 Found 1252 hits with Last Name = 'joshi' and Initial = 'n'
Found 1252 hits with Last Name = 'joshi' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM79800
(5-(methylamino)-2-(1-naphthalenyl)-4-oxazolecarbon...)Show InChI InChI=1S/C15H11N3O/c1-17-15-13(9-16)18-14(19-15)12-8-4-6-10-5-2-3-7-11(10)12/h2-8,17H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged human reticulocyte 12/15-lipoxygenase using arachidonic acid as substrate assessed as equilibrium co... |
J Med Chem 57: 4035-48 (2014)
Article DOI: 10.1021/jm401915r
BindingDB Entry DOI: 10.7270/Q26T0P58 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50447175
(CHEMBL3113165 | US10752581, Compound 35)Show SMILES COc1cccc(CNc2ccc(cc2)S(=O)(=O)Nc2nc3ccccc3s2)c1O Show InChI InChI=1S/C21H19N3O4S2/c1-28-18-7-4-5-14(20(18)25)13-22-15-9-11-16(12-10-15)30(26,27)24-21-23-17-6-2-3-8-19(17)29-21/h2-12,22,25H,13H2,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate assessed as 12-HPETE formation by Henri-... |
J Med Chem 57: 495-506 (2014)
Article DOI: 10.1021/jm4016476
BindingDB Entry DOI: 10.7270/Q27082XR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50447176
(CHEMBL3113166 | US10752581, Compound 36)Show SMILES COc1cccc(CNc2ccc(cc2)S(=O)(=O)Nc2nc3ccccc3o2)c1O Show InChI InChI=1S/C21H19N3O5S/c1-28-19-8-4-5-14(20(19)25)13-22-15-9-11-16(12-10-15)30(26,27)24-21-23-17-6-2-3-7-18(17)29-21/h2-12,22,25H,13H2,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate assessed as 12-HPETE formation by Henri-... |
J Med Chem 57: 495-506 (2014)
Article DOI: 10.1021/jm4016476
BindingDB Entry DOI: 10.7270/Q27082XR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human 15-lipoxygenase |
J Med Chem 54: 5485-97 (2011)
Article DOI: 10.1021/jm2005089
BindingDB Entry DOI: 10.7270/Q25T3KVT |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50447176

(CHEMBL3113166 | US10752581, Compound 36)Show SMILES COc1cccc(CNc2ccc(cc2)S(=O)(=O)Nc2nc3ccccc3o2)c1O Show InChI InChI=1S/C21H19N3O5S/c1-28-19-8-4-5-14(20(19)25)13-22-15-9-11-16(12-10-15)30(26,27)24-21-23-17-6-2-3-7-18(17)29-21/h2-12,22,25H,13H2,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate assessed as 12-HPETE formation by Henri-Mi... |
