 Found 829 hits with Last Name = 'jordan' and Initial = 's'
Found 829 hits with Last Name = 'jordan' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50137388
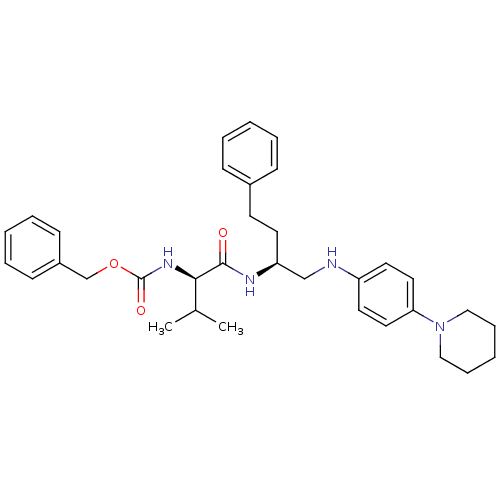
(((R)-2-Methyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES CC(C)[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H44N4O3/c1-26(2)32(37-34(40)41-25-28-14-8-4-9-15-28)33(39)36-30(17-16-27-12-6-3-7-13-27)24-35-29-18-20-31(21-19-29)38-22-10-5-11-23-38/h3-4,6-9,12-15,18-21,26,30,32,35H,5,10-11,16-17,22-25H2,1-2H3,(H,36,39)(H,37,40)/t30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137400
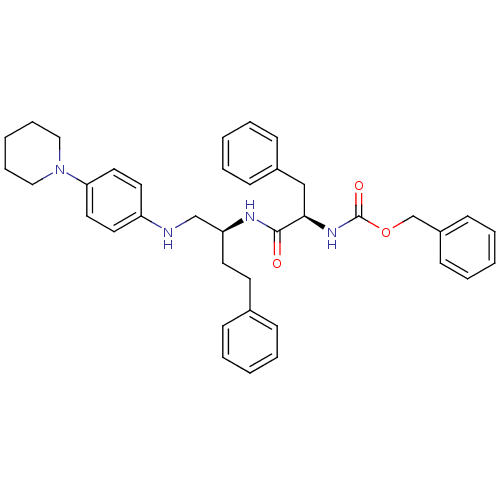
(((R)-2-Phenyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES O=C(N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1)OCc1ccccc1 Show InChI InChI=1S/C38H44N4O3/c43-37(36(27-31-15-7-2-8-16-31)41-38(44)45-29-32-17-9-3-10-18-32)40-34(20-19-30-13-5-1-6-14-30)28-39-33-21-23-35(24-22-33)42-25-11-4-12-26-42/h1-3,5-10,13-18,21-24,34,36,39H,4,11-12,19-20,25-29H2,(H,40,43)(H,41,44)/t34-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137398
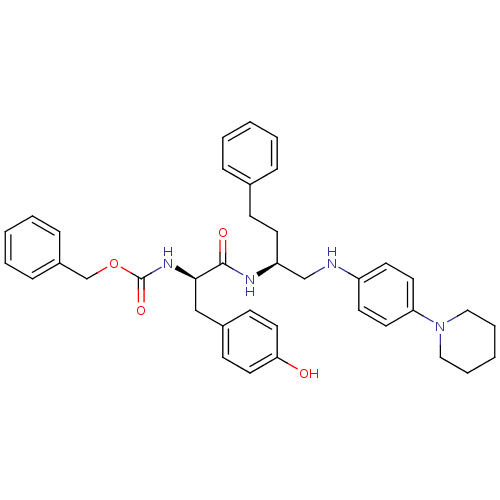
(((R)-2-(4-Hydroxy-phenyl)-1-{3-phenyl-1-[(4-piperi...)Show SMILES Oc1ccc(C[C@@H](NC(=O)OCc2ccccc2)C(=O)N[C@@H](CCc2ccccc2)CNc2ccc(cc2)N2CCCCC2)cc1 Show InChI InChI=1S/C38H44N4O4/c43-35-22-15-30(16-23-35)26-36(41-38(45)46-28-31-12-6-2-7-13-31)37(44)40-33(17-14-29-10-4-1-5-11-29)27-39-32-18-20-34(21-19-32)42-24-8-3-9-25-42/h1-2,4-7,10-13,15-16,18-23,33,36,39,43H,3,8-9,14,17,24-28H2,(H,40,44)(H,41,45)/t33-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137394
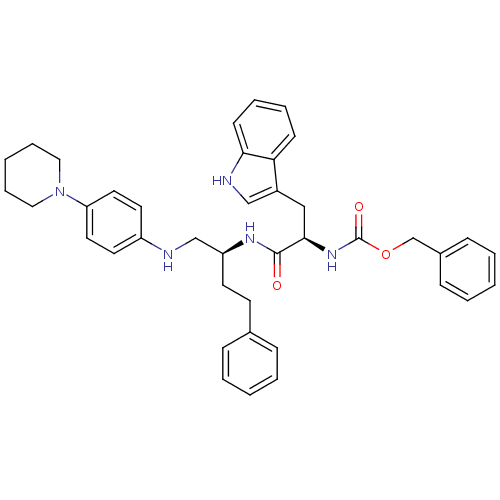
(((R)-2-(3H-Indol-3-yl)-1-{3-phenyl-1-[(4-piperidin...)Show SMILES O=C(N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1)OCc1ccccc1 Show InChI InChI=1S/C40H45N5O3/c46-39(38(26-32-27-42-37-17-9-8-16-36(32)37)44-40(47)48-29-31-14-6-2-7-15-31)43-34(19-18-30-12-4-1-5-13-30)28-41-33-20-22-35(23-21-33)45-24-10-3-11-25-45/h1-2,4-9,12-17,20-23,27,34,38,41-42H,3,10-11,18-19,24-26,28-29H2,(H,43,46)(H,44,47)/t34-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137392
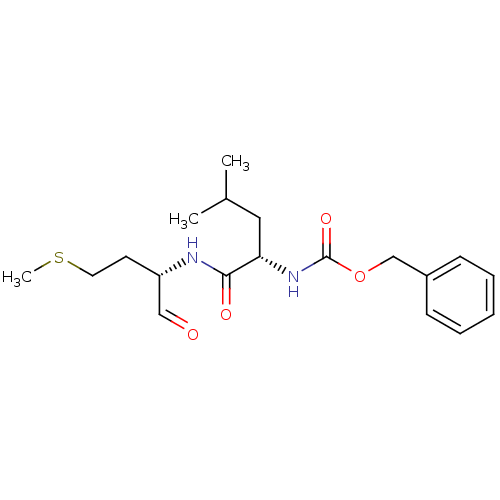
(CHEMBL99195 | [(S)-1-((S)-1-Formyl-3-methylsulfany...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C19H28N2O4S/c1-14(2)11-17(18(23)20-16(12-22)9-10-26-3)21-19(24)25-13-15-7-5-4-6-8-15/h4-8,12,14,16-17H,9-11,13H2,1-3H3,(H,20,23)(H,21,24)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137393
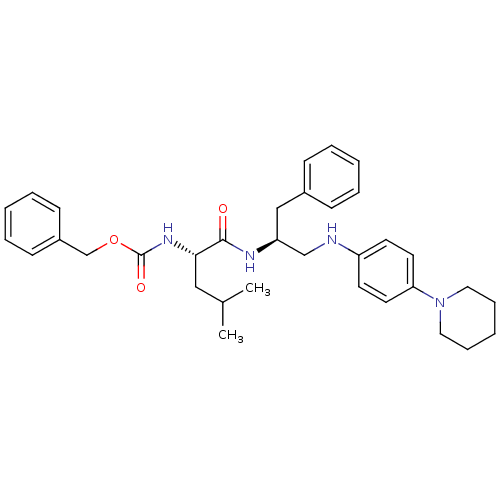
(CHEMBL353725 | {(S)-1-[(S)-1-Benzyl-2-(4-piperidin...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H](CNc1ccc(cc1)N1CCCCC1)Cc1ccccc1 Show InChI InChI=1S/C34H44N4O3/c1-26(2)22-32(37-34(40)41-25-28-14-8-4-9-15-28)33(39)36-30(23-27-12-6-3-7-13-27)24-35-29-16-18-31(19-17-29)38-20-10-5-11-21-38/h3-4,6-9,12-19,26,30,32,35H,5,10-11,20-25H2,1-2H3,(H,36,39)(H,37,40)/t30-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137389
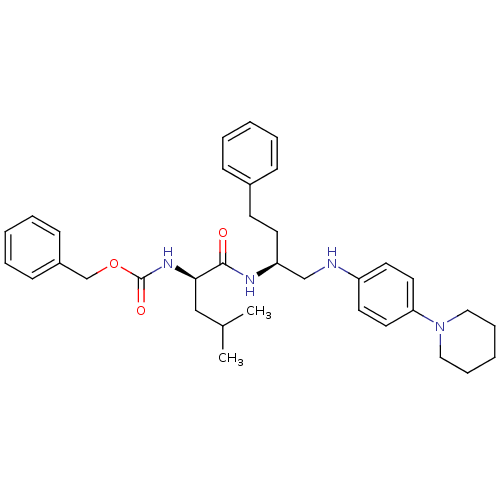
(((R)-3-Methyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C35H46N4O3/c1-27(2)24-33(38-35(41)42-26-29-14-8-4-9-15-29)34(40)37-31(17-16-28-12-6-3-7-13-28)25-36-30-18-20-32(21-19-30)39-22-10-5-11-23-39/h3-4,6-9,12-15,18-21,27,31,33,36H,5,10-11,16-17,22-26H2,1-2H3,(H,37,40)(H,38,41)/t31-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137395
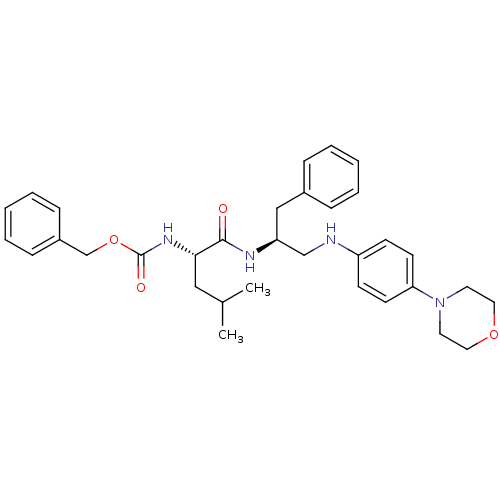
(CHEMBL170242 | {(S)-1-[(S)-1-Benzyl-2-(4-morpholin...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H](CNc1ccc(cc1)N1CCOCC1)Cc1ccccc1 Show InChI InChI=1S/C33H42N4O4/c1-25(2)21-31(36-33(39)41-24-27-11-7-4-8-12-27)32(38)35-29(22-26-9-5-3-6-10-26)23-34-28-13-15-30(16-14-28)37-17-19-40-20-18-37/h3-16,25,29,31,34H,17-24H2,1-2H3,(H,35,38)(H,36,39)/t29-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM22473
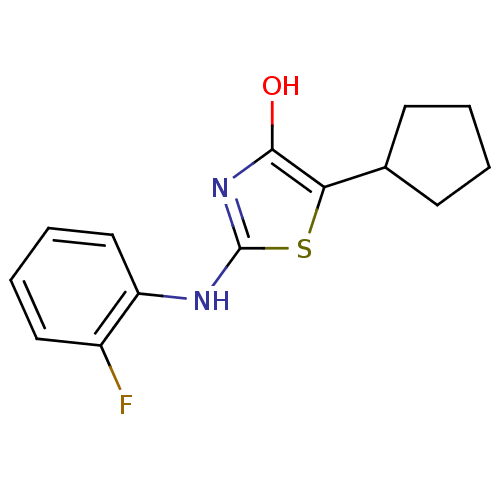
(2-(hexahydro-2,5-methanopentalen-3a(1H)-ylamino)-1...)Show SMILES O=C1N=C(NC23CC4CC2CC(C3)C4)SC11CCCC1 |t:2,TLB:12:11:5.6:8,4:5:8:11.10.13,4:5:10:7.8.13,THB:6:5:10:7.8.13,6:7:5.12:10,12:5:8:11.10.13| Show InChI InChI=1S/C16H22N2OS/c19-13-16(3-1-2-4-16)20-14(17-13)18-15-8-10-5-11(9-15)7-12(15)6-10/h10-12H,1-9H2,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB
| Assay Description
The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... |
J Med Chem 51: 2933-43 (2008)
Article DOI: 10.1021/jm701551j
BindingDB Entry DOI: 10.7270/Q2HM56Q8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137400

