

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018730 (3-(2-Phenethyl-octahydro-isoquinolin-4a-yl)-phenol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018728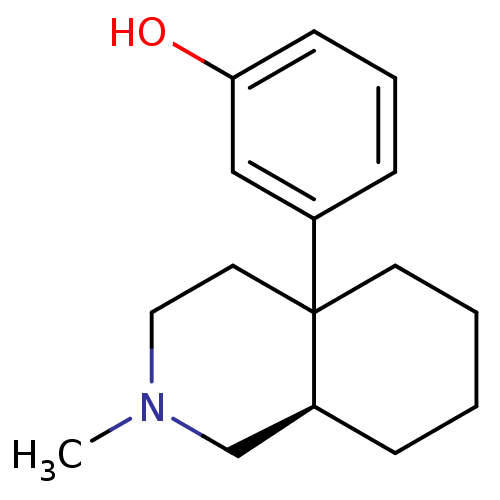 (3-(2-Methyl-octahydro-isoquinolin-4a-yl)-phenol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018728 (3-(2-Methyl-octahydro-isoquinolin-4a-yl)-phenol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001023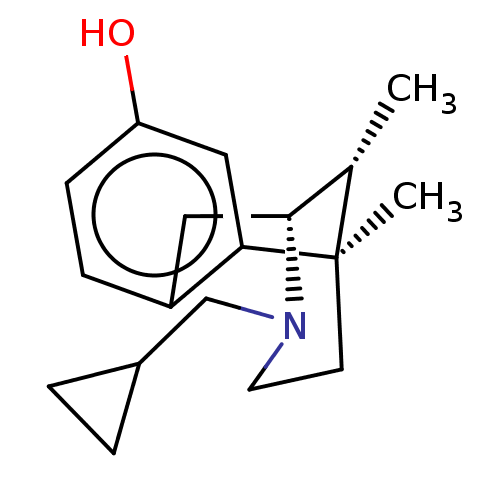 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000087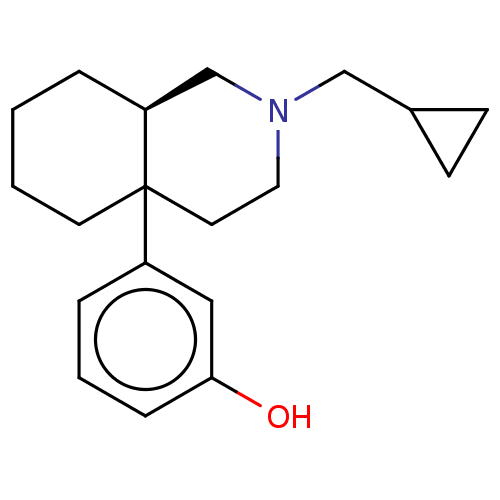 (3-(2-Cyclopropylmethyl-octahydro-isoquinolin-4a-yl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000087 (3-(2-Cyclopropylmethyl-octahydro-isoquinolin-4a-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092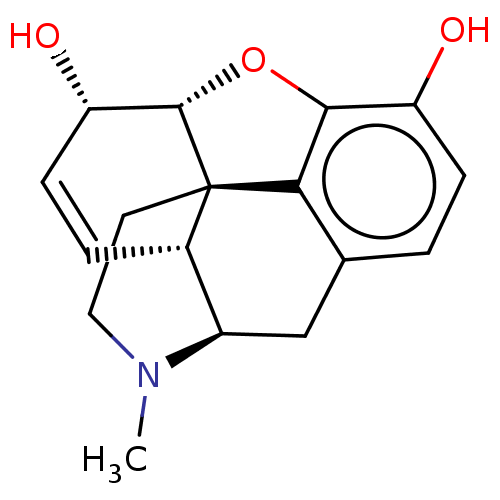 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50367061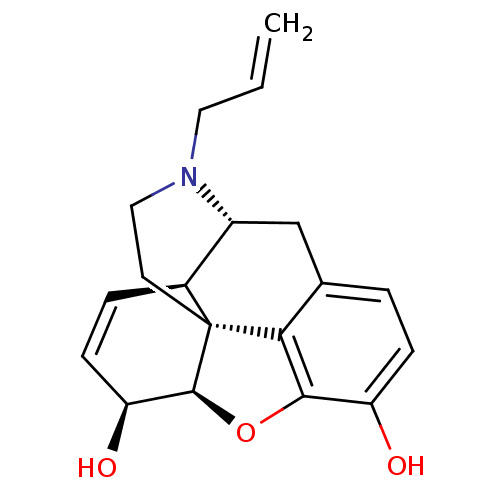 (NALORPHINE | NALORPHINE HYDROCHLORIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001028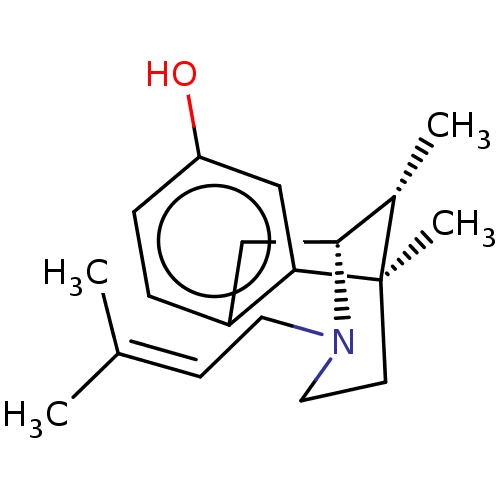 ((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50367061 (NALORPHINE | NALORPHINE HYDROCHLORIDE) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018733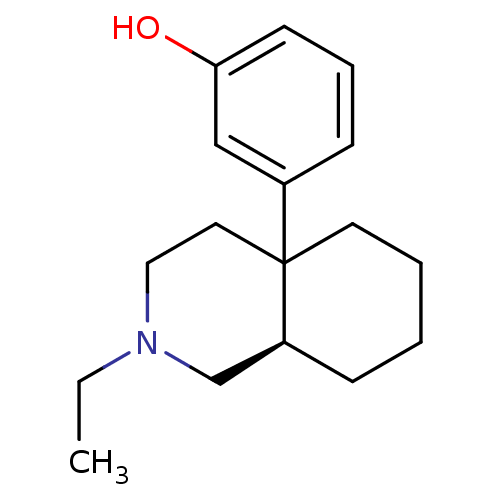 (3-(2-Ethyl-octahydro-isoquinolin-4a-yl)-phenol | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50018728 (3-(2-Methyl-octahydro-isoquinolin-4a-yl)-phenol | ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50018728 (3-(2-Methyl-octahydro-isoquinolin-4a-yl)-phenol | ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001028 ((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50018730 (3-(2-Phenethyl-octahydro-isoquinolin-4a-yl)-phenol...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018729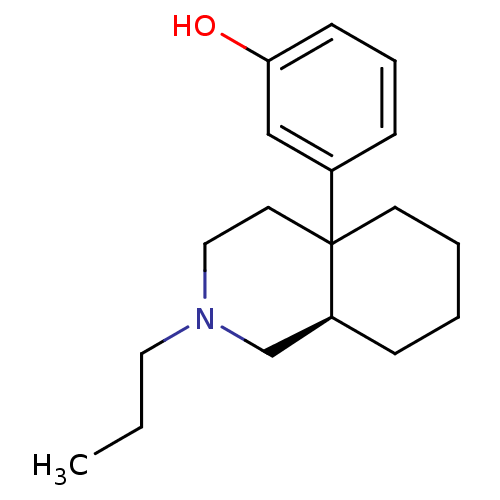 (3-(2-Propyl-octahydro-isoquinolin-4a-yl)-phenol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 473 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50018733 (3-(2-Ethyl-octahydro-isoquinolin-4a-yl)-phenol | C...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 529 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50017543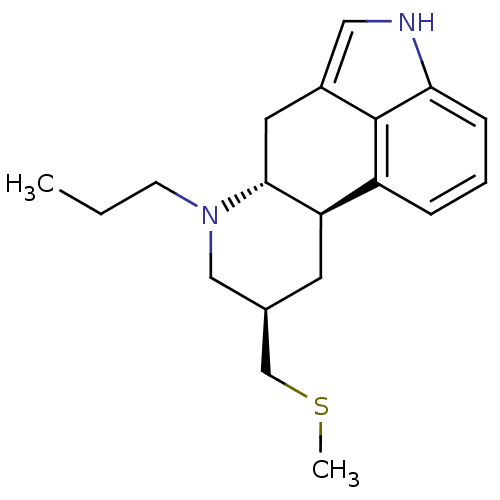 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]apomorphine binding to dopamine receptor of rat corpus striatum | J Med Chem 26: 1112-6 (1983) BindingDB Entry DOI: 10.7270/Q29G5Q18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055371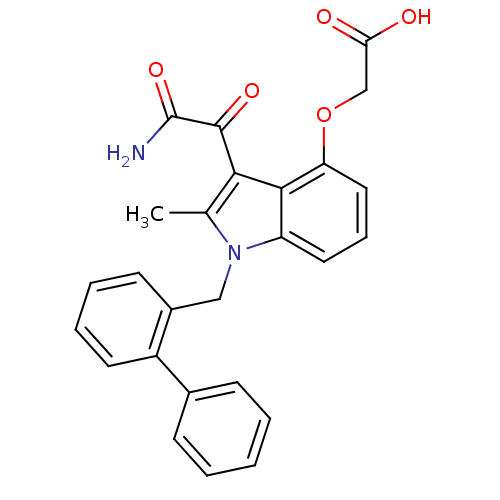 ((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50055367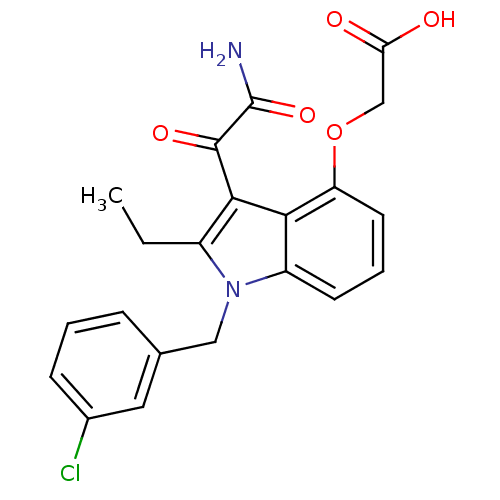 (CHEMBL345986 | [3-Aminooxalyl-1-(3-chloro-benzyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound