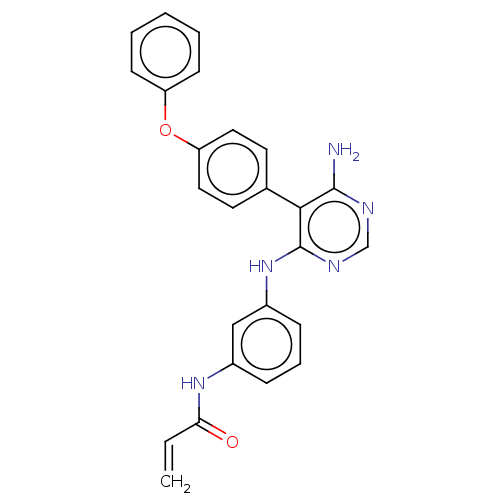 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291413 (1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291455 (N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291452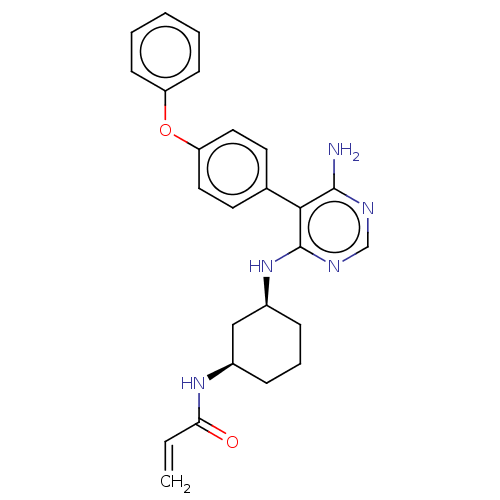 (N-((1R,3S)-3-((6-amino-5-(4-phenoxyphenyl)pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291522 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50519156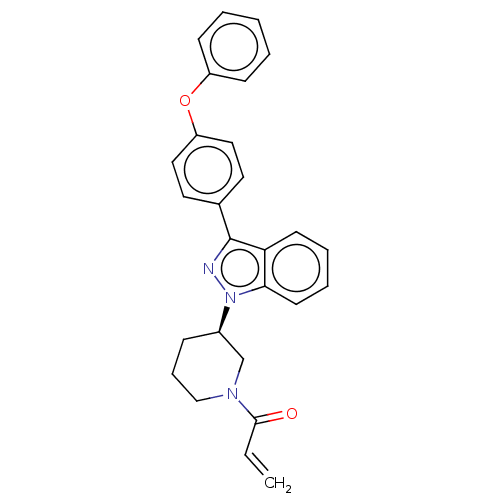 (CHEMBL4466205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291635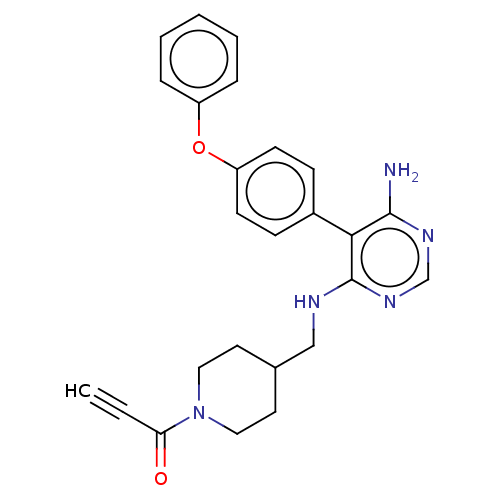 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291634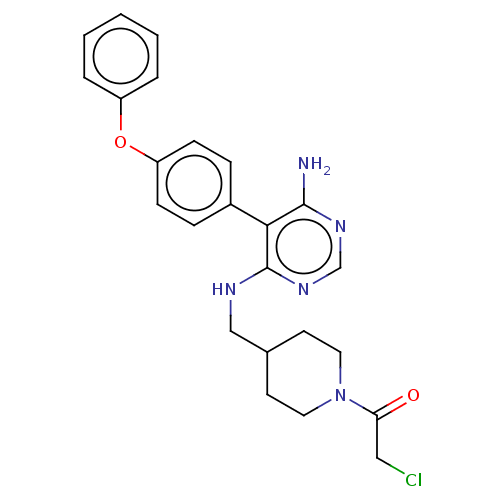 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387635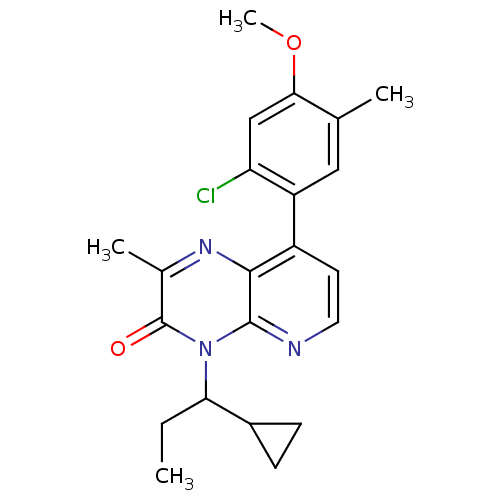 (CHEMBL2058338) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224498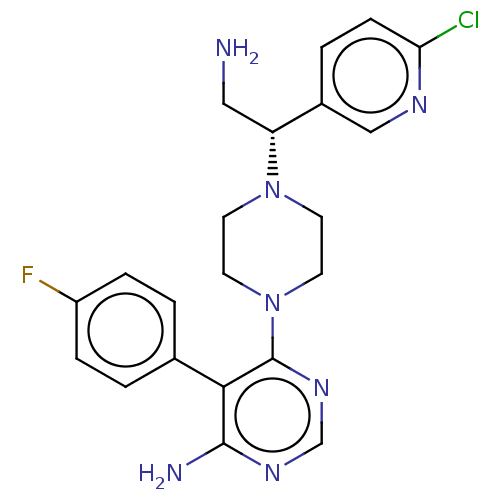 (US10080750, Compound 176 | US9321760, 176 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9321760 (2016) BindingDB Entry DOI: 10.7270/Q2R78D2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291389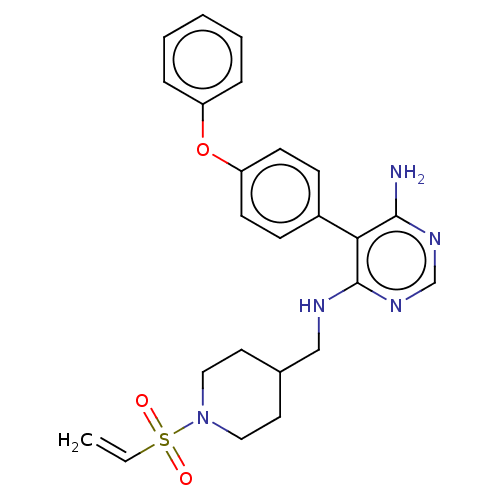 (5-(4-phenoxyphenyl)-N4-((1-(vinylsulfonyl)piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224498 (US10080750, Compound 176 | US9321760, 176 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224498 (US10080750, Compound 176 | US9321760, 176 | US9662...