(((R)-2-Phenyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES O=C(N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1)OCc1ccccc1 Show InChI InChI=1S/C38H44N4O3/c43-37(36(27-31-15-7-2-8-16-31)41-38(44)45-29-32-17-9-3-10-18-32)40-34(20-19-30-13-5-1-6-14-30)28-39-33-21-23-35(24-22-33)42-25-11-4-12-26-42/h1-3,5-10,13-18,21-24,34,36,39H,4,11-12,19-20,25-29H2,(H,40,43)(H,41,44)/t34-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137390
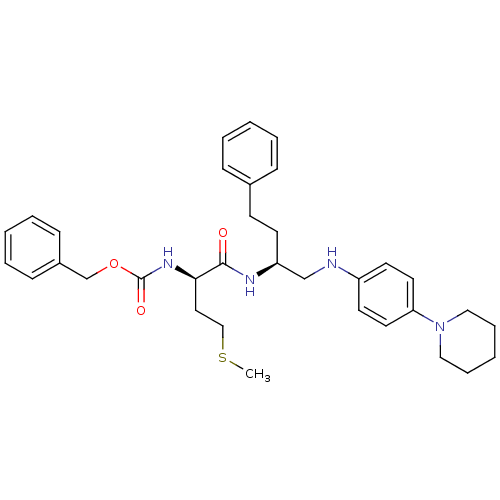
(((R)-3-Methylsulfanyl-1-{3-phenyl-1-[(4-piperidin-...)Show SMILES CSCC[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H44N4O3S/c1-42-24-21-32(37-34(40)41-26-28-13-7-3-8-14-28)33(39)36-30(16-15-27-11-5-2-6-12-27)25-35-29-17-19-31(20-18-29)38-22-9-4-10-23-38/h2-3,5-8,11-14,17-20,30,32,35H,4,9-10,15-16,21-26H2,1H3,(H,36,39)(H,37,40)/t30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137388