J Med Chem 57: 495-506 (2014)
Article DOI: 10.1021/jm4016476
BindingDB Entry DOI: 10.7270/Q27082XR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50350394
(CHEMBL528045)Show InChI InChI=1S/C16H13ClN2O2S/c1-9(20)19-14(13-5-3-7-22-13)11-8-12(17)10-4-2-6-18-15(10)16(11)21/h2-8,14,21H,1H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Competitive inhibition of human platelet-type N-terminally His6-tagged 12-lipoxygenase assessed as 12-HPETE formation using arachidonic acid by by Mi... |
J Med Chem 54: 5485-97 (2011)
Article DOI: 10.1021/jm2005089
BindingDB Entry DOI: 10.7270/Q25T3KVT |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50447175

(CHEMBL3113165 | US10752581, Compound 35)Show SMILES COc1cccc(CNc2ccc(cc2)S(=O)(=O)Nc2nc3ccccc3s2)c1O Show InChI InChI=1S/C21H19N3O4S2/c1-28-18-7-4-5-14(20(18)25)13-22-15-9-11-16(12-10-15)30(26,27)24-21-23-17-6-2-3-8-19(17)29-21/h2-12,22,25H,13H2,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate assessed as 12-HPETE formation by Henri-Mi... |
J Med Chem 57: 495-506 (2014)
Article DOI: 10.1021/jm4016476
BindingDB Entry DOI: 10.7270/Q27082XR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM79800

(5-(methylamino)-2-(1-naphthalenyl)-4-oxazolecarbon...)Show InChI InChI=1S/C15H11N3O/c1-17-15-13(9-16)18-14(19-15)12-8-4-6-10-5-2-3-7-11(10)12/h2-8,17H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged human reticulocyte 12/15-lipoxygenase using arachidonic acid as substrate assessed as equilibrium co... |
J Med Chem 57: 4035-48 (2014)
Article DOI: 10.1021/jm401915r
BindingDB Entry DOI: 10.7270/Q26T0P58 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human platelet-type 12-lipoxygenase |
J Med Chem 54: 5485-97 (2011)
Article DOI: 10.1021/jm2005089
BindingDB Entry DOI: 10.7270/Q25T3KVT |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130865

