 Found 5675 hits with Last Name = 'jones' and Initial = 'a'
Found 5675 hits with Last Name = 'jones' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1A adrenergic receptor
(Dog) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514220
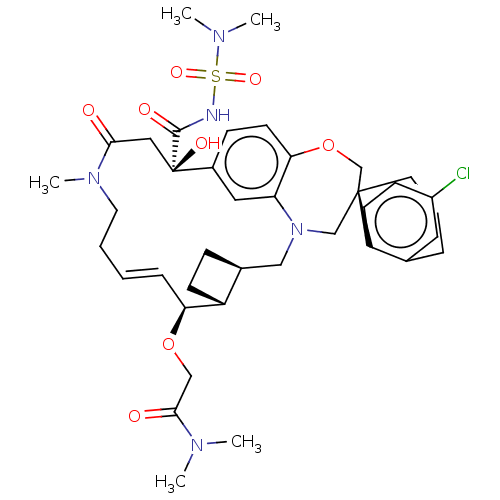
(CHEMBL4535151 | US11274105, Example 188)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCC(=O)N(C)C)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:15| Show InChI InChI=1S/C39H52ClN5O8S/c1-42(2)36(47)23-52-33-10-6-7-18-44(5)35(46)21-39(49,37(48)41-54(50,51)43(3)4)28-12-16-34-32(20-28)45(22-27-11-14-30(27)33)24-38(25-53-34)17-8-9-26-19-29(40)13-15-31(26)38/h6,10,12-13,15-16,19-20,27,30,33,49H,7-9,11,14,17-18,21-25H2,1-5H3,(H,41,48)/b10-6+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514203
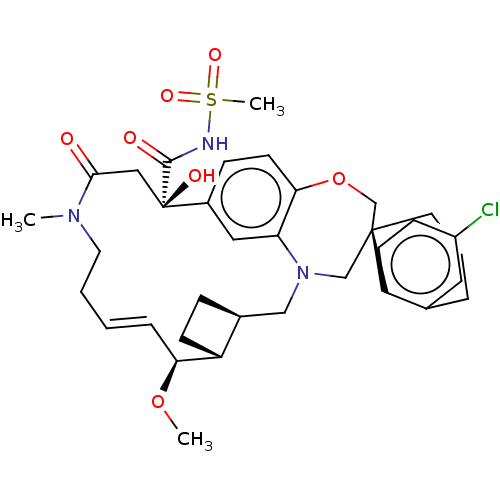
(CHEMBL4593361 | US11274105, Example 6)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(C)(=O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C35H44ClN3O7S/c1-38-16-5-4-8-30(45-2)27-12-9-24(27)20-39-21-34(15-6-7-23-17-26(36)11-13-28(23)34)22-46-31-14-10-25(18-29(31)39)35(42,19-32(38)40)33(41)37-47(3,43)44/h4,8,10-11,13-14,17-18,24,27,30,42H,5-7,9,12,15-16,19-22H2,1-3H3,(H,37,41)/b8-4+/t24-,27+,30-,34-,35+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50390841
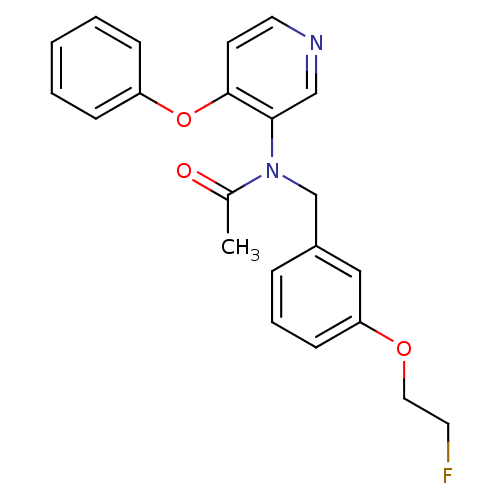
(CHEMBL2070787)Show InChI InChI=1S/C22H21FN2O3/c1-17(26)25(16-18-6-5-9-20(14-18)27-13-11-23)21-15-24-12-10-22(21)28-19-7-3-2-4-8-19/h2-10,12,14-15H,11,13,16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat heart incubated for 15 mins |
Bioorg Med Chem Lett 22: 5795-800 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.093
BindingDB Entry DOI: 10.7270/Q29K4CBT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50390853
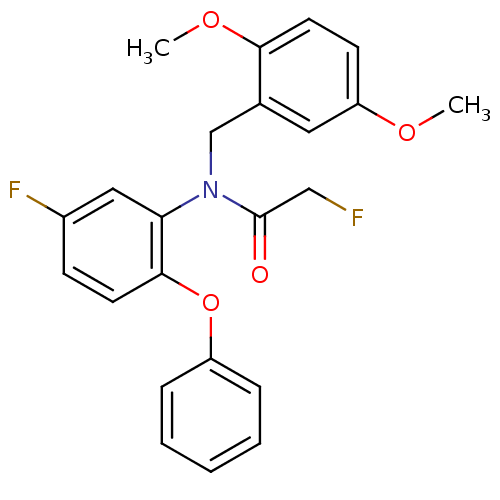
(CHEMBL2070800)Show SMILES COc1ccc(OC)c(CN(C(=O)CF)c2cc(F)ccc2Oc2ccccc2)c1 Show InChI InChI=1S/C23H21F2NO4/c1-28-19-9-11-21(29-2)16(12-19)15-26(23(27)14-24)20-13-17(25)8-10-22(20)30-18-6-4-3-5-7-18/h3-13H,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat heart incubated for 15 mins |
Bioorg Med Chem Lett 22: 5795-800 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.093
BindingDB Entry DOI: 10.7270/Q29K4CBT |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514222
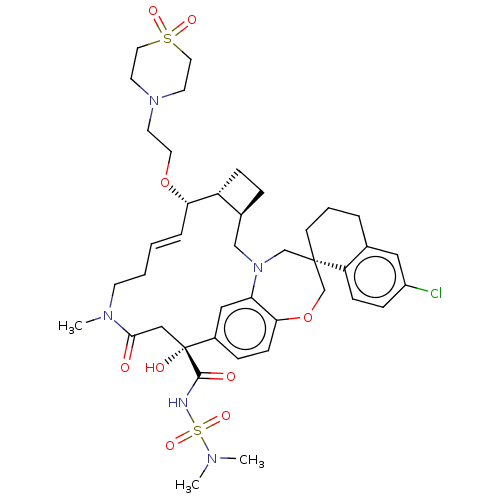
(CHEMBL4580244 | US11274105, Example 193)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCS(=O)(=O)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C41H56ClN5O9S2/c1-44(2)58(53,54)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(55-20-17-46-18-21-57(51,52)22-19-46)33-12-9-30(33)26-47-27-40(28-56-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514196
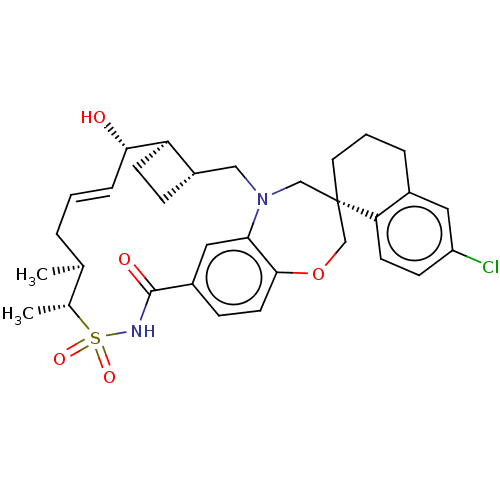
(CHEMBL4476472)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C32H39ClN2O5S/c1-20-5-3-7-29(36)26-11-8-24(26)17-35-18-32(14-4-6-22-15-25(33)10-12-27(22)32)19-40-30-13-9-23(16-28(30)35)31(37)34-41(38,39)21(20)2/h3,7,9-10,12-13,15-16,20-21,24,26,29,36H,4-6,8,11,14,17-19H2,1-2H3,(H,34,37)/b7-3+/t20-,21+,24-,26+,29-,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vesicular acetylcholine transporter
(Homo sapiens (Human)) | BDBM50039613
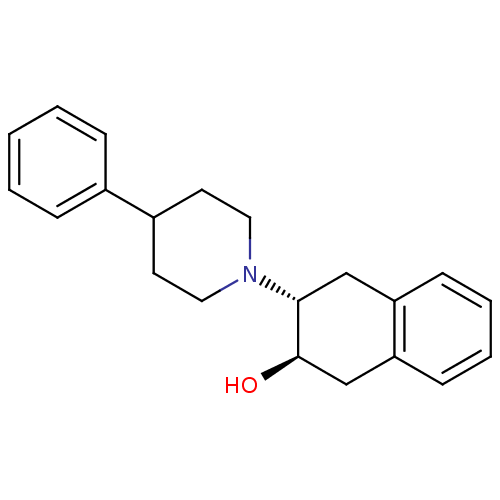
((2R,3R)-3-(4-Phenyl-piperidin-1-yl)-1,2,3,4-tetrah...)Show SMILES O[C@@H]1Cc2ccccc2C[C@H]1N1CCC(CC1)c1ccccc1 |r| Show InChI InChI=1S/C21H25NO/c23-21-15-19-9-5-4-8-18(19)14-20(21)22-12-10-17(11-13-22)16-6-2-1-3-7-16/h1-9,17,20-21,23H,10-15H2/t20-,21-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]vesamicol from human vesicular acetylcholine transporter expressed in rat PC12 cells by liquid scintillation spectrometry |