(((R)-2-Methyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES CC(C)[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H44N4O3/c1-26(2)32(37-34(40)41-25-28-14-8-4-9-15-28)33(39)36-30(17-16-27-12-6-3-7-13-27)24-35-29-18-20-31(21-19-29)38-22-10-5-11-23-38/h3-4,6-9,12-15,18-21,26,30,32,35H,5,10-11,16-17,22-25H2,1-2H3,(H,36,39)(H,37,40)/t30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005335
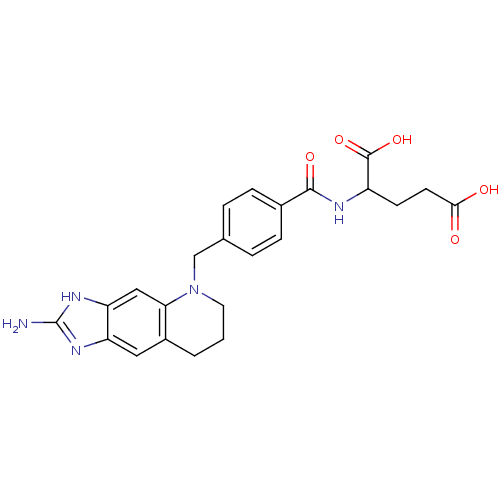
(2-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)C(=O)NC(CCC(O)=O)C(O)=O)c3cc2[nH]1 Show InChI InChI=1S/C23H25N5O5/c24-23-26-17-10-15-2-1-9-28(19(15)11-18(17)27-23)12-13-3-5-14(6-4-13)21(31)25-16(22(32)33)7-8-20(29)30/h3-6,10-11,16H,1-2,7-9,12H2,(H,25,31)(H,29,30)(H,32,33)(H3,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137389