(CHEMBL3634853)Show SMILES O=c1ccc(-c2ccc(OCc3ccc4[nH]ccc4n3)cc2)c([nH]1)-c1ccccc1 Show InChI InChI=1S/C25H19N3O2/c29-24-13-11-21(25(28-24)18-4-2-1-3-5-18)17-6-9-20(10-7-17)30-16-19-8-12-22-23(27-19)14-15-26-22/h1-15,26H,16H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50048260
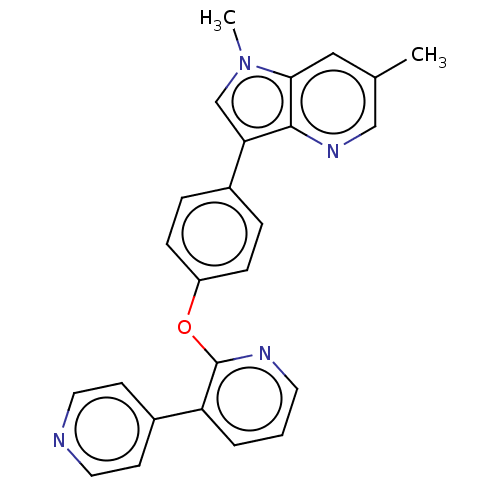
(CHEMBL3315045)Show SMILES Cc1cnc2c(cn(C)c2c1)-c1ccc(Oc2ncccc2-c2ccncc2)cc1 Show InChI InChI=1S/C25H20N4O/c1-17-14-23-24(28-15-17)22(16-29(23)2)18-5-7-20(8-6-18)30-25-21(4-3-11-27-25)19-9-12-26-13-10-19/h3-16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130760
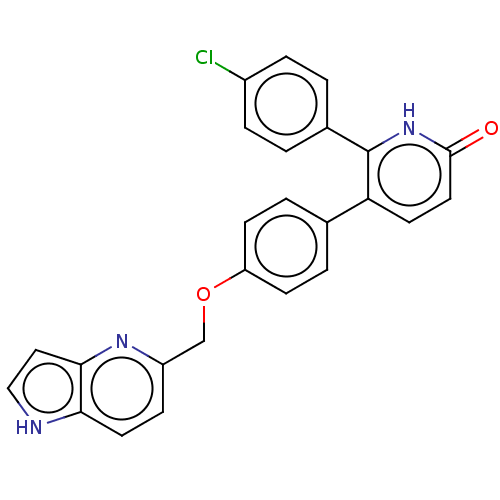
(CHEMBL3634855)Show SMILES Clc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3[nH]ccc3n2)cc1 Show InChI InChI=1S/C25H18ClN3O2/c26-18-5-1-17(2-6-18)25-21(10-12-24(30)29-25)16-3-8-20(9-4-16)31-15-19-7-11-22-23(28-19)13-14-27-22/h1-14,27H,15H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130759

(CHEMBL3634854)Show SMILES Fc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3[nH]ccc3n2)cc1 Show InChI InChI=1S/C25H18FN3O2/c26-18-5-1-17(2-6-18)25-21(10-12-24(30)29-25)16-3-8-20(9-4-16)31-15-19-7-11-22-23(28-19)13-14-27-22/h1-14,27H,15H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM31592
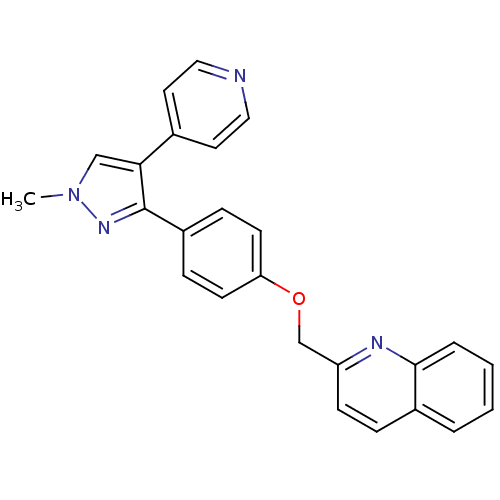
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human recombinant PDE10A assessed as substrate hydrolysis using [3H]cAMP as substrate after 30 mins by two-step r... |
Bioorg Med Chem Lett 24: 2073-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.054
BindingDB Entry DOI: 10.7270/Q22V2HNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130761
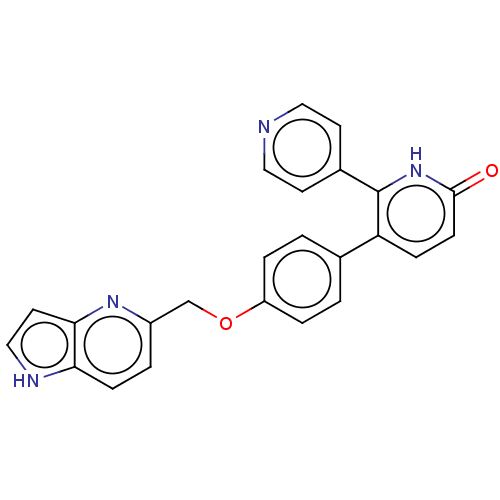
(CHEMBL3634856)Show SMILES O=c1ccc(-c2ccc(OCc3ccc4[nH]ccc4n3)cc2)c([nH]1)-c1ccncc1 Show InChI InChI=1S/C24H18N4O2/c29-23-8-6-20(24(28-23)17-9-12-25-13-10-17)16-1-4-19(5-2-16)30-15-18-3-7-21-22(27-18)11-14-26-21/h1-14,26H,15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM363753
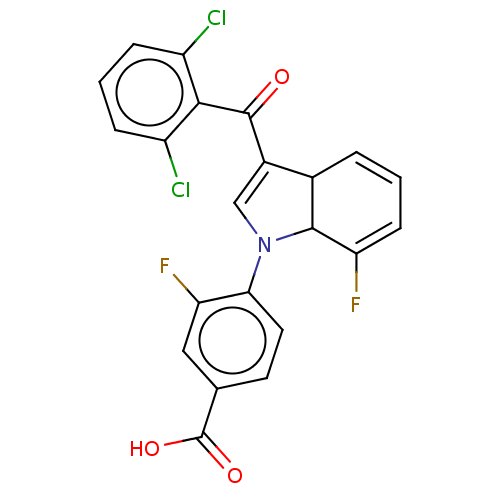