wastested for inhibition of porcine secreted pancreatic PLA2 | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055374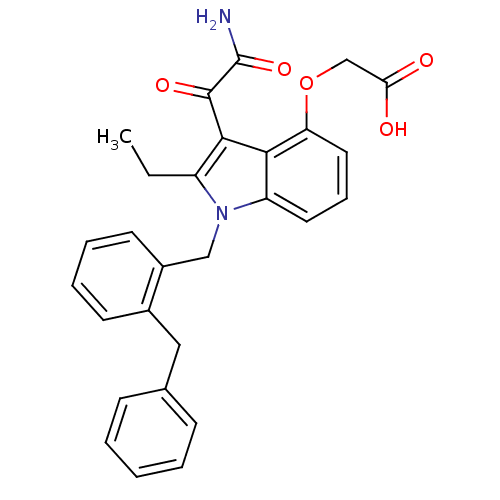 (CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055374 (CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50017543 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]dopamine binding to dopamine receptor of rat corpus striatum | J Med Chem 26: 1112-6 (1983) BindingDB Entry DOI: 10.7270/Q29G5Q18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055401 ((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055387 ((R)-2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055371 ((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055379 ((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-cyclopropyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055401 ((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055378 (CHEMBL345488 | [3-Aminooxalyl-1-(2,6-dichloro-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50055384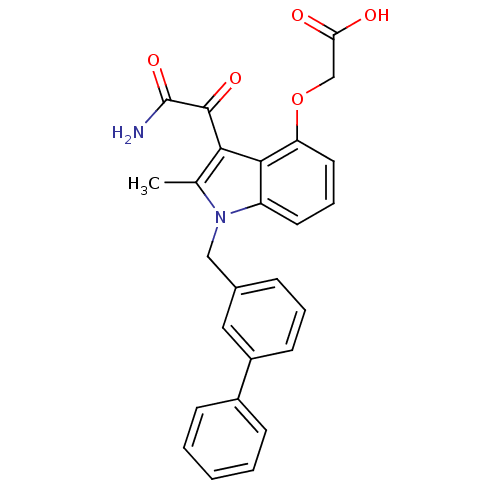 ((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine secretory pancreatic Phospholipase A2 | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366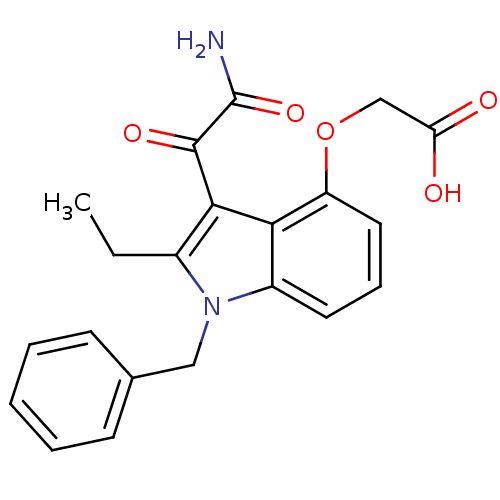 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055367 (CHEMBL345986 | [3-Aminooxalyl-1-(3-chloro-benzyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055370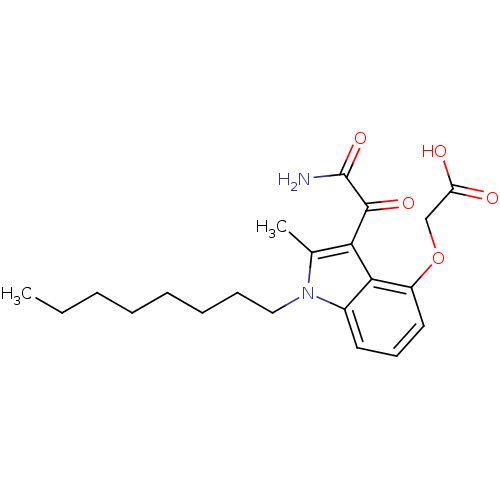 ((3-Aminooxalyl-2-methyl-1-octyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055380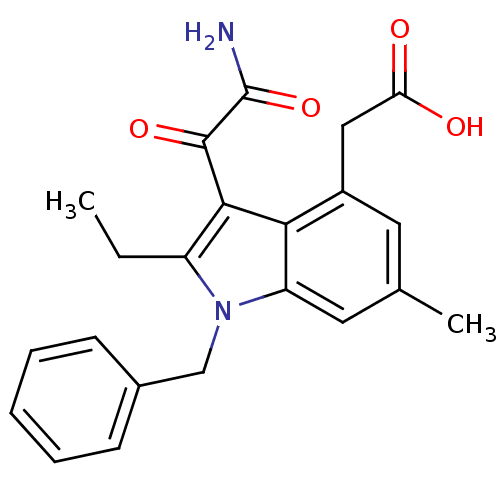 ((3-Aminooxalyl-1-benzyl-2-ethyl-6-methyl-1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055384 ((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055380 ((3-Aminooxalyl-1-benzyl-2-ethyl-6-methyl-1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50223718 (CHEMBL273788) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]QNB from Muscarinic acetylcholine receptor of rat brain membrane | J Med Chem 25: 1133-40 (1982) BindingDB Entry DOI: 10.7270/Q2K64M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055368 ((3-Aminooxalyl-2-methyl-1-naphthalen-1-ylmethyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055394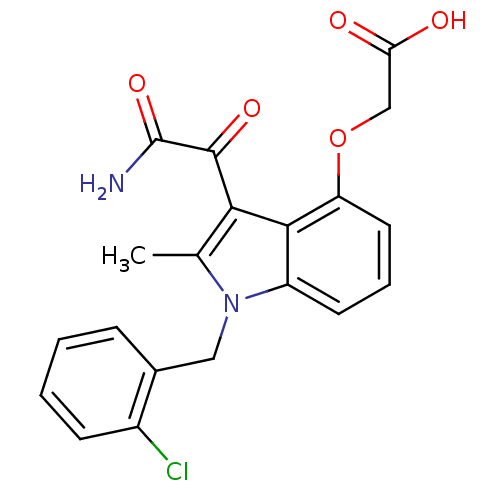 (CHEMBL148795 | [3-Aminooxalyl-1-(2-chloro-benzyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055385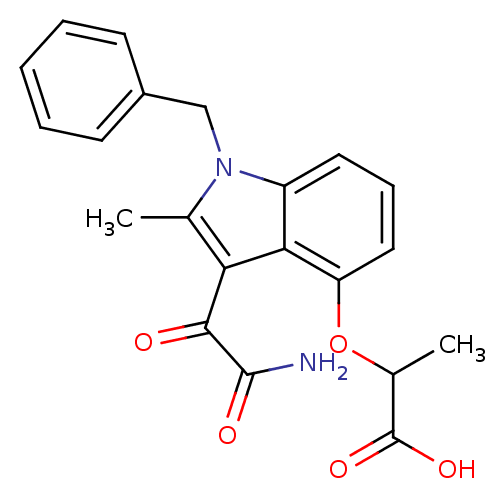 (2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-ylox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055384 ((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]QNB from Muscarinic acetylcholine receptor of rat brain membrane | J