J Med Chem 52: 1358-69 (2009)
Article DOI: 10.1021/jm8012344
BindingDB Entry DOI: 10.7270/Q26W9B3B |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514202

(CHEMBL4446369 | US11274105, Example 179)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C40H52ClF2N5O7S/c1-45(2)56(52,53)44-37(50)40(51)21-36(49)46(3)16-5-4-8-34(54-18-17-47-24-39(42,43)25-47)31-12-9-28(31)22-48-23-38(26-55-35-14-10-29(40)20-33(35)48)15-6-7-27-19-30(41)11-13-32(27)38/h4,8,10-11,13-14,19-20,28,31,34,51H,5-7,9,12,15-18,21-26H2,1-3H3,(H,44,50)/b8-4+/t28-,31+,34-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514199
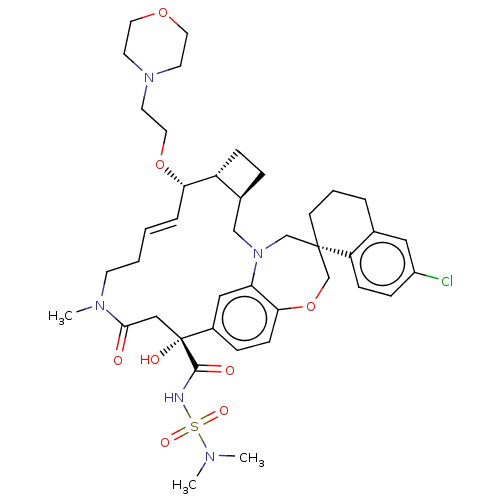
(CHEMBL4553660 | US11274105, Example 182)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCOCC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(54-22-19-46-17-20-53-21-18-46)33-12-9-30(33)26-47-27-40(28-55-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514200
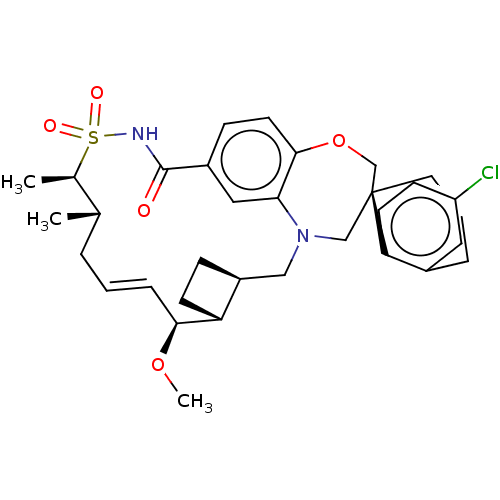
(CHEMBL4446378 | US10703733, Comparative Example 1)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C33H41ClN2O5S/c1-21-6-4-8-30(40-3)27-12-9-25(27)18-36-19-33(15-5-7-23-16-26(34)11-13-28(23)33)20-41-31-14-10-24(17-29(31)36)32(37)35-42(38,39)22(21)2/h4,8,10-11,13-14,16-17,21-22,25,27,30H,5-7,9,12,15,18-20H2,1-3H3,(H,35,37)/b8-4+/t21-,22+,25-,27+,30-,33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514215
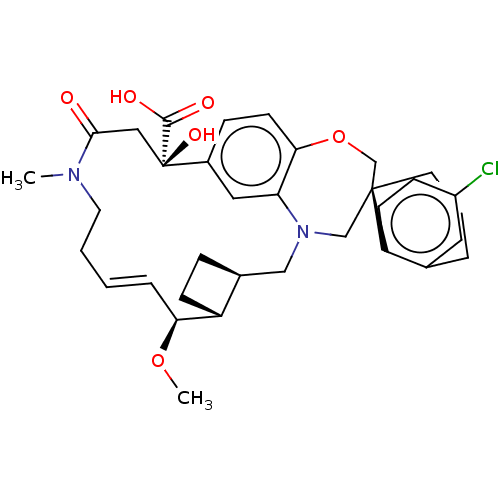
(CHEMBL4577379 | US11274105, Example 4)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(42-2)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(41,32(39)40)18-31(36)38/h3,7,9-10,12-13,16-17,23,26,29,41H,4-6,8,11,14-15,18-21H2,1-2H3,(H,39,40)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212159
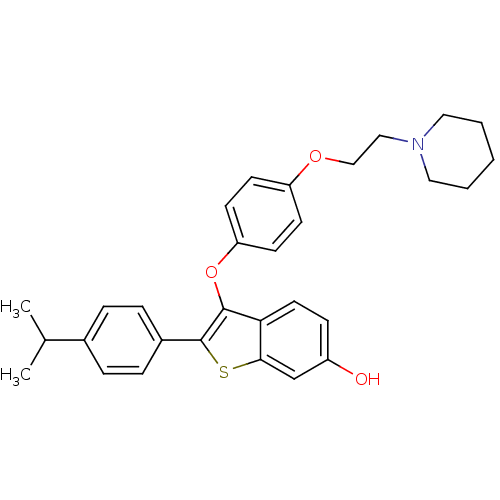
(2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...)Show SMILES CC(C)c1ccc(cc1)-c1sc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H33NO3S/c1-21(2)22-6-8-23(9-7-22)30-29(27-15-10-24(32)20-28(27)35-30)34-26-13-11-25(12-14-26)33-19-18-31-16-4-3-5-17-31/h6-15,20-21,32H,3-5,16-19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514201

(CHEMBL4547370 | US11274105, Example 191)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCOCC(F)F)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:16| Show InChI InChI=1S/C39H51ClF2N4O8S/c1-44(2)55(50,51)43-37(48)39(49)21-36(47)45(3)16-5-4-8-33(53-18-17-52-23-35(41)42)30-12-9-27(30)22-46-24-38(25-54-34-14-10-28(39)20-32(34)46)15-6-7-26-19-29(40)11-13-31(26)38/h4,8,10-11,13-14,19-20,27,30,33,35,49H,5-7,9,12,15-18,21-25H2,1-3H3,(H,43,48)/b8-4+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514206
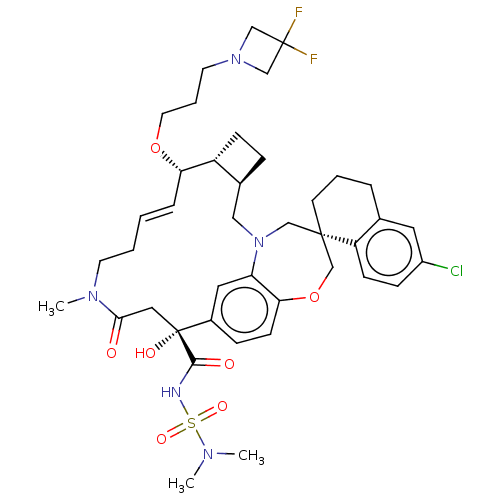
(CHEMBL4588330 | US11274105, Example 187)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:19| Show InChI InChI=1S/C41H54ClF2N5O7S/c1-46(2)57(53,54)45-38(51)41(52)22-37(50)47(3)17-5-4-9-35(55-19-7-18-48-25-40(43,44)26-48)32-13-10-29(32)23-49-24-39(27-56-36-15-11-30(41)21-34(36)49)16-6-8-28-20-31(42)12-14-33(28)39/h4,9,11-12,14-15,20-21,29,32,35,52H,5-8,10,13,16-19,22-27H2,1-3H3,(H,45,51)/b9-4+/t29-,32+,35-,39-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514214

(CHEMBL4542646 | US11274105, Example 41)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C33H39ClN2O6/c1-35-14-3-2-6-28(37)25-10-7-22(25)18-36-19-32(13-4-5-21-15-24(34)9-11-26(21)32)20-42-29-12-8-23(16-27(29)36)33(41,31(39)40)17-30(35)38/h2,6,8-9,11-12,15-16,22,25,28,37,41H,3-5,7,10,13-14,17-20H2,1H3,(H,39,40)/b6-2+/t22-,25+,28-,32-,33+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514219