(US9855229, Compound 7)Show SMILES OC(=O)c1ccc(N2C=C(C3C=CC=C(F)C23)C(=O)c2c(Cl)cccc2Cl)c(F)c1 |c:8,11,t:13| Show InChI InChI=1S/C22H13Cl2F2NO3/c23-14-4-2-5-15(24)19(14)21(28)13-10-27(20-12(13)3-1-6-16(20)25)18-8-7-11(22(29)30)9-17(18)26/h1-10,12,20H,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.615 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
| Assay Description
The assay is based on the principle that binding of the agonist to the RORγ causes a conformational change around helix 12 in the ligand binding... |
J Med Chem 46: 3709-27 (2003)
BindingDB Entry DOI: 10.7270/Q20867MM |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130763
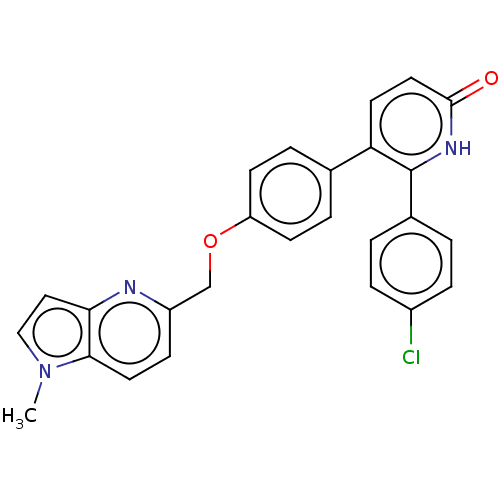
(CHEMBL3634858)Show SMILES Cn1ccc2nc(COc3ccc(cc3)-c3ccc(=O)[nH]c3-c3ccc(Cl)cc3)ccc12 Show InChI InChI=1S/C26H20ClN3O2/c1-30-15-14-23-24(30)12-8-20(28-23)16-32-21-9-4-17(5-10-21)22-11-13-25(31)29-26(22)18-2-6-19(27)7-3-18/h2-15H,16H2,1H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50048260

(CHEMBL3315045)Show SMILES Cc1cnc2c(cn(C)c2c1)-c1ccc(Oc2ncccc2-c2ccncc2)cc1 Show InChI InChI=1S/C25H20N4O/c1-17-14-23-24(28-15-17)22(16-29(23)2)18-5-7-20(8-6-18)30-25-21(4-3-11-27-25)19-9-12-26-13-10-19/h3-16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GST-tagged PDE10A using [3H]-cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50494753
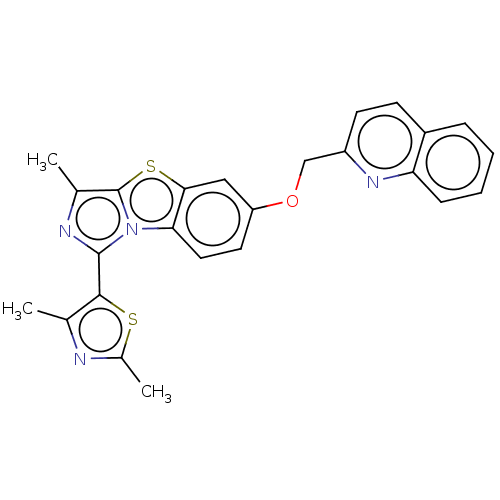
(CHEMBL3094144)Show SMILES Cc1nc(C)c(s1)-c1nc(C)c2sc3cc(OCc4ccc5ccccc5n4)ccc3n12 Show InChI InChI=1S/C25H20N4OS2/c1-14-23(31-16(3)26-14)24-27-15(2)25-29(24)21-11-10-19(12-22(21)32-25)30-13-18-9-8-17-6-4-5-7-20(17)28-18/h4-12H,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A using [3H]-cAMP/[3H]-cGMP as substrate after 30 mins by radiometric assay |
Bioorg Med Chem Lett 23: 6747-54 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.027
BindingDB Entry DOI: 10.7270/Q2RV0RP4 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130849
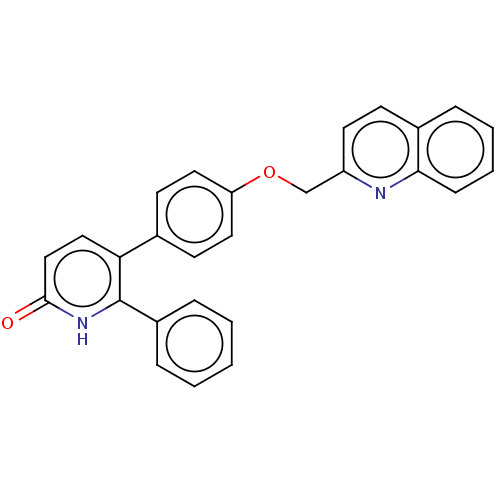
(CHEMBL3634744)Show SMILES O=c1ccc(-c2ccc(OCc3ccc4ccccc4n3)cc2)c([nH]1)-c1ccccc1 Show InChI InChI=1S/C27H20N2O2/c30-26-17-16-24(27(29-26)21-7-2-1-3-8-21)19-11-14-23(15-12-19)31-18-22-13-10-20-6-4-5-9-25(20)28-22/h1-17H,18H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130850