(((R)-3-Methyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C35H46N4O3/c1-27(2)24-33(38-35(41)42-26-29-14-8-4-9-15-29)34(40)37-31(17-16-28-12-6-3-7-13-28)25-36-30-18-20-32(21-19-30)39-22-10-5-11-23-39/h3-4,6-9,12-15,18-21,27,31,33,36H,5,10-11,16-17,22-26H2,1-2H3,(H,37,40)(H,38,41)/t31-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224221
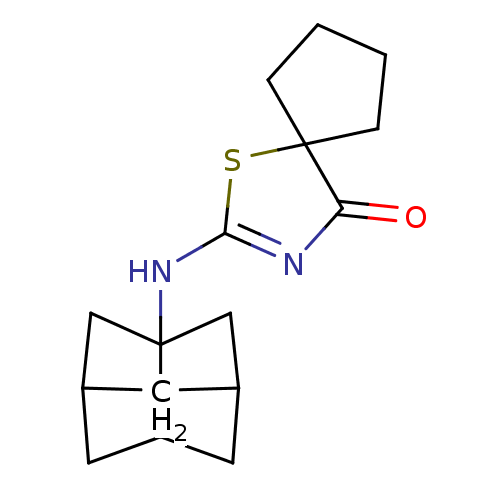
(5-cyclopentyl-2-(2-fluorophenylamino)thiazol-4(5H)...)Show InChI InChI=1S/C14H15FN2OS/c15-10-7-3-4-8-11(10)16-14-17-13(18)12(19-14)9-5-1-2-6-9/h3-4,7-9,18H,1-2,5-6H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005329
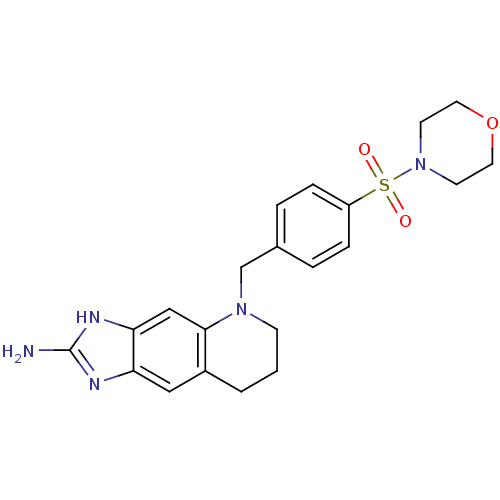
(5-[4-(Morpholine-4-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)N4CCOCC4)c3cc2[nH]1 Show InChI InChI=1S/C21H25N5O3S/c22-21-23-18-12-16-2-1-7-25(20(16)13-19(18)24-21)14-15-3-5-17(6-4-15)30(27,28)26-8-10-29-11-9-26/h3-6,12-13H,1-2,7-11,14H2,(H3,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224216
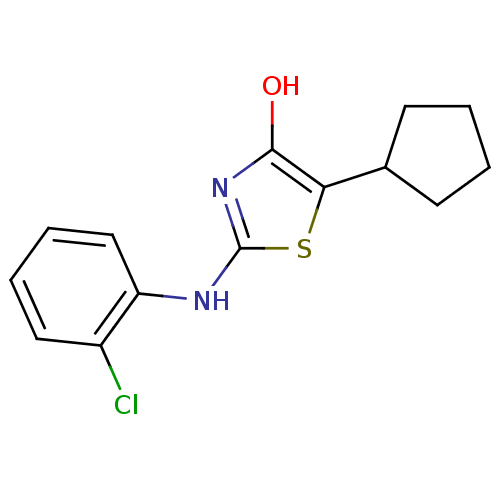
(2-(2-chlorophenylamino)-5-cyclopentylthiazol-4(5H)...)Show InChI InChI=1S/C14H15ClN2OS/c15-10-7-3-4-8-11(10)16-14-17-13(18)12(19-14)9-5-1-2-6-9/h3-4,7-9,18H,1-2,5-6H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224207
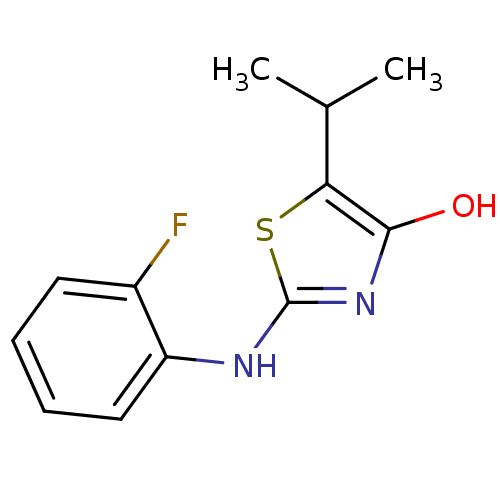
(2-(2-fluorophenylamino)-5-isopropylthiazol-4(5H)-o...)Show InChI InChI=1S/C12H13FN2OS/c1-7(2)10-11(16)15-12(17-10)14-9-6-4-3-5-8(9)13/h3-7,16H,1-2H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137388

(((R)-2-Methyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES CC(C)[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H44N4O3/c1-26(2)32(37-34(40)41-25-28-14-8-4-9-15-28)33(39)36-30(17-16-27-12-6-3-7-13-27)24-35-29-18-20-31(21-19-29)38-22-10-5-11-23-38/h3-4,6-9,12-15,18-21,26,30,32,35H,5,10-11,16-17,22-25H2,1-2H3,(H,36,39)(H,37,40)/t30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin B |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224208

(2-(2-chlorophenylamino)-5-isopropylthiazol-4(5H)-o...)Show InChI InChI=1S/C12H13ClN2OS/c1-7(2)10-11(16)15-12(17-10)14-9-6-4-3-5-8(9)13/h3-7,16H,1-2H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224202

(5-cyclohexyl-2-(2-fluorophenylamino)thiazol-4(5H)-...)Show InChI InChI=1S/C15H17FN2OS/c16-11-8-4-5-9-12(11)17-15-18-14(19)13(20-15)10-6-2-1-3-7-10/h4-5,8-10,19H,1-3,6-7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005324

(4-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccc(O)cc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O3S/c24-23-25-20-12-16-2-1-11-27(22(16)13-21(20)26-23)14-15-3-7-18(8-4-15)31(29,30)19-9-5-17(28)6-10-19/h3-10,12-13,28H,1-2,11,14H2,(H3,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137398

(((R)-2-(4-Hydroxy-phenyl)-1-{3-phenyl-1-[(4-piperi...)Show SMILES Oc1ccc(C[C@@H](NC(=O)OCc2ccccc2)C(=O)N[C@@H](CCc2ccccc2)CNc2ccc(cc2)N2CCCCC2)cc1 Show InChI InChI=1S/C38H44N4O4/c43-35-22-15-30(16-23-35)26-36(41-38(45)46-28-31-12-6-2-7-13-31)37(44)40-33(17-14-29-10-4-1-5-11-29)27-39-32-18-20-34(21-19-32)42-24-8-3-9-25-42/h1-2,4-7,10-13,15-16,18-23,33,36,39,43H,3,8-9,14,17,24-28H2,(H,40,44)(H,41,45)/t33-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137391