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387601 (CHEMBL2058596) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224487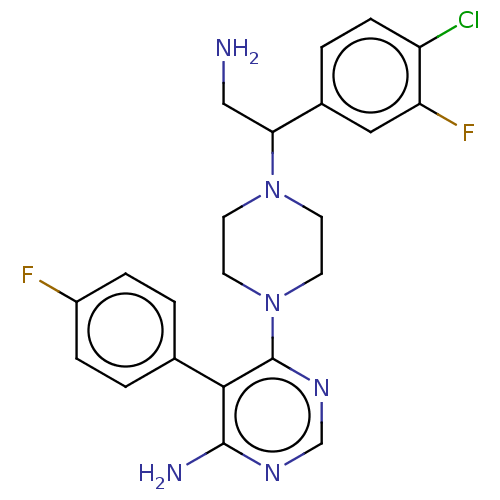 (US10080750, Compound 165 | US9321760, 165 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224487 (US10080750, Compound 165 | US9321760, 165 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9321760 (2016) BindingDB Entry DOI: 10.7270/Q2R78D2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224487 (US10080750, Compound 165 | US9321760, 165 | US9662...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM221860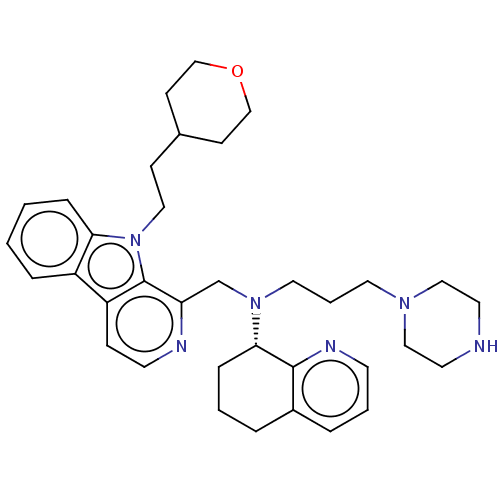 (US9314468, Table 7, Compound 147) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc. US Patent | Assay Description Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... | US Patent US9314468 (2016) BindingDB Entry DOI: 10.7270/Q2DF6Q2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM291512 (N-(3-(4-amino-6-((4-phenoxyphenyl)amino)pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cells | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM221760 (US9314468, Table 7, Compound 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc. US Patent | Assay