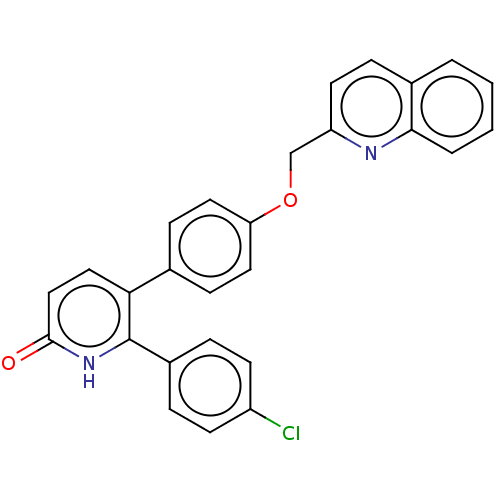
(CHEMBL3634746)Show SMILES Clc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H19ClN2O2/c28-21-10-5-20(6-11-21)27-24(15-16-26(31)30-27)18-8-13-23(14-9-18)32-17-22-12-7-19-3-1-2-4-25(19)29-22/h1-16H,17H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130853

(CHEMBL3634748)Show SMILES Fc1cccc(c1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H19FN2O2/c28-21-6-3-5-20(16-21)27-24(14-15-26(31)30-27)18-9-12-23(13-10-18)32-17-22-11-8-19-4-1-2-7-25(19)29-22/h1-16H,17H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Mus musculus) | BDBM50048260

(CHEMBL3315045)Show SMILES Cc1cnc2c(cn(C)c2c1)-c1ccc(Oc2ncccc2-c2ccncc2)cc1 Show InChI InChI=1S/C25H20N4O/c1-17-14-23-24(28-15-17)22(16-29(23)2)18-5-7-20(8-6-18)30-25-21(4-3-11-27-25)19-9-12-26-13-10-19/h3-16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130860
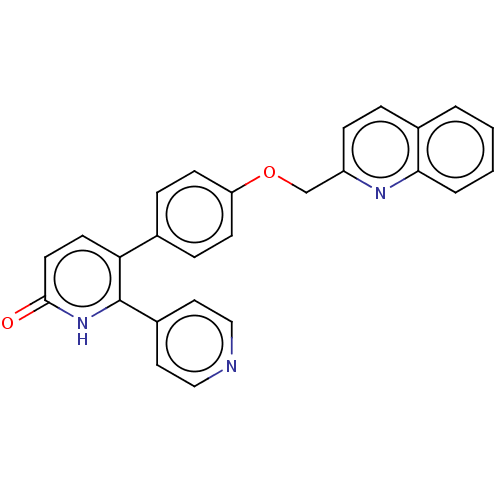
(CHEMBL3634847)Show SMILES O=c1ccc(-c2ccc(OCc3ccc4ccccc4n3)cc2)c([nH]1)-c1ccncc1 Show InChI InChI=1S/C26H19N3O2/c30-25-12-11-23(26(29-25)20-13-15-27-16-14-20)18-6-9-22(10-7-18)31-17-21-8-5-19-3-1-2-4-24(19)28-21/h1-16H,17H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50048261
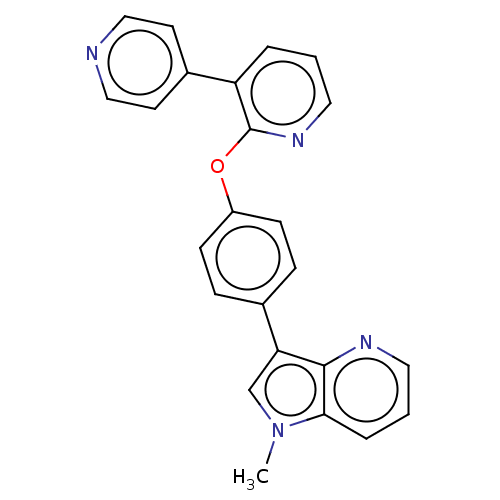
(CHEMBL3315044)Show SMILES Cn1cc(-c2ccc(Oc3ncccc3-c3ccncc3)cc2)c2ncccc12 Show InChI InChI=1S/C24H18N4O/c1-28-16-21(23-22(28)5-3-12-26-23)17-6-8-19(9-7-17)29-24-20(4-2-13-27-24)18-10-14-25-15-11-18/h2-16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM31596
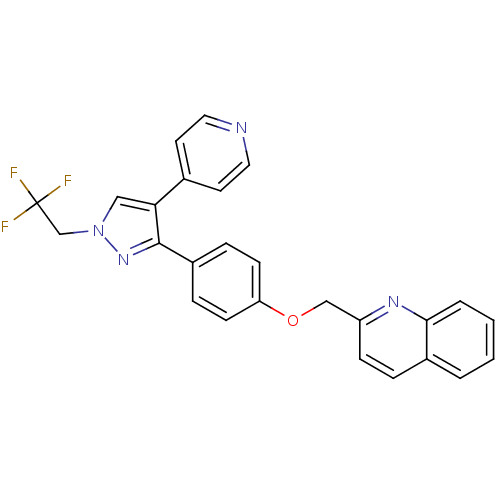
(substituted pyrazole, 13)Show SMILES FC(F)(F)Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H19F3N4O/c27-26(28,29)17-33-15-23(18-11-13-30-14-12-18)25(32-33)20-6-9-22(10-7-20)34-16-21-8-5-19-3-1-2-4-24(19)31-21/h1-15H,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human recombinant PDE10A assessed as substrate hydrolysis using [3H]cAMP as substrate after 30 mins by two-step r... |