(((R)-1-{3-Phenyl-1-[(4-piperidin-1-yl-phenylamino)...)Show SMILES C[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C32H40N4O3/c1-25(34-32(38)39-24-27-13-7-3-8-14-27)31(37)35-29(16-15-26-11-5-2-6-12-26)23-33-28-17-19-30(20-18-28)36-21-9-4-10-22-36/h2-3,5-8,11-14,17-20,25,29,33H,4,9-10,15-16,21-24H2,1H3,(H,34,38)(H,35,37)/t25-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137387

(((R)-3-Phenyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES O=C(N[C@H](CCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1)OCc1ccccc1 Show InChI InChI=1S/C39H46N4O3/c44-38(37(26-20-32-15-7-2-8-16-32)42-39(45)46-30-33-17-9-3-10-18-33)41-35(21-19-31-13-5-1-6-14-31)29-40-34-22-24-36(25-23-34)43-27-11-4-12-28-43/h1-3,5-10,13-18,22-25,35,37,40H,4,11-12,19-21,26-30H2,(H,41,44)(H,42,45)/t35-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137393

(CHEMBL353725 | {(S)-1-[(S)-1-Benzyl-2-(4-piperidin...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H](CNc1ccc(cc1)N1CCCCC1)Cc1ccccc1 Show InChI InChI=1S/C34H44N4O3/c1-26(2)22-32(37-34(40)41-25-28-14-8-4-9-15-28)33(39)36-30(23-27-12-6-3-7-13-27)24-35-29-16-18-31(19-17-29)38-20-10-5-11-21-38/h3-4,6-9,12-19,26,30,32,35H,5,10-11,20-25H2,1-2H3,(H,36,39)(H,37,40)/t30-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005329

(5-[4-(Morpholine-4-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)N4CCOCC4)c3cc2[nH]1 Show InChI InChI=1S/C21H25N5O3S/c22-21-23-18-12-16-2-1-7-25(20(16)13-19(18)24-21)14-15-3-5-17(6-4-15)30(27,28)26-8-10-29-11-9-26/h3-6,12-13H,1-2,7-11,14H2,(H3,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005335

(2-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)C(=O)NC(CCC(O)=O)C(O)=O)c3cc2[nH]1 Show InChI InChI=1S/C23H25N5O5/c24-23-26-17-10-15-2-1-9-28(19(15)11-18(17)27-23)12-13-3-5-14(6-4-13)21(31)25-16(22(32)33)7-8-20(29)30/h3-6,10-11,16H,1-2,7-9,12H2,(H,25,31)(H,29,30)(H,32,33)(H3,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224203

(2-(2-chlorophenylamino)-5-cyclohexylthiazol-4(5H)-...)Show InChI InChI=1S/C15H17ClN2OS/c16-11-8-4-5-9-12(11)17-15-18-14(19)13(20-15)10-6-2-1-3-7-10/h4-5,8-10,19H,1-3,6-7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137390

(((R)-3-Methylsulfanyl-1-{3-phenyl-1-[(4-piperidin-...)Show SMILES CSCC[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H44N4O3S/c1-42-24-21-32(37-34(40)41-26-28-13-7-3-8-14-28)33(39)36-30(16-15-27-11-5-2-6-12-27)25-35-29-17-19-31(20-18-29)38-22-9-4-10-23-38/h2-3,5-8,11-14,17-20,30,32,35H,4,9-10,15-16,21-26H2,1H3,(H,36,39)(H,37,40)/t30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin B |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137386
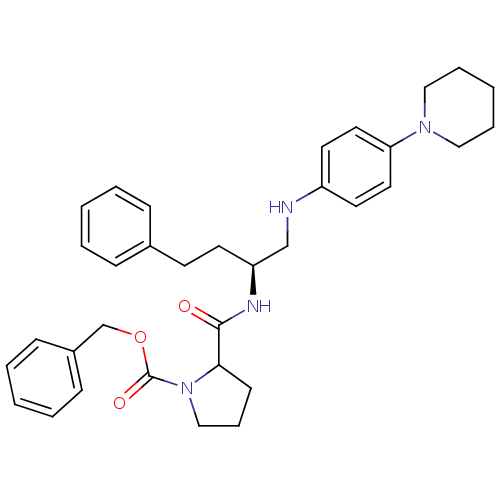
(2-{(S)-3-Phenyl-1-[(4-piperidin-1-yl-phenylamino)-...)Show SMILES O=C(N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1)C1CCCN1C(=O)OCc1ccccc1 Show InChI InChI=1S/C34H42N4O3/c39-33(32-15-10-24-38(32)34(40)41-26-28-13-6-2-7-14-28)36-30(17-16-27-11-4-1-5-12-27)25-35-29-18-20-31(21-19-29)37-22-8-3-9-23-37/h1-2,4-7,11-14,18-21,30,32,35H,3,8-10,15-17,22-26H2,(H,36,39)/t30-,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224217
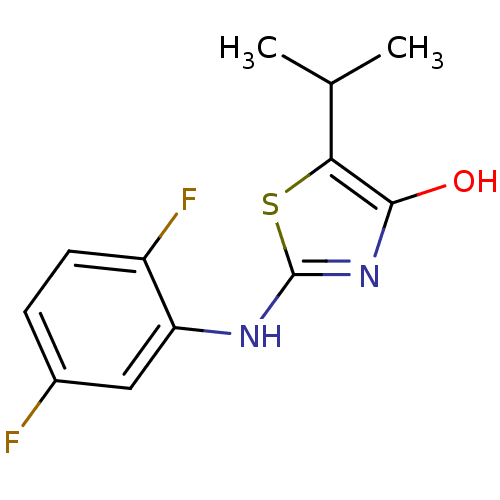
(2-(2,5-difluorophenylamino)-5-isopropylthiazol-4(5...)Show InChI InChI=1S/C12H12F2N2OS/c1-6(2)10-11(17)16-12(18-10)15-9-5-7(13)3-4-8(9)14/h3-6,17H,1-2H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
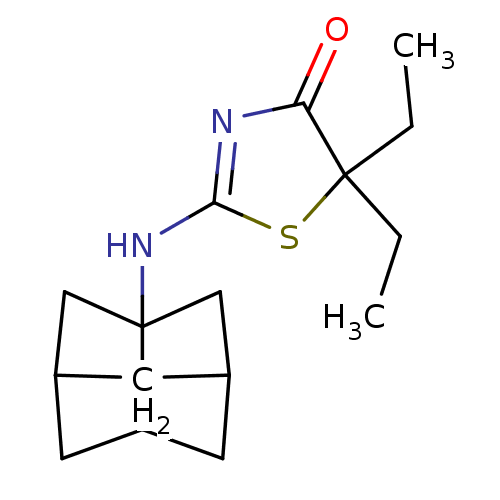
(Homo sapiens (Human)) | BDBM22467
(2-Amino-1,3-thiazol-4(5H)-one, 6h | 5-methyl-5-pro...)Show SMILES CCCC1(C)SC(NC23CC4CC2CC(C3)C4)=NC1=O |c:19,TLB:15:14:8.9:11,7:8:11:14.13.16,7:8:13:10.11.16,THB:9:8:13:10.11.16,9:10:8.15:13,15:8:11:14.13.16| Show InChI InChI=1S/C16H24N2OS/c1-3-4-15(2)13(19)17-14(20-15)18-16-8-10-5-11(9-16)7-12(16)6-10/h10-12H,3-9H2,1-2H3,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB
| Assay Description
The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... |
J Med Chem 51: 2933-43 (2008)
Article DOI: 10.1021/jm701551j
BindingDB Entry DOI: 10.7270/Q2HM56Q8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319660
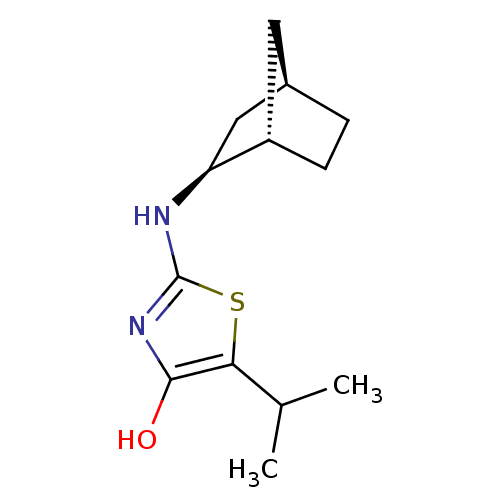
((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)c1sc(N[C@H]2C[C@@H]3CC[C@H]2C3)nc1O |r| Show InChI InChI=1S/C13H20N2OS/c1-7(2)11-12(16)15-13(17-11)14-10-6-8-3-4-9(10)5-8/h7-10,16H,3-6H2,1-2H3,(H,14,15)/t8-,9+,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319665