Description Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... | US Patent US9314468 (2016) BindingDB Entry DOI: 10.7270/Q2DF6Q2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387603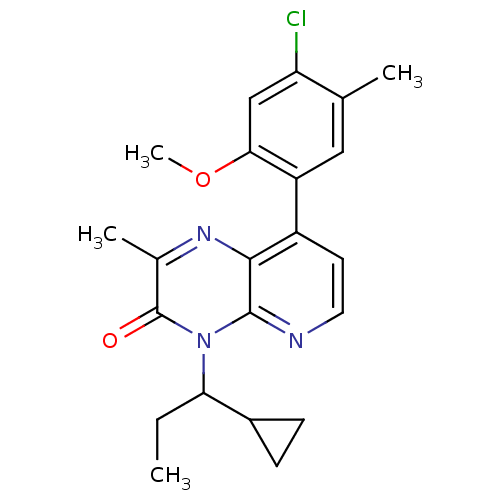 (CHEMBL2058598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224485 (US10080750, Compound 163 | US9321760, 163 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224485 (US10080750, Compound 163 | US9321760, 163 | US9662...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224485 (US10080750, Compound 163 | US9321760, 163 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9321760 (2016) BindingDB Entry DOI: 10.7270/Q2R78D2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224508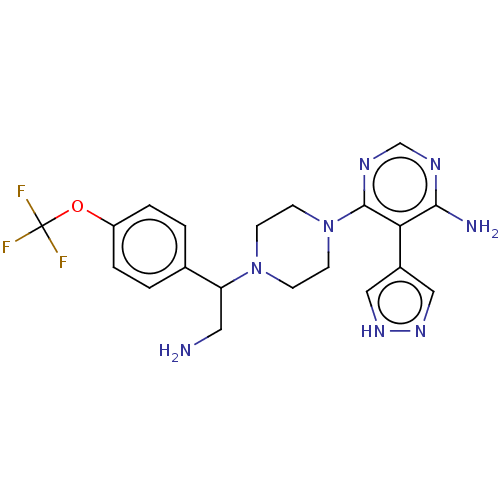 (US10080750, Compound 186 | US9321760, 186 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9321760 (2016) BindingDB Entry DOI: 10.7270/Q2R78D2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224508 (US10080750, Compound 186 | US9321760, 186 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224508 (US10080750, Compound 186 | US9321760, 186 | US9662...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387618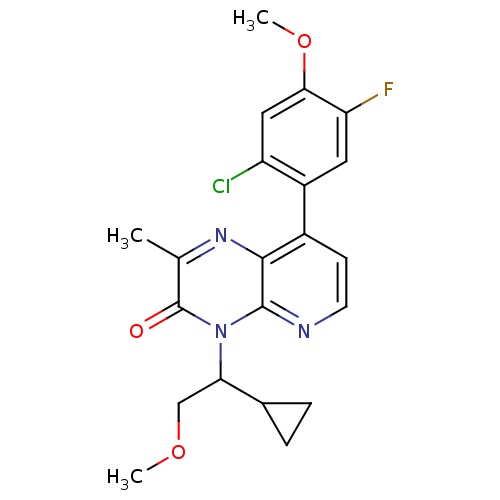 (CHEMBL2057479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224340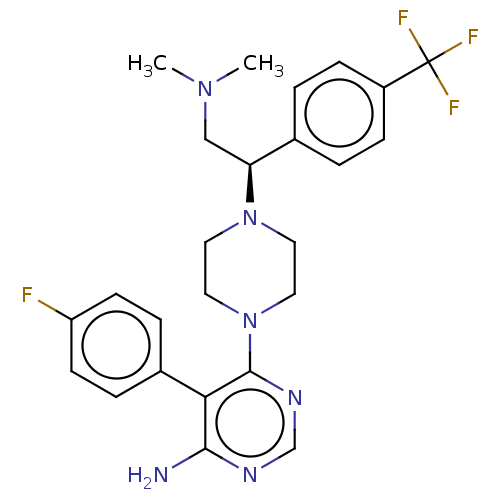 (US10080750, Compound 17 | US9321760, 17 | US966233...