Bioorg Med Chem Lett 24: 2073-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.054
BindingDB Entry DOI: 10.7270/Q22V2HNB |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130854
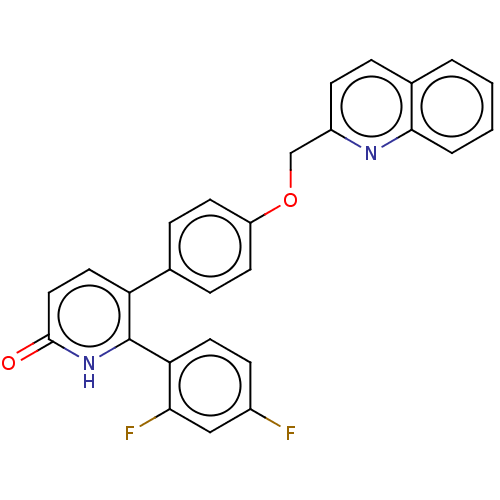
(CHEMBL3634749)Show SMILES Fc1ccc(c(F)c1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H18F2N2O2/c28-19-8-12-23(24(29)15-19)27-22(13-14-26(32)31-27)17-6-10-21(11-7-17)33-16-20-9-5-18-3-1-2-4-25(18)30-20/h1-15H,16H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM191653

(US9186360, 57)Show SMILES Cn1c2occ(CC(=O)Nc3nc(cs3)-c3cc(Cl)c(OCC(F)(F)F)c(Cl)c3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C21H15Cl2F3N4O5S/c1-29-17(32)15-10(6-34-18(15)30(2)20(29)33)5-14(31)28-19-27-13(7-36-19)9-3-11(22)16(12(23)4-9)35-8-21(24,25)26/h3-4,6-7H,5,8H2,1-2H3,(H,27,28,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | 25 |
GLENMARK PHARMACEUTICALS S.A.
US Patent
| Assay Description
The inhibition of TRPA1 receptor activation was measured as inhibition of allylisothiocyanate (AITC) induced cellular uptake of radioactive calcium. ... |
US Patent US9186360 (2015)
BindingDB Entry DOI: 10.7270/Q2TX3D5Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
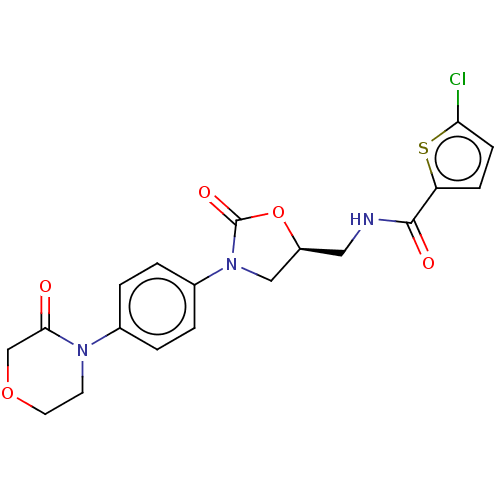
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130841
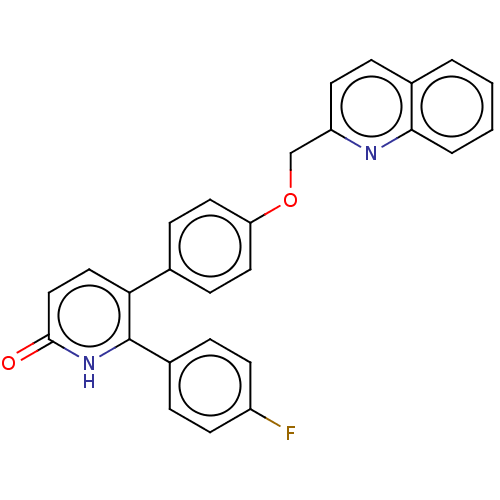
(CHEMBL3634745)Show SMILES Fc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H19FN2O2/c28-21-10-5-20(6-11-21)27-24(15-16-26(31)30-27)18-8-13-23(14-9-18)32-17-22-12-7-19-3-1-2-4-25(19)29-22/h1-16H,17H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM60874
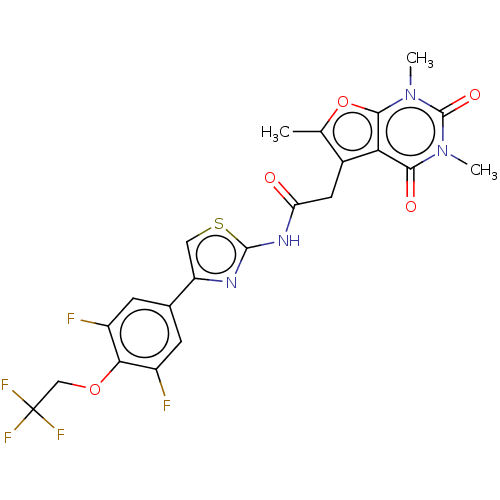
(US9186360, 62)Show SMILES Cc1oc2n(C)c(=O)n(C)c(=O)c2c1CC(=O)Nc1nc(cs1)-c1cc(F)c(OCC(F)(F)F)c(F)c1 Show InChI InChI=1S/C22H17F5N4O5S/c1-9-11(16-18(33)30(2)21(34)31(3)19(16)36-9)6-15(32)29-20-28-14(7-37-20)10-4-12(23)17(13(24)5-10)35-8-22(25,26)27/h4-5,7H,6,8H2,1-3H3,(H,28,29,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.68 | n/a | n/a | n/a | n/a | n/a | 25 |
GLENMARK PHARMACEUTICALS S.A.