(CHEMBL4438074 | US11274105, Example 181)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:17| Show InChI InChI=1S/C40H53ClFN5O7S/c1-44(2)55(51,52)43-38(49)40(50)21-37(48)45(3)16-5-4-8-35(53-18-17-46-23-31(42)24-46)32-12-9-28(32)22-47-25-39(26-54-36-14-10-29(40)20-34(36)47)15-6-7-27-19-30(41)11-13-33(27)39/h4,8,10-11,13-14,19-20,28,31-32,35,50H,5-7,9,12,15-18,21-26H2,1-3H3,(H,43,49)/b8-4+/t28-,32+,35-,39-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514216
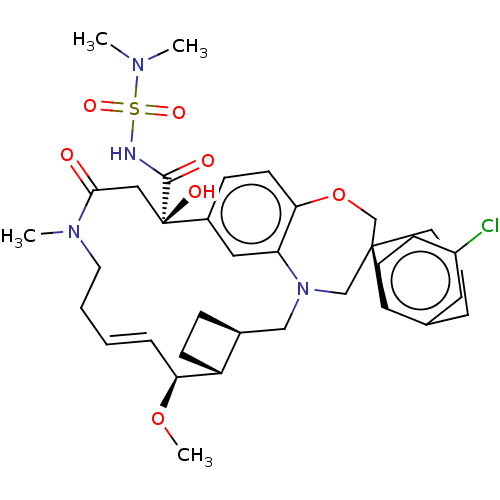
(CHEMBL4528051 | US11274105, Example 5)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C36H47ClN4O7S/c1-39(2)49(45,46)38-34(43)36(44)20-33(42)40(3)17-6-5-9-31(47-4)28-13-10-25(28)21-41-22-35(23-48-32-15-11-26(36)19-30(32)41)16-7-8-24-18-27(37)12-14-29(24)35/h5,9,11-12,14-15,18-19,25,28,31,44H,6-8,10,13,16-17,20-23H2,1-4H3,(H,38,43)/b9-5+/t25-,28+,31-,35-,36+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514218
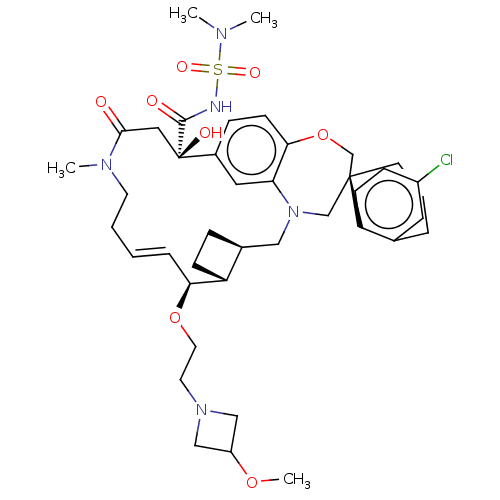
(CHEMBL4539543 | US11274105, Example 197)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(C1)OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)22-38(48)45(3)17-6-5-9-36(54-19-18-46-24-32(25-46)53-4)33-13-10-29(33)23-47-26-40(27-55-37-15-11-30(41)21-35(37)47)16-7-8-28-20-31(42)12-14-34(28)40/h5,9,11-12,14-15,20-21,29,32-33,36,50H,6-8,10,13,16-19,22-27H2,1-4H3,(H,43,49)/b9-5+/t29-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514204
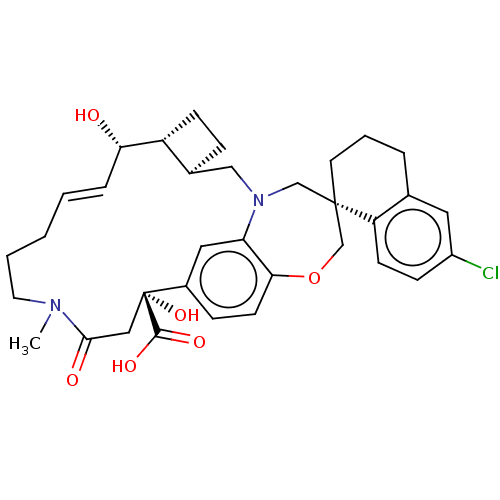
(CHEMBL4437832)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-2-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38,42H,2,4-6,8,11,14-15,18-21H2,1H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50273370
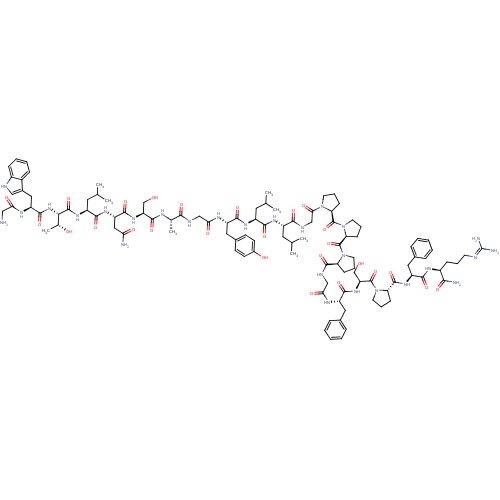
(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50212148
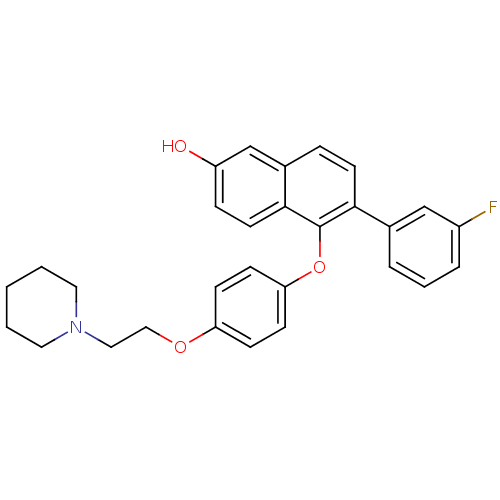
(6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(F)c1 Show InChI InChI=1S/C29H28FNO3/c30-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-31-15-2-1-3-16-31/h4-14,19-20,32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERbeta |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212148

(6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(F)c1 Show InChI InChI=1S/C29H28FNO3/c30-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-31-15-2-1-3-16-31/h4-14,19-20,32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19968
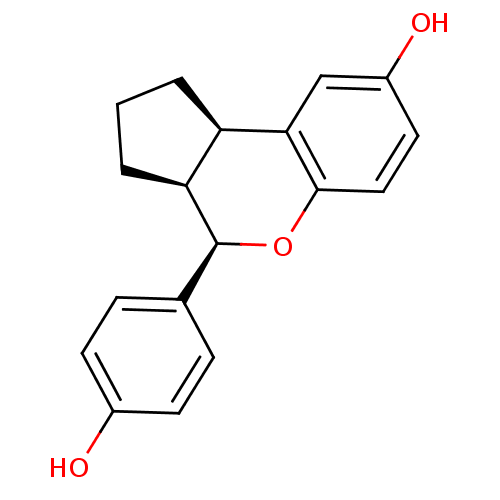
((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...)Show SMILES Oc1ccc(cc1)[C@@H]1Oc2ccc(O)cc2[C@@H]2CCC[C@H]12 |r| Show InChI InChI=1S/C18H18O3/c19-12-6-4-11(5-7-12)18-15-3-1-2-14(15)16-10-13(20)8-9-17(16)21-18/h4-10,14-15,18-20H,1-3H2/t14-,15+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.190 | -54.9 | n/a | n/a | 0.660 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... |
J Med Chem 49: 6155-7 (2006)
Article DOI: 10.1021/jm060491j
BindingDB Entry DOI: 10.7270/Q2N014SV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50390840
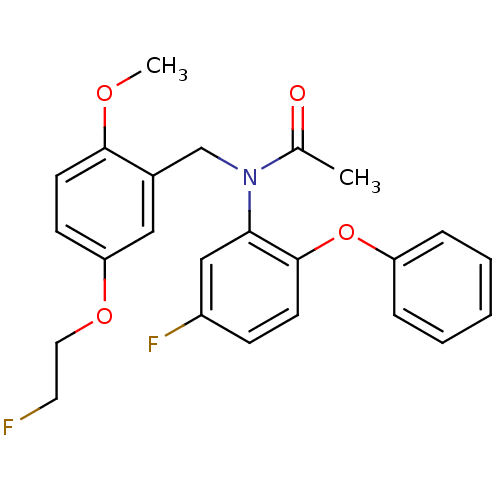
(CHEMBL2070786)Show SMILES COc1ccc(OCCF)cc1CN(C(C)=O)c1cc(F)ccc1Oc1ccccc1 Show InChI InChI=1S/C24H23F2NO4/c1-17(28)27(16-18-14-21(30-13-12-25)9-11-23(18)29-2)22-15-19(26)8-10-24(22)31-20-6-4-3-5-7-20/h3-11,14-15H,12-13,16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat heart incubated for 15 mins |