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224340 (US10080750, Compound 17 | US9321760, 17 | US966233...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224340 (US10080750, Compound 17 | US9321760, 17 | US966233...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9321760 (2016) BindingDB Entry DOI: 10.7270/Q2R78D2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387639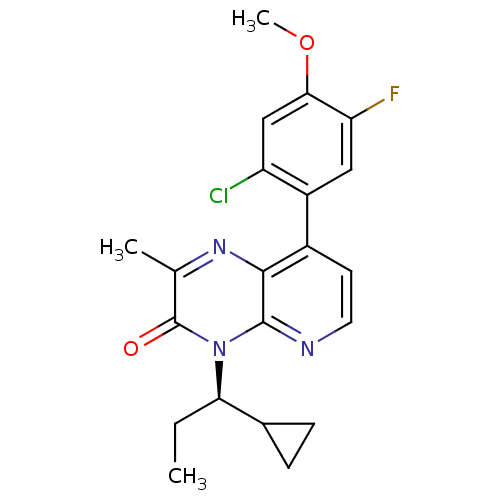 (CHEMBL2058593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84796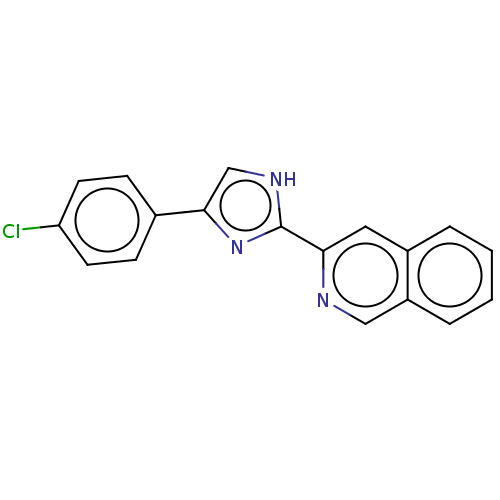 (PF-00215924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. | Assay Description Inhibition of active p38a MAP kinase by inhibitors was determinedusing a p38a cascade activity assay. A 30-lL reaction mixture wasprepared containing... | Chem Biol Drug Des 74: 547-59 (2009) Article DOI: 10.1111/j.1747-0285.2009.00884.x BindingDB Entry DOI: 10.7270/Q2930RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224469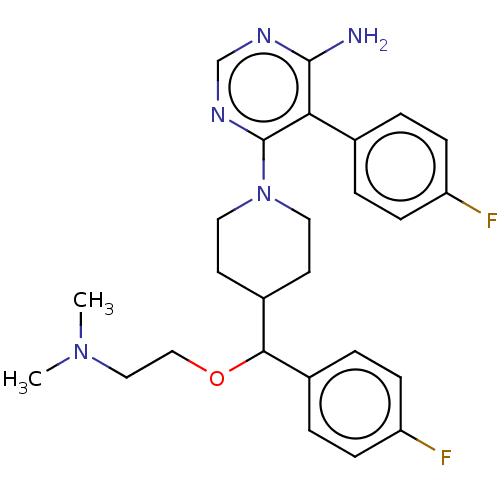 (US10080750, Compound 147 | US9321760, 147 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9321760 (2016) BindingDB Entry DOI: 10.7270/Q2R78D2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224469 (US10080750, Compound 147 | US9321760, 147 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387636 (CHEMBL2058589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224469 (US10080750, Compound 147 | US9321760, 147 | US9662...