 Found 55 hits with Last Name = 'joud' and Initial = 'c'
Found 55 hits with Last Name = 'joud' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235402
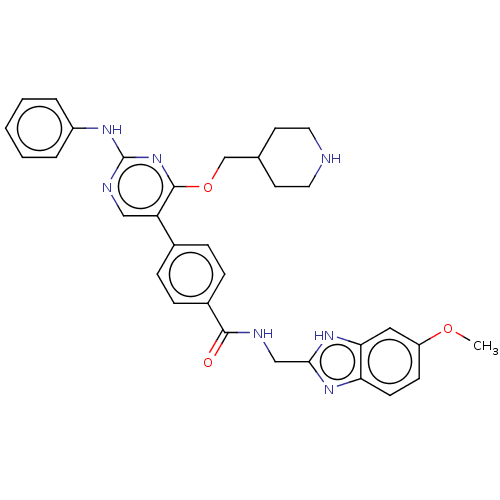
(CHEMBL4077934)Show SMILES COc1ccc2nc(CNC(=O)c3ccc(cc3)-c3cnc(Nc4ccccc4)nc3OCC3CCNCC3)[nH]c2c1 Show InChI InChI=1S/C32H33N7O3/c1-41-25-11-12-27-28(17-25)38-29(37-27)19-34-30(40)23-9-7-22(8-10-23)26-18-35-32(36-24-5-3-2-4-6-24)39-31(26)42-20-21-13-15-33-16-14-21/h2-12,17-18,21,33H,13-16,19-20H2,1H3,(H,34,40)(H,37,38)(H,35,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235407
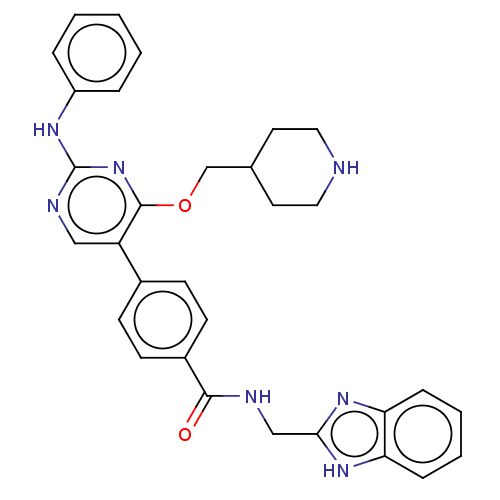
(CHEMBL4088246)Show SMILES O=C(NCc1nc2ccccc2[nH]1)c1ccc(cc1)-c1cnc(Nc2ccccc2)nc1OCC1CCNCC1 Show InChI InChI=1S/C31H31N7O2/c39-29(33-19-28-36-26-8-4-5-9-27(26)37-28)23-12-10-22(11-13-23)25-18-34-31(35-24-6-2-1-3-7-24)38-30(25)40-20-21-14-16-32-17-15-21/h1-13,18,21,32H,14-17,19-20H2,(H,33,39)(H,36,37)(H,34,35,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235399
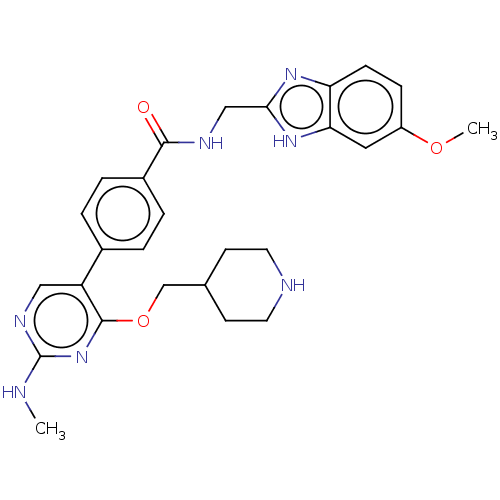
(CHEMBL4092605)Show SMILES CNc1ncc(-c2ccc(cc2)C(=O)NCc2nc3ccc(OC)cc3[nH]2)c(OCC2CCNCC2)n1 Show InChI InChI=1S/C27H31N7O3/c1-28-27-31-14-21(26(34-27)37-16-17-9-11-29-12-10-17)18-3-5-19(6-4-18)25(35)30-15-24-32-22-8-7-20(36-2)13-23(22)33-24/h3-8,13-14,17,29H,9-12,15-16H2,1-2H3,(H,30,35)(H,32,33)(H,28,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235401
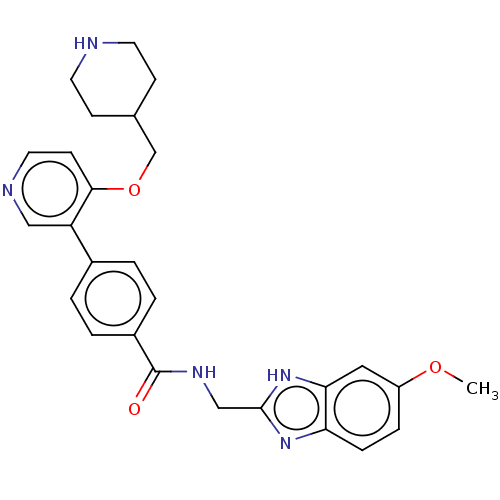
(CHEMBL4071823)Show SMILES COc1ccc2nc(CNC(=O)c3ccc(cc3)-c3cnccc3OCC3CCNCC3)[nH]c2c1 Show InChI InChI=1S/C27H29N5O3/c1-34-21-6-7-23-24(14-21)32-26(31-23)16-30-27(33)20-4-2-19(3-5-20)22-15-29-13-10-25(22)35-17-18-8-11-28-12-9-18/h2-7,10,13-15,18,28H,8-9,11-12,16-17H2,1H3,(H,30,33)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235404