Med Chem 25: 1133-40 (1982) BindingDB Entry DOI: 10.7270/Q2K64M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055305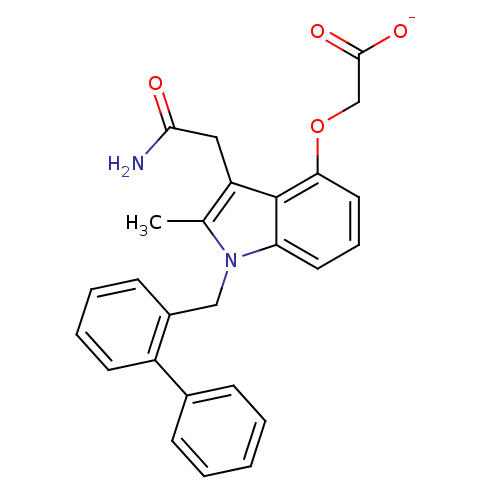 (CHEMBL423762 | Sodium; (1-biphenyl-2-ylmethyl-3-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human non-pancreatic secretory phospholipase A2 (PLA2) in a chromogenic assay | J Med Chem 39: 5137-58 (1997) Article DOI: 10.1021/jm960486n BindingDB Entry DOI: 10.7270/Q2639NV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055385 (2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-ylox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50223771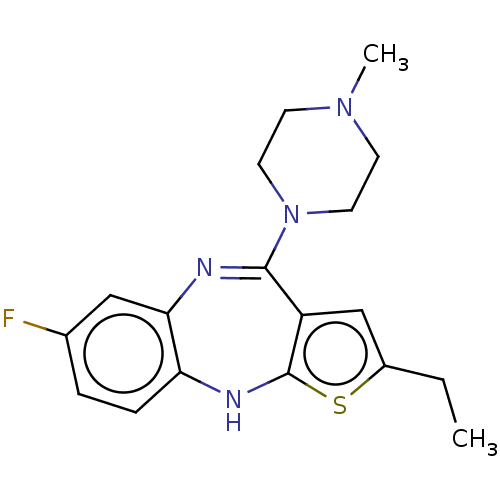 (CHEMBL14668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]spiroperidol from Dopamine receptor of rat striatum membrane | J Med Chem 25: 1133-40 (1982) BindingDB Entry DOI: 10.7270/Q2K64M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]spiroperidol from Dopamine receptor of rat striatum membrane | J Med Chem 25: 1133-40 (1982) BindingDB Entry DOI: 10.7270/Q2K64M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055383 ((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay | J Med Chem 39: 5159-75 (1997) Article DOI: 10.1021/jm960487f BindingDB Entry DOI: 10.7270/Q22B8X54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 345 total ) | Next | Last >> |