Bioorg Med Chem Lett 22: 5795-800 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.093
BindingDB Entry DOI: 10.7270/Q29K4CBT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50491017
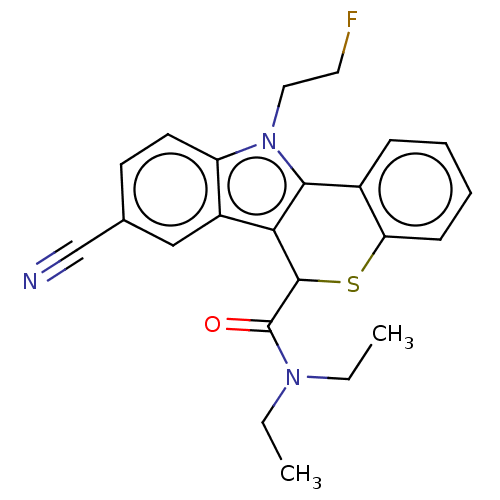
(CHEMBL2377343)Show SMILES CCN(CC)C(=O)C1Sc2ccccc2-c2c1c1cc(ccc1n2CCF)C#N Show InChI InChI=1S/C23H22FN3OS/c1-3-26(4-2)23(28)22-20-17-13-15(14-25)9-10-18(17)27(12-11-24)21(20)16-7-5-6-8-19(16)29-22/h5-10,13,22H,3-4,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from PBR receptor in Wistar rat heart homogenates |
Bioorg Med Chem Lett 23: 2368-72 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.057
BindingDB Entry DOI: 10.7270/Q2PR7ZW9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19964
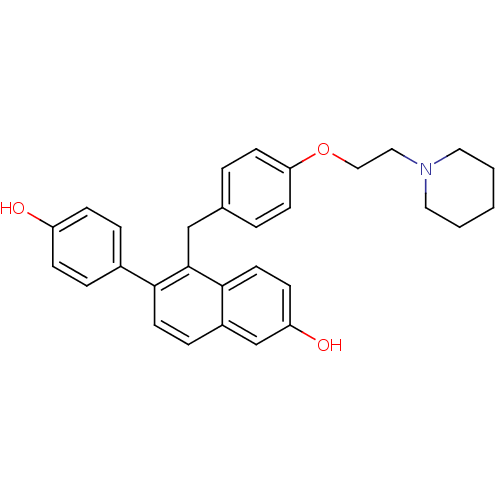
(6-(4-hydroxyphenyl)-5-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1ccc2cc(O)ccc2c1Cc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H31NO3/c32-25-9-6-23(7-10-25)28-14-8-24-21-26(33)11-15-29(24)30(28)20-22-4-12-27(13-5-22)34-19-18-31-16-2-1-3-17-31/h4-15,21,32-33H,1-3,16-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | 1.14 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... |
J Med Chem 48: 6772-5 (2005)
Article DOI: 10.1021/jm050723z
BindingDB Entry DOI: 10.7270/Q2RR1WHW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514217

(CHEMBL4452794 | US11274105, Example 196)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCC(F)(F)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C42H56ClF2N5O7S/c1-47(2)58(54,55)46-39(52)42(53)25-38(51)48(3)18-5-4-8-36(56-22-21-49-19-16-41(44,45)17-20-49)33-12-9-30(33)26-50-27-40(28-57-37-14-10-31(42)24-35(37)50)15-6-7-29-23-32(43)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,53H,5-7,9,12,15-22,25-28H2,1-3H3,(H,46,52)/b8-4+/t30-,33+,36-,40-,42+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212157

(6-(3,4-difluorophenyl)-5-(4-(2-(piperidin-1-yl)eth...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)c(F)c1 Show InChI InChI=1S/C29H27F2NO3/c30-27-13-5-21(19-28(27)31)25-11-4-20-18-22(33)6-12-26(20)29(25)35-24-9-7-23(8-10-24)34-17-16-32-14-2-1-3-15-32/h4-13,18-19,33H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212149
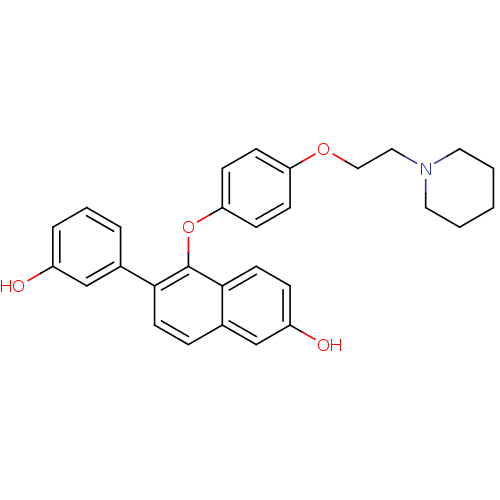
(6-(3-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...)Show SMILES Oc1cccc(c1)-c1ccc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C29H29NO4/c31-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-30-15-2-1-3-16-30/h4-14,19-20,31-32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50336931
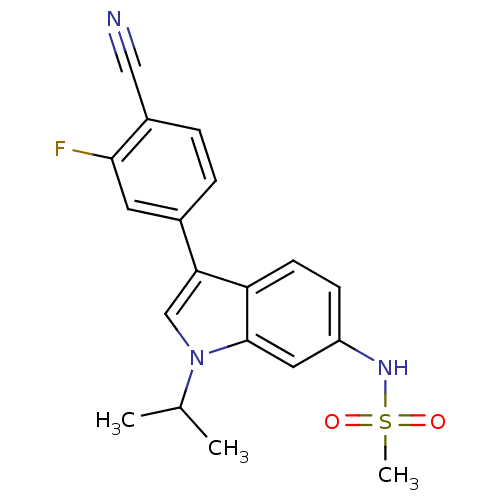
(CHEMBL1672547 | N-(3-(4-cyano-3-fluorophenyl)-1-is...)Show SMILES CC(C)n1cc(-c2ccc(C#N)c(F)c2)c2ccc(NS(C)(=O)=O)cc12 Show InChI InChI=1S/C19H18FN3O2S/c1-12(2)23-11-17(13-4-5-14(10-21)18(20)8-13)16-7-6-15(9-19(16)23)22-26(3,24)25/h4-9,11-12,22H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from human progesterone receptor expressed in HEK293 cells |
ACS Med Chem Lett 2: 148-153 (2011)
Article DOI: 10.1021/ml100220b
BindingDB Entry DOI: 10.7270/Q2Z038G1 |
More data for this
Ligand-Target Pair | |
Vesicular acetylcholine transporter
(Homo sapiens (Human)) | BDBM50046952
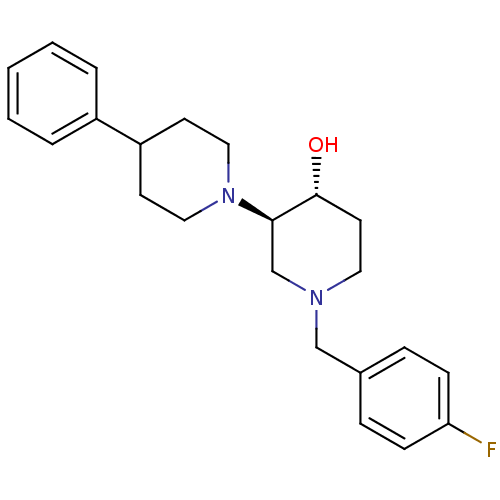
((3'R,4'R)-1'-(4-Fluoro-benzyl)-4-phenyl-[1,3']bipi...)Show SMILES O[C@@H]1CCN(Cc2ccc(F)cc2)C[C@H]1N1CCC(CC1)c1ccccc1 |r| Show InChI InChI=1S/C23H29FN2O/c24-21-8-6-18(7-9-21)16-25-13-12-23(27)22(17-25)26-14-10-20(11-15-26)19-4-2-1-3-5-19/h1-9,20,22-23,27H,10-17H2/t22-,23-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]vesamicol from human vesicular acetylcholine transporter expressed in rat PC12 cells by liquid scintillation spectrometry |