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291413 (1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50387617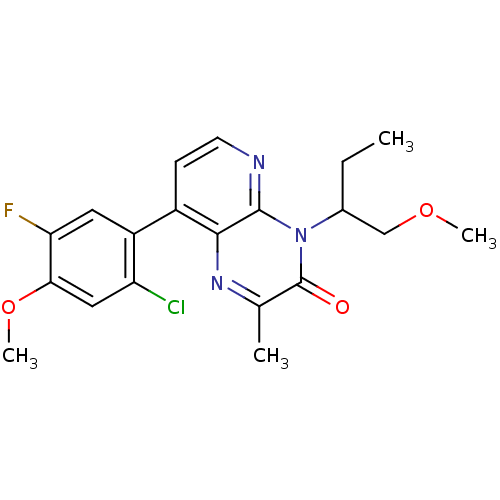 (CHEMBL2057478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Antagonist activity at rat CFR1R | Bioorg Med Chem Lett 22: 4986-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.034 BindingDB Entry DOI: 10.7270/Q23X87Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224325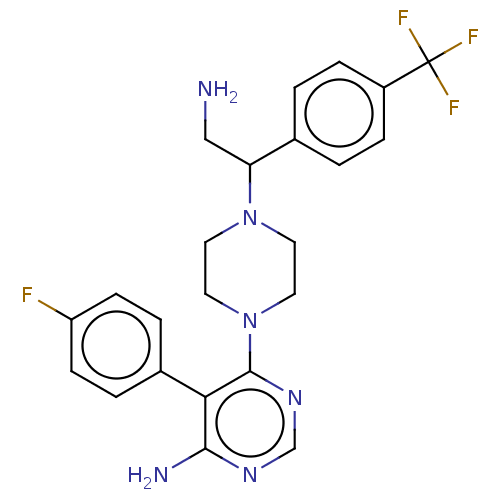 (US10080750, Compound 2 | US9321760, 2 | US9662330,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224325 (US10080750, Compound 2 | US9321760, 2 | US9662330,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9321760 (2016) BindingDB Entry DOI: 10.7270/Q2R78D2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224325 (US10080750, Compound 2 | US9321760, 2 | US9662330,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224460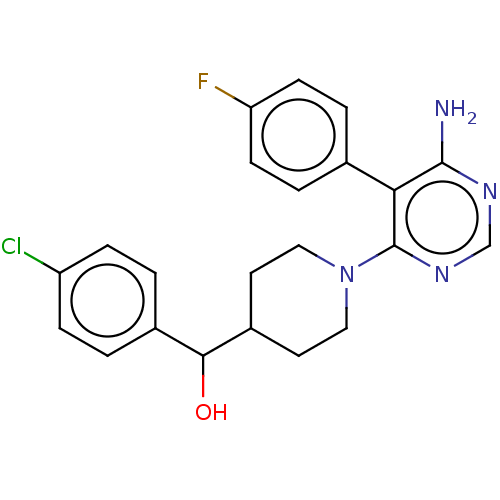 (US10080750, Compound 138 | US9321760, 138 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9321760 (2016) BindingDB Entry DOI: 10.7270/Q2R78D2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224460 (US10080750, Compound 138 | US9321760, 138 | US9662...