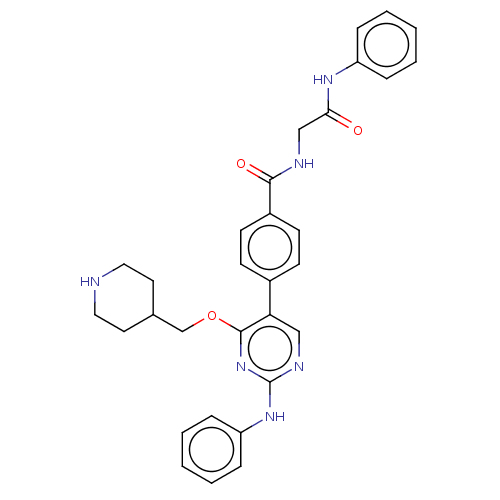
(CHEMBL4085799)Show SMILES O=C(CNC(=O)c1ccc(cc1)-c1cnc(Nc2ccccc2)nc1OCC1CCNCC1)Nc1ccccc1 Show InChI InChI=1S/C31H32N6O3/c38-28(35-25-7-3-1-4-8-25)20-33-29(39)24-13-11-23(12-14-24)27-19-34-31(36-26-9-5-2-6-10-26)37-30(27)40-21-22-15-17-32-18-16-22/h1-14,19,22,32H,15-18,20-21H2,(H,33,39)(H,35,38)(H,34,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235406

(CHEMBL4066968)Show SMILES CNc1ncc(-c2ccc(cc2)C(=O)NCc2nc3ccccc3[nH]2)c(OCC2CCNCC2)n1 Show InChI InChI=1S/C26H29N7O2/c1-27-26-30-14-20(25(33-26)35-16-17-10-12-28-13-11-17)18-6-8-19(9-7-18)24(34)29-15-23-31-21-4-2-3-5-22(21)32-23/h2-9,14,17,28H,10-13,15-16H2,1H3,(H,29,34)(H,31,32)(H,27,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235405
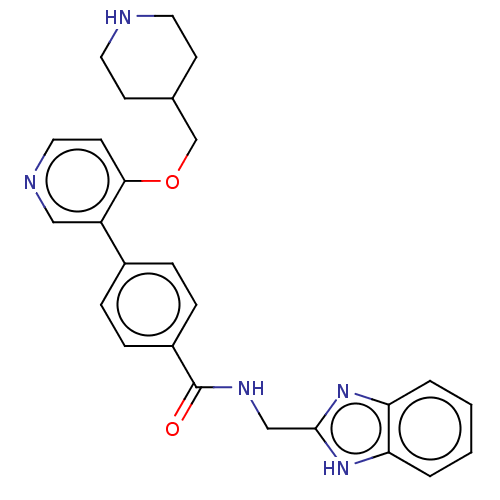
(CHEMBL4095977)Show SMILES O=C(NCc1nc2ccccc2[nH]1)c1ccc(cc1)-c1cnccc1OCC1CCNCC1 Show InChI InChI=1S/C26H27N5O2/c32-26(29-16-25-30-22-3-1-2-4-23(22)31-25)20-7-5-19(6-8-20)21-15-28-14-11-24(21)33-17-18-9-12-27-13-10-18/h1-8,11,14-15,18,27H,9-10,12-13,16-17H2,(H,29,32)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426420
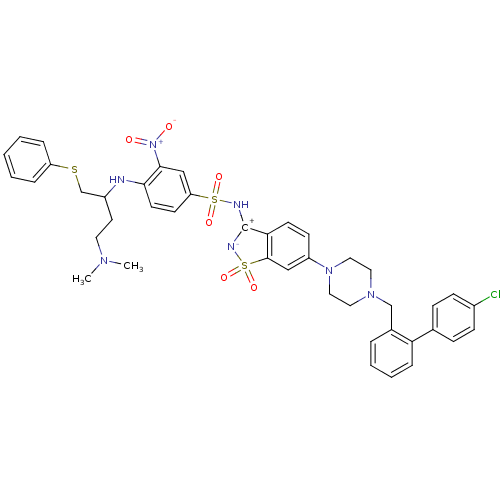
(CHEMBL2322027)Show SMILES CN(C)CCC(CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)N[C+]1[N-]S(=O)(=O)c2cc(ccc12)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C42H44ClN7O6S3/c1-47(2)21-20-33(29-57-35-9-4-3-5-10-35)44-39-19-17-36(27-40(39)50(51)52)58(53,54)45-42-38-18-16-34(26-41(38)59(55,56)46-42)49-24-22-48(23-25-49)28-31-8-6-7-11-37(31)30-12-14-32(43)15-13-30/h3-19,26-27,33,44-45H,20-25,28-29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426417