J Med Chem 52: 1358-69 (2009)
Article DOI: 10.1021/jm8012344
BindingDB Entry DOI: 10.7270/Q26W9B3B |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50363527
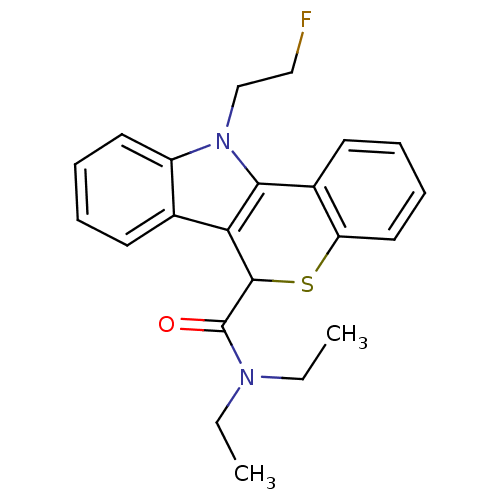
(CHEMBL1945412 | US9481685, [18F]FE-PBR)Show InChI InChI=1S/C22H23FN2OS/c1-3-24(4-2)22(26)21-19-15-9-5-7-11-17(15)25(14-13-23)20(19)16-10-6-8-12-18(16)27-21/h5-12,21H,3-4,13-14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from PBR receptor in Wistar rat heart homogenates |
Bioorg Med Chem Lett 23: 2368-72 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.057
BindingDB Entry DOI: 10.7270/Q2PR7ZW9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441
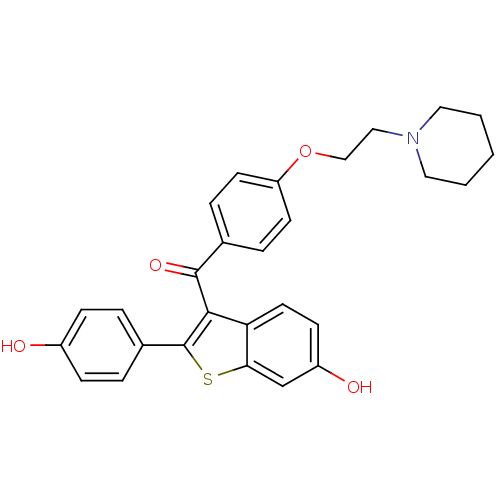
(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-17-beta-estradiol from Estrogen receptor alpha by scintillation proximity assay. |
Bioorg Med Chem Lett 11: 1939-42 (2001)
BindingDB Entry DOI: 10.7270/Q2ZK5FZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50491024

(CHEMBL2377352)Show SMILES CCN(CC)C(=O)C1Sc2ccccc2-c2c1c1cc(Cl)ccc1n2CCF Show InChI InChI=1S/C22H22ClFN2OS/c1-3-25(4-2)22(27)21-19-16-13-14(23)9-10-17(16)26(12-11-24)20(19)15-7-5-6-8-18(15)28-21/h5-10,13,21H,3-4,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from PBR receptor in Wistar rat heart homogenates |
Bioorg Med Chem Lett 23: 2368-72 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.057
BindingDB Entry DOI: 10.7270/Q2PR7ZW9 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85070
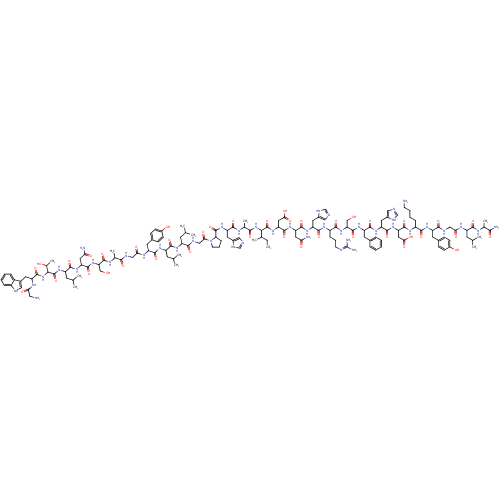
(Galanin, Porcine)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CC(O)=O)C(=O)NC(CCCCN)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(C)C(N)=O |(22.15,12.84,;20.71,12.32,;20.44,10.8,;21.61,9.81,;18.99,10.28,;18.72,8.76,;19.9,7.77,;21.34,8.3,;19.63,6.25,;18.18,5.73,;20.8,5.26,;20.53,3.75,;19.09,3.22,;21.71,2.75,;21.44,1.24,;19.99,.71,;18.72,1.58,;17.5,.63,;18.03,-.82,;19.57,-.77,;23.16,3.28,;24.34,2.29,;24.07,.77,;25.79,2.81,;26.21,4.29,;27.75,4.34,;28.28,2.89,;27.06,1.95,;27.11,.41,;25.81,-.41,;28.47,-.32,;28.52,-1.86,;29.88,-2.58,;31.19,-1.77,;29.93,-4.12,;31.29,-4.85,;32.6,-4.03,;33.96,-4.76,;32.55,-2.49,;28.62,-4.93,;28.68,-6.47,;30.2,-6.69,;27.73,-7.69,;26.22,-8,;25.19,-6.86,;23.68,-7.17,;25.67,-5.39,;28.3,-9.11,;27.35,-10.33,;25.82,-10.11,;27.92,-11.76,;26.97,-12.97,;25.45,-12.75,;24.87,-11.32,;23.35,-11.1,;22.4,-12.32,;20.88,-12.1,;22.97,-13.75,;24.5,-13.96,;29.45,-11.97,;30.02,-13.4,;29.07,-14.61,;31.55,-13.62,;32.12,-15.05,;33.65,-15.26,;34.6,-14.05,;34.22,-16.69,;33.27,-17.9,;35.75,-16.91,;36.32,-18.34,;35.37,-19.55,;37.85,-18.56,;38.8,-17.34,;38.22,-15.91,;38.42,-19.98,;39.94,-20.2,;40.89,-18.99,;40.52,-21.63,;42.04,-21.85,;42.99,-20.64,;44.52,-20.85,;42.42,-19.21,;39.57,-22.84,;40.14,-24.27,;41.65,-23.95,;39.66,-25.74,;38.36,-26.55,;37,-25.82,;35.69,-26.64,;36.95,-24.28,;40.69,-26.88,;40.21,-28.35,;38.71,-28.66,;41.24,-29.49,;40.76,-30.96,;41.79,-32.1,;43.3,-31.78,;41.31,-33.57,;39.81,-33.88,;39.33,-35.35,;40.23,-36.59,;39.33,-37.83,;37.87,-37.36,;36.54,-38.13,;35.2,-37.36,;35.2,-35.82,;36.54,-35.05,;37.87,-35.82,;42.34,-34.71,;43.85,-34.39,;44.33,-32.93,;44.88,-35.54,;46.39,-35.22,;42.75,-29.17,;43.78,-30.32,;43.23,-27.71,;17.81,11.27,;16.36,10.75,;18.08,12.79,;16.9,13.78,;15.45,13.25,;14.28,14.25,;12.83,13.72,;14.55,15.76,;17.17,15.3,;15.99,16.29,;18.62,15.82,;18.89,17.34,;17.37,17.61,;16.85,19.05,;15.33