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224460 (US10080750, Compound 138 | US9321760, 138 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224326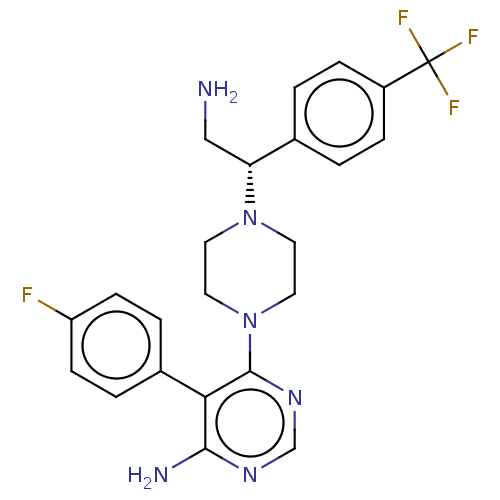 (US10080750, Compound 3 | US9321760, 3 | US9662330,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM224497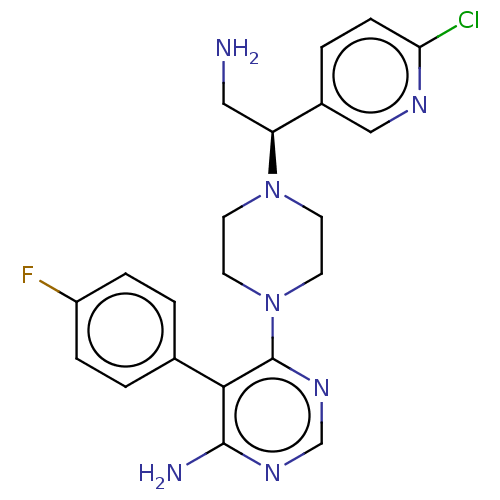 (US10080750, Compound 175 | US9321760, 175 | US9662...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US9662330 (2017) BindingDB Entry DOI: 10.7270/Q2MK6G04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224326 (US10080750, Compound 3 | US9321760, 3 | US9662330,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224526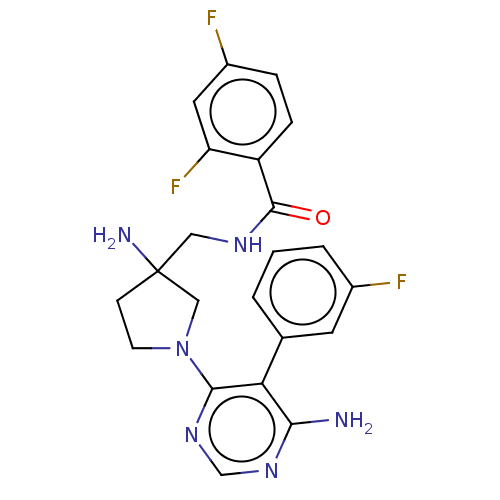 (US10080750, Compound 204 | US9321760, 204 | US9662...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 [T412E] (Homo sapiens (Human)) | BDBM224497 (US10080750, Compound 175 | US9321760, 175 | US9662...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Patent GmbH US Patent | Assay Description P70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components was then added to the com... | US Patent US10080750 (2018) BindingDB Entry DOI: 10.7270/Q2WS8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3151 total ) | Next | Last >> |