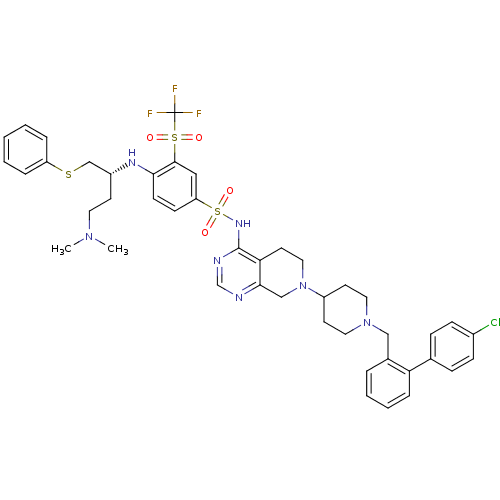
(CHEMBL2326745)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1ncnc2CN(CCc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H49ClF3N7O4S3/c1-53(2)22-18-34(29-60-36-9-4-3-5-10-36)51-40-17-16-37(26-42(40)61(56,57)44(46,47)48)62(58,59)52-43-39-21-25-55(28-41(39)49-30-50-43)35-19-23-54(24-20-35)27-32-8-6-7-11-38(32)31-12-14-33(45)15-13-31/h3-17,26,30,34-35,51H,18-25,27-29H2,1-2H3,(H,49,50,52)/t34-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50426413

(CHEMBL2322021)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1nc(Cl)nc2CN(CCc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H48Cl2F3N7O4S3/c1-54(2)22-18-33(29-61-35-9-4-3-5-10-35)50-39-17-16-36(26-41(39)62(57,58)44(47,48)49)63(59,60)53-42-38-21-25-56(28-40(38)51-43(46)52-42)34-19-23-55(24-20-34)27-31-8-6-7-11-37(31)30-12-14-32(45)15-13-30/h3-17,26,33-34,50H,18-25,27-29H2,1-2H3,(H,51,52,53)/t33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL/BAK (unknown origin) interaction |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50426414
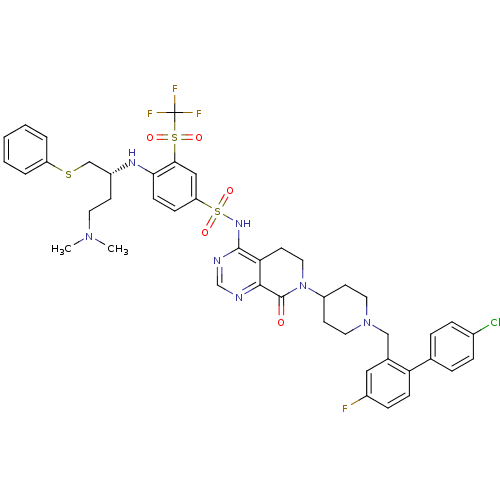
(CHEMBL2322020)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1ncnc2C(=O)N(CCc12)C1CCN(Cc2cc(F)ccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H46ClF4N7O5S3/c1-54(2)20-16-33(27-62-35-6-4-3-5-7-35)52-39-15-13-36(25-40(39)63(58,59)44(47,48)49)64(60,61)53-42-38-19-23-56(43(57)41(38)50-28-51-42)34-17-21-55(22-18-34)26-30-24-32(46)12-14-37(30)29-8-10-31(45)11-9-29/h3-15,24-25,28,33-34,52H,16-23,26-27H2,1-2H3,(H,50,51,53)/t33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL/BAK (unknown origin) interaction |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50426417