,19.32,;17.84,20.23,;20.34,17.86,;21.52,16.87,;21.33,19.04,;22.85,18.77,;23.37,17.32,;24.89,17.05,;26,18.12,;27.36,17.39,;27.09,15.88,;25.56,15.66,;23.84,19.95,;25.35,19.68,;23.31,21.39,;24.31,22.57,;25.82,22.3,;26.81,23.48,;28.33,23.21,;29.32,24.39,;30.84,24.12,;31.83,25.3,;31.36,22.67,;23.78,24.02,;22.27,24.29,;24.77,25.2,;24.25,26.65,;22.73,26.92,;22.21,28.36,;25.24,27.82,;26.76,27.55,;24.72,29.27,;25.71,30.45,;27.23,30.18,;28.22,31.36,;29.73,31.09,;30.73,32.27,;30.2,33.71,;28.69,33.98,;27.69,32.81,;25.19,31.9,;26.18,33.08,;23.67,32.17,;23.14,33.62,;24.14,34.79,;23.61,36.24,;22.13,36.67,;22.08,38.21,;23.53,38.73,;24.48,37.52,;21.63,33.89,;21.1,35.33,;20.64,32.71,;19.12,32.98,;18.6,34.43,;17.42,35.42,;17.69,36.93,;15.97,34.89,;18.13,31.8,;18.65,30.35,;16.61,32.07,;15.62,30.89,;16.14,29.44,;15.15,28.27,;15.68,26.82,;14.69,25.64,;15.21,24.19,;14.1,31.16,;13.58,32.61,;13.11,29.98,;11.6,30.25,;11.07,31.7,;9.56,31.97,;9.03,33.42,;7.52,33.69,;6.52,32.51,;5.01,32.78,;7.05,31.06,;8.56,30.79,;10.6,29.08,;9.09,29.35,;11.13,27.63,;10.14,26.45,;8.62,26.72,;8.1,28.17,;7.63,25.54,;6.11,25.81,;5.59,27.26,;4.07,27.53,;3.55,28.98,;3.08,26.35,;5.12,24.63,;3.6,24.9,;5.64,23.19,;4.65,22.01,;3.14,22.28,;5.18,20.56,;4.19,19.38,;6.69,20.29,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19967
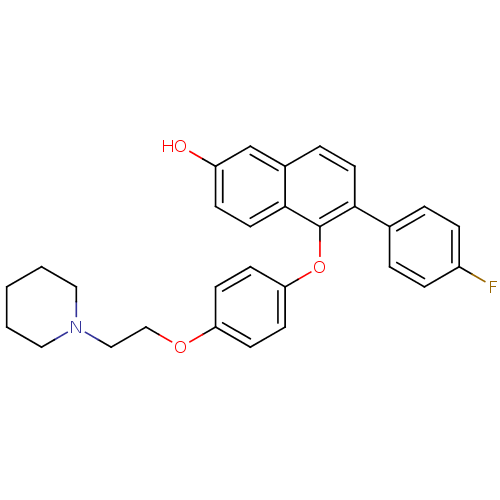
(6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO3/c30-23-7-4-21(5-8-23)27-14-6-22-20-24(32)9-15-28(22)29(27)34-26-12-10-25(11-13-26)33-19-18-31-16-2-1-3-17-31/h4-15,20,32H,1-3,16-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212160
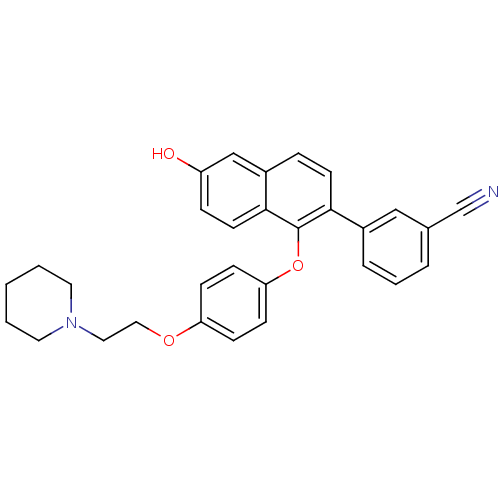
(3-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(c1)C#N Show InChI InChI=1S/C30H28N2O3/c31-21-22-5-4-6-23(19-22)28-13-7-24-20-25(33)8-14-29(24)30(28)35-27-11-9-26(10-12-27)34-18-17-32-15-2-1-3-16-32/h4-14,19-20,33H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85073
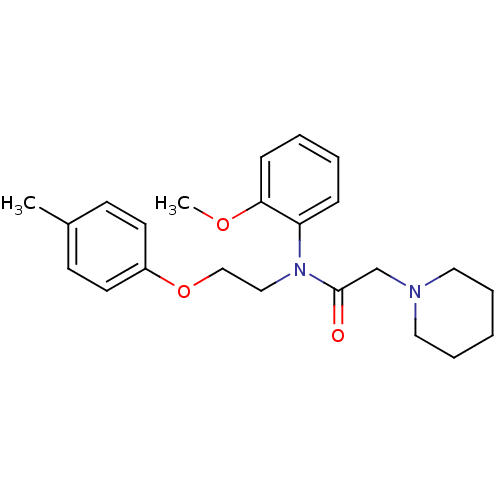
(CAS_3043476 | Galantide (M15) | NSC_3043476)Show InChI InChI=1S/C23H30N2O3/c1-19-10-12-20(13-11-19)28-17-16-25(21-8-4-5-9-22(21)27-2)23(26)18-24-14-6-3-7-15-24/h4-5,8-13H,3,6-7,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Vesicular acetylcholine transporter
(Homo sapiens (Human)) | BDBM50292908
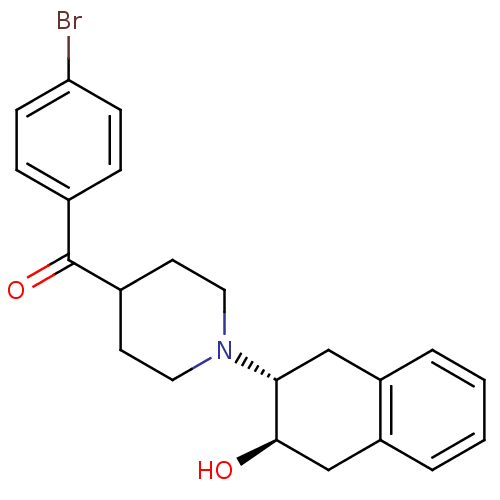
((+/-)-trans-2-Hydroxy-3-(4-(4-bromobenzoyl)piperid...)Show SMILES O[C@@H]1Cc2ccccc2C[C@H]1N1CCC(CC1)C(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C22H24BrNO2/c23-19-7-5-15(6-8-19)22(26)16-9-11-24(12-10-16)20-13-17-3-1-2-4-18(17)14-21(20)25/h1-8,16,20-21,25H,9-14H2/t20-,21-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]vesamicol from human vesicular acetylcholine transporter expressed in rat PC12 cells by liquid scintillation spectrometry |
J Med Chem 52: 1358-69 (2009)
Article DOI: 10.1021/jm8012344
BindingDB Entry DOI: 10.7270/Q26W9B3B |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19967

(6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO3/c30-23-7-4-21(5-8-23)27-14-6-22-20-24(32)9-15-28(22)29(27)34-26-12-10-25(11-13-26)33-19-18-31-16-2-1-3-17-31/h4-15,20,32H,1-3,16-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | -54.3 | n/a | n/a | 3.18 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... |
J Med Chem 48: 6772-5 (2005)
Article DOI: 10.1021/jm050723z
BindingDB Entry DOI: 10.7270/Q2RR1WHW |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50491021
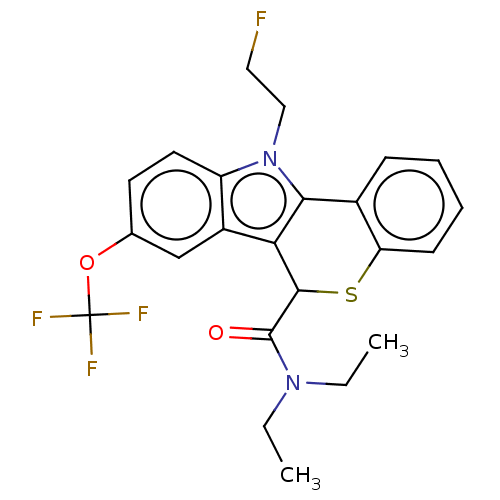