((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
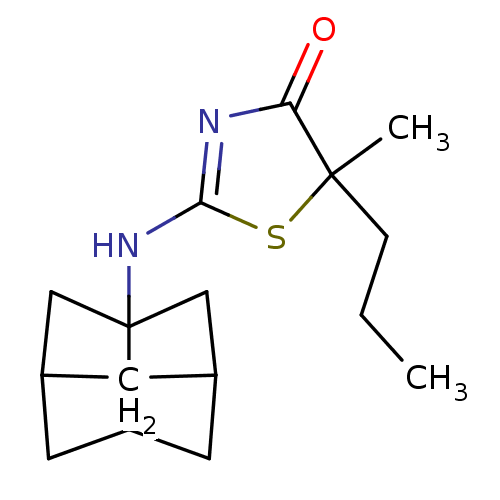
(Homo sapiens (Human)) | BDBM22472
(2-Amino-1,3-thiazol-4(5H)-one, 7b | 5,5-diethyl-2-...)Show SMILES CCC1(CC)SC(NC23CC4CC2CC(C3)C4)=NC1=O |c:19,TLB:15:14:8.9:11,7:8:11:14.13.16,7:8:13:10.11.16,THB:9:8:13:10.11.16,9:10:8.15:13,15:8:11:14.13.16| Show InChI InChI=1S/C16H24N2OS/c1-3-16(4-2)13(19)17-14(20-16)18-15-8-10-5-11(9-15)7-12(15)6-10/h10-12H,3-9H2,1-2H3,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB
| Assay Description
The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... |
J Med Chem 51: 2933-43 (2008)
Article DOI: 10.1021/jm701551j
BindingDB Entry DOI: 10.7270/Q2HM56Q8 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005330
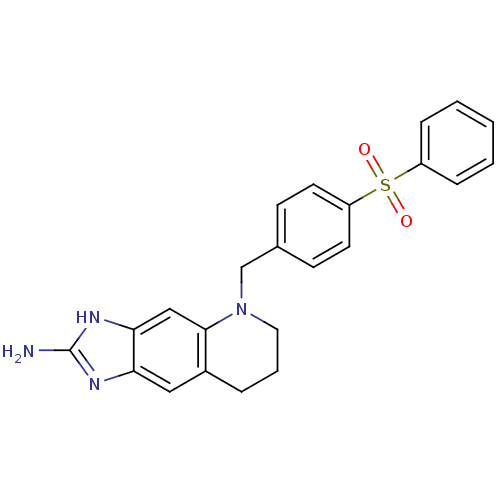
(5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccccc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O2S/c24-23-25-20-13-17-5-4-12-27(22(17)14-21(20)26-23)15-16-8-10-19(11-9-16)30(28,29)18-6-2-1-3-7-18/h1-3,6-11,13-14H,4-5,12,15H2,(H3,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224205
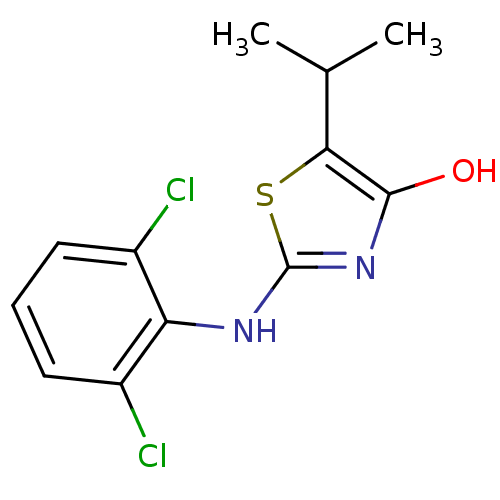
(2-(2,6-dichlorophenylamino)-5-isopropylthiazol-4(5...)Show InChI InChI=1S/C12H12Cl2N2OS/c1-6(2)10-11(17)16-12(18-10)15-9-7(13)4-3-5-8(9)14/h3-6,17H,1-2H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM22466
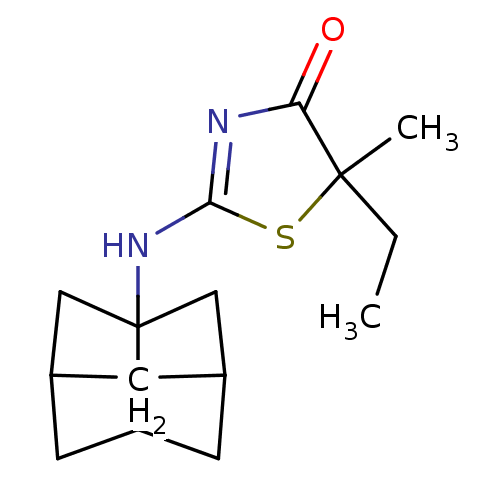
(2-Amino-1,3-thiazol-4(5H)-one, 5h | 5-ethyl-5-meth...)Show SMILES CCC1(C)SC(NC23CC4CC2CC(C3)C4)=NC1=O |c:18,TLB:14:13:7.8:10,6:7:10:13.12.15,6:7:12:9.10.15,THB:8:7:12:9.10.15,8:9:7.14:12,14:7:10:13.12.15| Show InChI InChI=1S/C15H22N2OS/c1-3-14(2)12(18)16-13(19-14)17-15-7-9-4-10(8-15)6-11(15)5-9/h9-11H,3-8H2,1-2H3,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB
| Assay Description
The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... |
J Med Chem 51: 2933-43 (2008)
Article DOI: 10.1021/jm701551j
BindingDB Entry DOI: 10.7270/Q2HM56Q8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137387