US Patent
| Assay Description
The inhibition of TRPA1 receptor activation was measured as inhibition of allylisothiocyanate (AITC) induced cellular uptake of radioactive calcium. ... |
US Patent US9186360 (2015)
BindingDB Entry DOI: 10.7270/Q2TX3D5Q |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130762
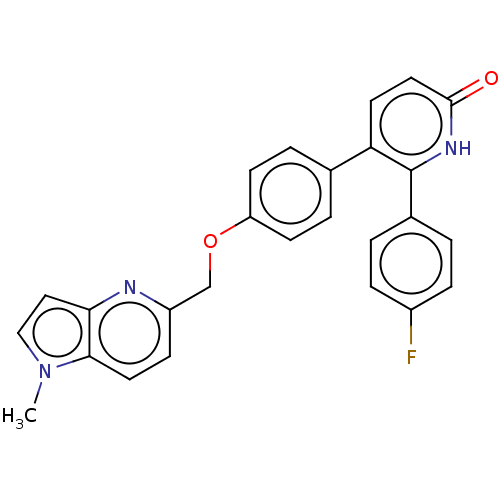
(CHEMBL3634857)Show SMILES Cn1ccc2nc(COc3ccc(cc3)-c3ccc(=O)[nH]c3-c3ccc(F)cc3)ccc12 Show InChI InChI=1S/C26H20FN3O2/c1-30-15-14-23-24(30)12-8-20(28-23)16-32-21-9-4-17(5-10-21)22-11-13-25(31)29-26(22)18-2-6-19(27)7-3-18/h2-15H,16H2,1H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130858

(CHEMBL3634845)Show SMILES COc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H22N2O3/c1-32-23-12-9-21(10-13-23)28-25(16-17-27(31)30-28)19-7-14-24(15-8-19)33-18-22-11-6-20-4-2-3-5-26(20)29-22/h2-17H,18H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50227631
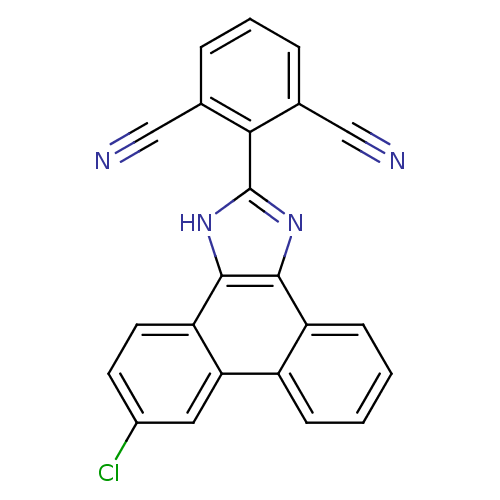
(2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...)Show SMILES Clc1ccc2c3[nH]c(nc3c3ccccc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C23H11ClN4/c24-15-8-9-18-19(10-15)16-6-1-2-7-17(16)21-22(18)28-23(27-21)20-13(11-25)4-3-5-14(20)12-26/h1-10H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES-1 expressed in CHO cells assessed as reduction in PGE2 formation using PGH2 a substrate preincubated for 10 min... |
Bioorg Med Chem Lett 27: 2594-2601 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.068
BindingDB Entry DOI: 10.7270/Q24B33Q3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50227631

(2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...)Show SMILES Clc1ccc2c3[nH]c(nc3c3ccccc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C23H11ClN4/c24-15-8-9-18-19(10-15)16-6-1-2-7-17(16)21-22(18)28-23(27-21)20-13(11-25)4-3-5-14(20)12-26/h1-10H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 expressed in CHO cells assessed as reduction in conversion of PGH2 to PGE2 incubated for 10 mins followed by ... |
Bioorg Med Chem Lett 26: 5977-5984 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.079
BindingDB Entry DOI: 10.7270/Q2XK8HJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130856