(CHEMBL2377344)Show SMILES CCN(CC)C(=O)C1Sc2ccccc2-c2c1c1cc(OC(F)(F)F)ccc1n2CCF Show InChI InChI=1S/C23H22F4N2O2S/c1-3-28(4-2)22(30)21-19-16-13-14(31-23(25,26)27)9-10-17(16)29(12-11-24)20(19)15-7-5-6-8-18(15)32-21/h5-10,13,21H,3-4,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from PBR receptor in Wistar rat heart homogenates |
Bioorg Med Chem Lett 23: 2368-72 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.057
BindingDB Entry DOI: 10.7270/Q2PR7ZW9 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85168

(M32)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r,wU:111.115,131.138,95.103,84.88,68.80,52.59,20.28,4.4,2.2,145.150,161.166,162.169,179.184,188.193,199.204,wD:115.131,103.111,89.95,60.67,41.42,30.39,8.17,137.142,153.158,154.160,168.173,(13.69,11.51,;13.72,9.97,;12.46,9.14,;11.11,9.89,;12.54,7.63,;11.23,6.8,;9.89,7.48,;9.85,9.02,;8.62,6.65,;8.66,5.14,;10.01,4.43,;10.12,2.93,;11.51,2.18,;12.74,3.05,;14.16,2.37,;12.66,4.59,;11.27,5.26,;7.28,7.36,;5.97,6.57,;5.97,4.98,;4.67,7.32,;4.63,8.82,;5.93,9.65,;7.36,9.02,;8.39,10.12,;7.71,11.47,;6.13,11.19,;3.36,6.53,;2.02,7.16,;2.02,8.62,;.63,6.37,;.59,4.82,;1.94,3.99,;1.94,2.45,;3.24,1.66,;3.2,.08,;4.55,-.67,;1.86,-.67,;-.71,7.2,;-2.06,6.49,;-3.56,7.12,;-2.06,5.02,;-.75,4.11,;-1.19,2.65,;-2.69,2.61,;-3.16,4.03,;-4.63,4.43,;-4.98,5.93,;-5.77,3.36,;-7.24,3.84,;-8.27,2.73,;-7.91,1.19,;-9.73,3.08,;-10.12,4.59,;-9.02,5.7,;-9.41,7.2,;-7.51,5.26,;-10.84,2.06,;-12.34,2.37,;-12.66,3.8,;-13.41,1.27,;-13.05,-.2,;-11.59,-.59,;-10.48,.51,;-11.15,-2.06,;-14.87,1.7,;-15.98,.67,;-15.62,-.87,;-17.56,1.07,;-17.92,2.61,;-16.85,3.64,;-15.35,3.28,;-14.24,4.39,;-14.6,5.85,;-13.49,6.96,;-16.1,6.29,;-17.24,5.22,;-18.59,0,;-20.05,.44,;-20.45,1.9,;-21.12,-.67,;-22.54,-.28,;-23.65,-1.34,;-23.26,-2.81,;-25.19,-.99,;-25.55,.55,;-26.26,-2.02,;-27.73,-1.62,;-28.08,-.16,;-28.83,-2.69,;-28.44,-4.15,;-26.97,-4.63,;-30.3,-2.25,;-31.44,-3.28,;-31.09,-4.71,;-32.95,-2.81,;-33.3,-1.34,;-32.19,-.28,;-30.73,-.71,;-32.55,1.23,;-34.05,-3.92,;-35.56,-3.56,;-35.87,-2.14,;-36.63,-4.71,;-36.27,-6.13,;-34.8,-6.57,;-33.7,-5.5,;-34.41,-8.03,;-38.09,-4.23,;-39.23,-5.3,;-38.84,-6.76,;-40.7,-4.87,;-41.85,-5.93,;-43.27,-5.54,;-43.66,-4.03,;-44.46,-6.6,;-44.18,-8.11,;-42.64,-8.54,;-41.45,-7.59,;-40.11,-8.39,;-40.54,-9.93,;-39.71,-11.27,;-40.46,-12.66,;-41.96,-12.66,;-42.87,-11.35,;-42.12,-10.05,;-45.92,-6.13,;-47.07,-7.2,;-46.71,-8.66,;-48.57,-6.76,;-49.68,-7.83,;-41.05,-3.36,;-42.6,-2.89,;-39.91,-2.29,;13.88,6.88,;13.88,5.42,;15.19,7.67,;16.53,6.88,;16.53,5.38,;17.92,4.67,;19.18,5.46,;17.96,3.12,;17.84,7.71,;17.84,9.18,;19.18,7.04,;20.45,7.75,;20.49,9.25,;21.79,10.09,;21.83,11.59,;23.14,9.25,;21.79,7.04,;21.79,5.46,;23.14,7.75,;24.44,7,;24.48,5.5,;23.18,4.71,;25.79,4.75,;25.83,3.24,;25.71,7.75,;25.71,9.29,;27.01,6.92,;28.32,7.71,;28.32,9.25,;27.01,10.05,;29.66,10.01,;29.66,6.96,;29.66,5.46,;30.97,7.67,;32.31,6.88,;32.27,5.38,;33.62,4.59,;33.62,3.08,;34.88,2.37,;34.92,.83,;36.27,.04,;33.62,0,;33.66,7.63,;33.66,9.1,;35,6.88,;36.27,7.67,;36.27,9.18,;37.53,9.97,;37.53,11.47,;38.8,12.26,;36.19,12.26,;37.61,6.92,;37.61,5.38,;38.88,7.75,;40.18,7.04,;40.26,5.5,;41.61,4.71,;41.69,3.16,;42.99,2.49,;43.07,.95,;44.46,.16,;41.77,.04,;41.53,7.87,;41.49,9.25,;42.91,7.24,;44.22,7.99,;44.22,9.57,;45.56,10.32,;45.6,11.87,;46.99,12.66,;48.33,11.83,;49.68,12.58,;48.29,10.32,;46.91,9.53,;45.52,7.24,;46.79,8.07,;45.52,5.66,)| Show InChI