(((R)-3-Phenyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES O=C(N[C@H](CCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1)OCc1ccccc1 Show InChI InChI=1S/C39H46N4O3/c44-38(37(26-20-32-15-7-2-8-16-32)42-39(45)46-30-33-17-9-3-10-18-33)41-35(21-19-31-13-5-1-6-14-31)29-40-34-22-24-36(25-23-34)43-27-11-4-12-28-43/h1-3,5-10,13-18,22-25,35,37,40H,4,11-12,19-21,26-30H2,(H,41,44)(H,42,45)/t35-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM22471
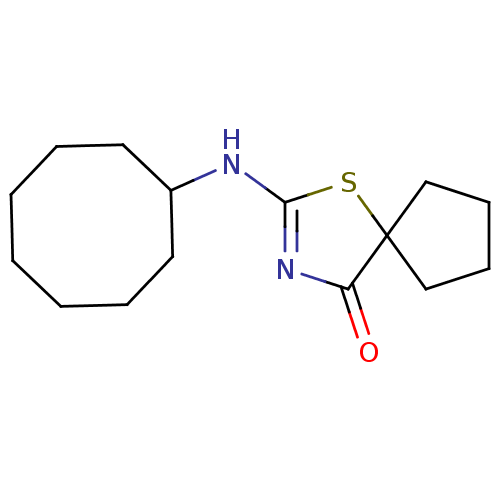
(2-(cyclooctylamino)-1-thia-3-azaspiro[4.4]non-2-en...)Show InChI InChI=1S/C15H24N2OS/c18-13-15(10-6-7-11-15)19-14(17-13)16-12-8-4-2-1-3-5-9-12/h12H,1-11H2,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB
| Assay Description
The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... |
J Med Chem 51: 2933-43 (2008)
Article DOI: 10.1021/jm701551j
BindingDB Entry DOI: 10.7270/Q2HM56Q8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137395

(CHEMBL170242 | {(S)-1-[(S)-1-Benzyl-2-(4-morpholin...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H](CNc1ccc(cc1)N1CCOCC1)Cc1ccccc1 Show InChI InChI=1S/C33H42N4O4/c1-25(2)21-31(36-33(39)41-24-27-11-7-4-8-12-27)32(38)35-29(22-26-9-5-3-6-10-26)23-34-28-13-15-30(16-14-28)37-17-19-40-20-18-37/h3-16,25,29,31,34H,17-24H2,1-2H3,(H,35,38)(H,36,39)/t29-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224207

(2-(2-fluorophenylamino)-5-isopropylthiazol-4(5H)-o...)Show InChI InChI=1S/C12H13FN2OS/c1-7(2)10-11(16)15-12(17-10)14-9-6-4-3-5-8(9)13/h3-7,16H,1-2H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319664