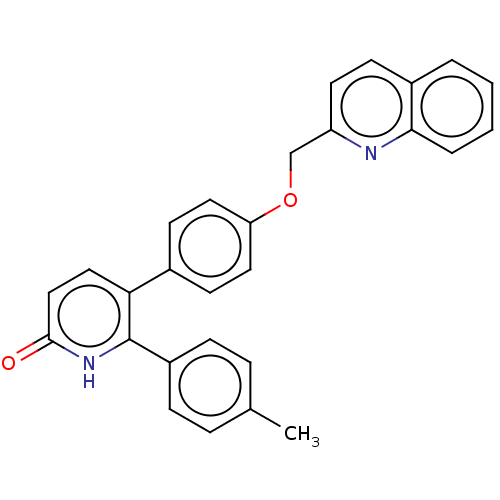
(CHEMBL3634751)Show SMILES Cc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H22N2O2/c1-19-6-8-22(9-7-19)28-25(16-17-27(31)30-28)20-11-14-24(15-12-20)32-18-23-13-10-21-4-2-3-5-26(21)29-23/h2-17H,18H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM363754

(US9855229, Compound 8)Show SMILES OC(=O)c1ccc(cc1)N1N=C(C2C=CC=NC12)C(=O)c1c(Cl)cccc1C1CC1 |c:11,14,16| Show InChI InChI=1S/C23H18ClN3O3/c24-18-5-1-3-16(13-6-7-13)19(18)21(28)20-17-4-2-12-25-22(17)27(26-20)15-10-8-14(9-11-15)23(29)30/h1-5,8-13,17,22H,6-7H2,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
| Assay Description
The assay is based on the principle that binding of the agonist to the RORγ causes a conformational change around helix 12 in the ligand binding... |
J Med Chem 46: 3709-27 (2003)
BindingDB Entry DOI: 10.7270/Q20867MM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM191709
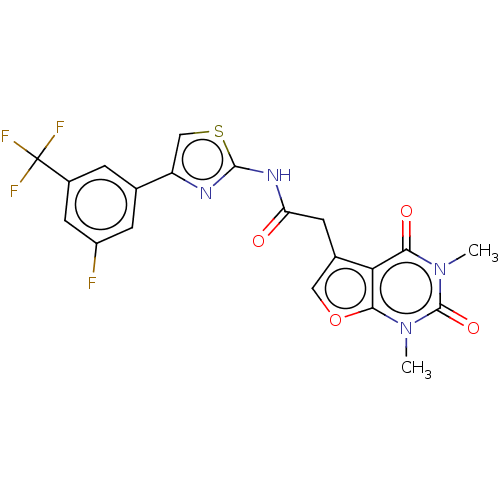
(US9186360, 55)Show SMILES Cn1c2occ(CC(=O)Nc3nc(cs3)-c3cc(F)cc(c3)C(F)(F)F)c2c(=O)n(C)c1=O Show InChI InChI=1S/C20H14F4N4O4S/c1-27-16(30)15-10(7-32-17(15)28(2)19(27)31)5-14(29)26-18-25-13(8-33-18)9-3-11(20(22,23)24)6-12(21)4-9/h3-4,6-8H,5H2,1-2H3,(H,25,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | n/a | 25 |
GLENMARK PHARMACEUTICALS S.A.
US Patent
| Assay Description
The inhibition of TRPA1 receptor activation was measured as inhibition of allylisothiocyanate (AITC) induced cellular uptake of radioactive calcium. ... |
US Patent US9186360 (2015)
BindingDB Entry DOI: 10.7270/Q2TX3D5Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM363752

(US9855229, Compound 6)Show SMILES OC(=O)c1ccc(N2C=C(C3C=CC=CC23)C(=O)c2c(Cl)cccc2Cl)c(F)c1 |c:8,11,13| Show InChI InChI=1S/C22H14Cl2FNO3/c23-15-5-3-6-16(24)20(15)21(27)14-11-26(18-7-2-1-4-13(14)18)19-9-8-12(22(28)29)10-17(19)25/h1-11,13,18H,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
| Assay Description
The assay is based on the principle that binding of the agonist to the RORγ causes a conformational change around helix 12 in the ligand binding... |
J Med Chem 46: 3709-27 (2003)
BindingDB Entry DOI: 10.7270/Q20867MM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data