InChI=1S/C136H209N41O34/c1-16-70(11)108(130(208)171-99(58-104(140)186)123(201)166-93(51-69(9)10)125(203)174-109(71(12)17-2)131(209)176-111(74(15)180)132(210)162-87(28-22-46-151-136(146)147)115(193)160-88(42-43-102(138)184)118(196)159-85(26-20-44-149-134(142)143)116(194)163-89(112(141)190)52-75-30-36-80(181)37-31-75)173-126(204)95(54-77-34-40-82(183)41-35-77)167-122(200)97(56-79-61-148-65-155-79)168-117(195)86(27-21-45-150-135(144)145)161-129(207)101-29-23-47-177(101)107(189)63-154-114(192)90(48-66(3)4)164-119(197)91(49-67(5)6)165-121(199)94(53-76-32-38-81(182)39-33-76)158-106(188)62-153-113(191)72(13)156-128(206)100(64-178)172-124(202)98(57-103(139)185)169-120(198)92(50-68(7)8)170-133(211)110(73(14)179)175-127(205)96(157-105(187)59-137)55-78-60-152-84-25-19-18-24-83(78)84/h18-19,24-25,30-41,60-61,65-74,85-101,108-111,152,178-183H,16-17,20-23,26-29,42-59,62-64,137H2,1-15H3,(H2,138,184)(H2,139,185)(H2,140,186)(H2,141,190)(H,148,155)(H,153,191)(H,154,192)(H,156,206)(H,157,187)(H,158,188)(H,159,196)(H,160,193)(H,161,207)(H,162,210)(H,163,194)(H,164,197)(H,165,199)(H,166,201)(H,167,200)(H,168,195)(H,169,198)(H,170,211)(H,171,208)(H,172,202)(H,173,204)(H,174,203)(H,175,205)(H,176,209)(H4,142,143,149)(H4,144,145,150)(H4,146,147,151)/t70-,71-,72-,73+,74+,85+,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,108-,109-,110-,111-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19967

(6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO3/c30-23-7-4-21(5-8-23)27-14-6-22-20-24(32)9-15-28(22)29(27)34-26-12-10-25(11-13-26)33-19-18-31-16-2-1-3-17-31/h4-15,20,32H,1-3,16-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERbeta |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50255877
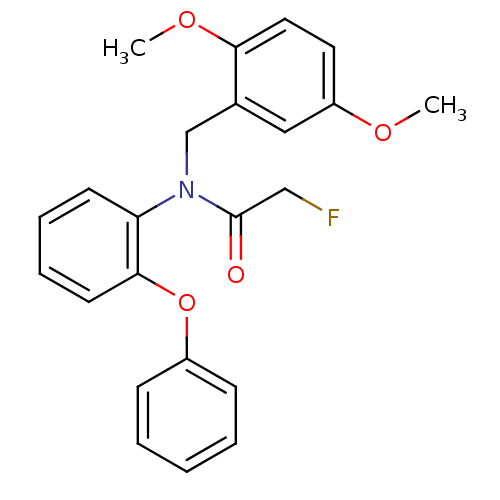
(CHEMBL473435 | N-Fluoroacetyl-N-(2,5-dimethoxybenz...)Show SMILES COc1ccc(OC)c(CN(C(=O)CF)c2ccccc2Oc2ccccc2)c1 Show InChI InChI=1S/C23H22FNO4/c1-27-19-12-13-21(28-2)17(14-19)16-25(23(26)15-24)20-10-6-7-11-22(20)29-18-8-4-3-5-9-18/h3-14H,15-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GE Healthcare
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Wistar rat heart incubated for 15 mins |
Bioorg Med Chem Lett 22: 5795-800 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.093
BindingDB Entry DOI: 10.7270/Q29K4CBT |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514208
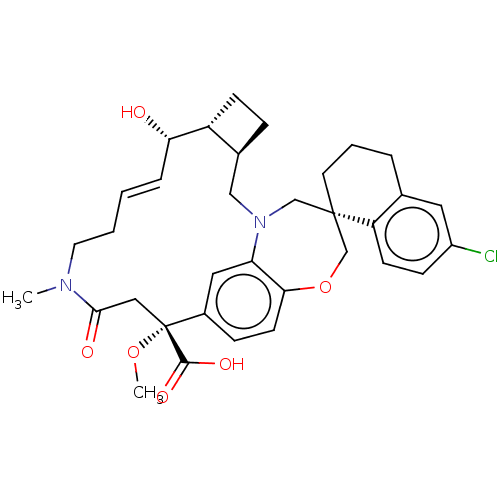
(CHEMBL4469850 | US11274105, Example 61)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](OC)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42-2,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38H,4-6,8,11,14-15,18-21H2,1-2H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85200
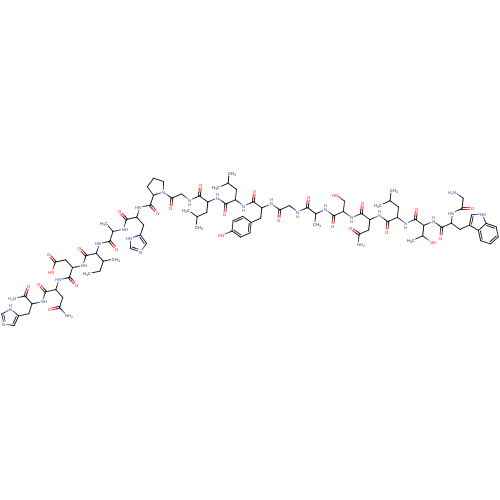
(Galanin (1-19), rat | Galanin rat)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(N)=O Show InChI InChI=1S/C92H135N27O26/c1-12-46(8)75(91(144)115-66(33-74(128)129)87(140)112-64(31-69(94)123)85(138)108-57(77(96)130)29-52-36-97-41-102-52)117-79(132)48(10)105-81(134)63(30-53-37-98-42-103-53)113-90(143)68-18-15-23-119(68)73(127)39-101-80(133)58(24-43(2)3)109-82(135)59(25-44(4)5)110-84(137)61(27-50-19-21-54(122)22-20-50)107-72(126)38-100-78(131)47(9)104-89(142)67(40-120)116-86(139)65(32-70(95)124)111-83(136)60(26-45(6)7)114-92(145)76(49(11)121)118-88(141)62(106-71(125)34-93)28-51-35-99-56-17-14-13-16-55(51)56/h13-14,16-17,19-22,35-37,41-49,57-68,75-76,99,120-122H,12,15,18,23-34,38-40,93H2,1-11H3,(H2,94,123)(H2,95,124)(H2,96,130)(H,97,102)(H,98,103)(H,100,131)(H,101,133)(H,104,142)(H,105,134)(H,106,125)(H,107,126)(H,108,138)(H,109,135)(H,110,137)(H,111,136)(H,112,140)(H,113,143)(H,114,145)(H,115,144)(H,116,139)(H,117,132)(H,118,141)(H,128,129) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data