((R,S)-2-((+/-)-exo-Bicyclo[2.2.1]heptan-2-ylamino)...)Show SMILES CC(C)C1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224222
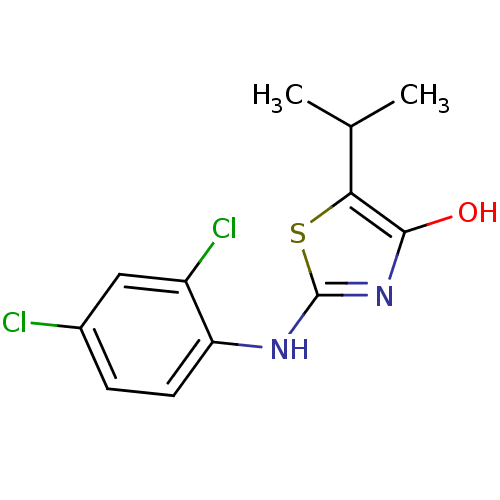
(2-(2,4-dichlorophenylamino)-5-isopropylthiazol-4(5...)Show InChI InChI=1S/C12H12Cl2N2OS/c1-6(2)10-11(17)16-12(18-10)15-9-4-3-7(13)5-8(9)14/h3-6,17H,1-2H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137397
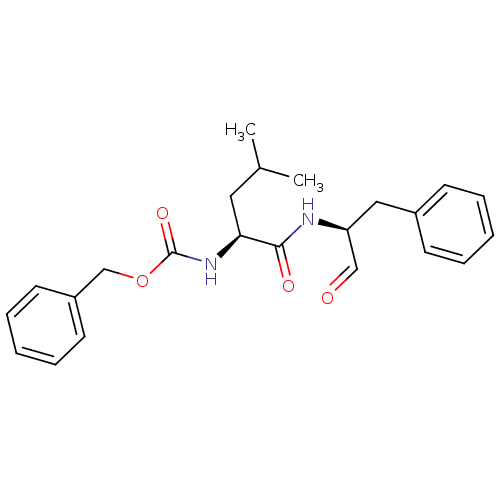
(CHEMBL341014 | [(S)-1-((S)-1-Benzyl-2-oxo-ethylcar...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H28N2O4/c1-17(2)13-21(25-23(28)29-16-19-11-7-4-8-12-19)22(27)24-20(15-26)14-18-9-5-3-6-10-18/h3-12,15,17,20-21H,13-14,16H2,1-2H3,(H,24,27)(H,25,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM22474
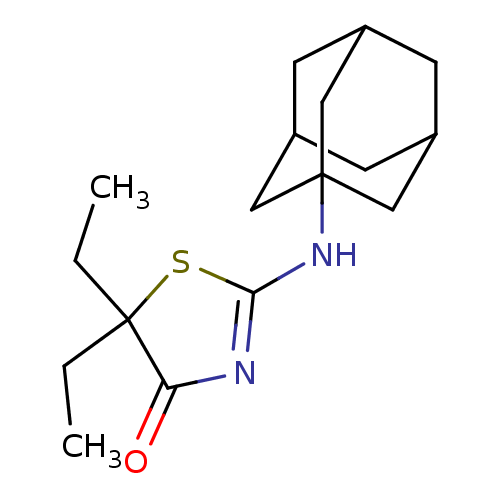
(2-(adamantan-1-ylamino)-5,5-diethyl-4,5-dihydro-1,...)Show SMILES CCC1(CC)SC(NC23CC4CC(CC(C4)C2)C3)=NC1=O |c:20,TLB:17:8:15:11.12.13,THB:17:12:15:9.8.16,16:8:11:15.14.13,16:14:11:9.8.17,7:8:15:11.12.13| Show InChI InChI=1S/C17H26N2OS/c1-3-17(4-2)14(20)18-15(21-17)19-16-8-11-5-12(9-16)7-13(6-11)10-16/h11-13H,3-10H2,1-2H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB
| Assay Description
The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... |
J Med Chem 51: 2933-43 (2008)
Article DOI: 10.1021/jm701551j
BindingDB Entry DOI: 10.7270/Q2HM56Q8 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005324

(4-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccc(O)cc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O3S/c24-23-25-20-12-16-2-1-11-27(22(16)13-21(20)26-23)14-15-3-7-18(8-4-15)31(29,30)19-9-5-17(28)6-10-19/h3-10,12-13,28H,1-2,11,14H2,(H3,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224212
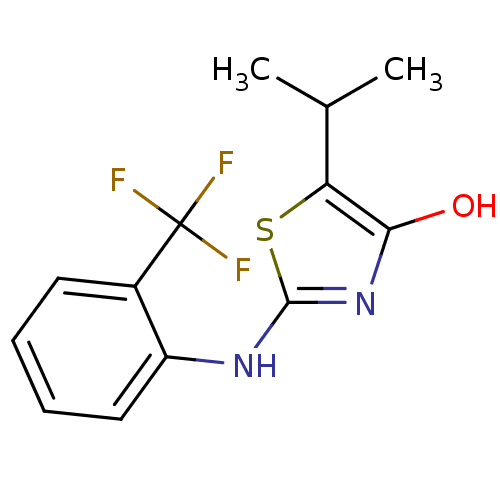
(5-isopropyl-2-(2-(trifluoromethyl)phenylamino)thia...)Show InChI InChI=1S/C13H13F3N2OS/c1-7(2)10-11(19)18-12(20-10)17-9-6-4-3-5-8(9)13(14,15)16/h3-7,19H,1-2H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005332
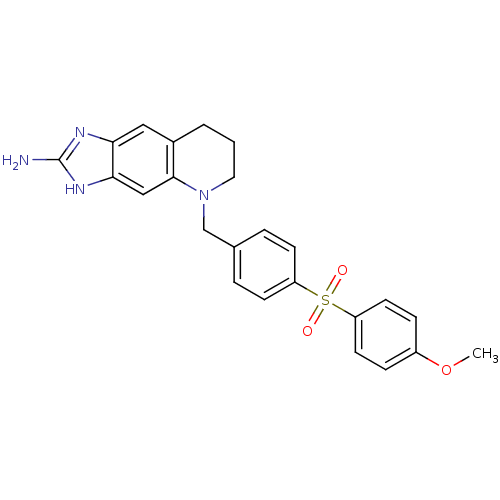
(5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-5,6,7,8-t...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(CN2CCCc3cc4nc(N)[nH]c4cc23)cc1 Show InChI InChI=1S/C24H24N4O3S/c1-31-18-6-10-20(11-7-18)32(29,30)19-8-4-16(5-9-19)15-28-12-2-3-17-13-21-22(14-23(17)28)27-24(25)26-21/h4-11,13-14H,2-3,12,15H2,1H3,(H3,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data