(CHEMBL2326745)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1ncnc2CN(CCc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H49ClF3N7O4S3/c1-53(2)22-18-34(29-60-36-9-4-3-5-10-36)51-40-17-16-37(26-42(40)61(56,57)44(46,47)48)62(58,59)52-43-39-21-25-55(28-41(39)49-30-50-43)35-19-23-54(24-20-35)27-32-8-6-7-11-38(32)31-12-14-33(45)15-13-31/h3-17,26,30,34-35,51H,18-25,27-29H2,1-2H3,(H,49,50,52)/t34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL/BAK (unknown origin) interaction |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426421

(CHEMBL2322026)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)Nc1nnc2cc(cnn12)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C40H43ClN10O4S2/c1-47(2)19-18-32(28-56-34-9-4-3-5-10-34)43-37-17-16-35(25-38(37)51(52)53)57(54,55)46-40-45-44-39-24-33(26-42-50(39)40)49-22-20-48(21-23-49)27-30-8-6-7-11-36(30)29-12-14-31(41)15-13-29/h3-17,24-26,32,43H,18-23,27-28H2,1-2H3,(H,45,46)/t32-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426418
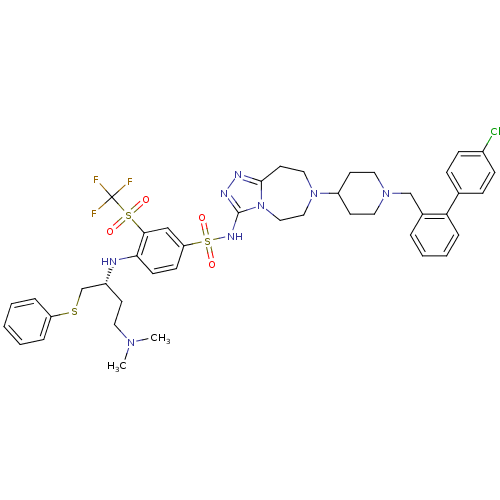
(CHEMBL2326744)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1nnc2CCN(CCn12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C43H50ClF3N8O4S3/c1-52(2)22-18-34(30-60-36-9-4-3-5-10-36)48-39-17-16-37(28-40(39)61(56,57)43(45,46)47)62(58,59)51-42-50-49-41-21-25-54(26-27-55(41)42)35-19-23-53(24-20-35)29-32-8-6-7-11-38(32)31-12-14-33(44)15-13-31/h3-17,28,34-35,48H,18-27,29-30H2,1-2H3,(H,50,51)/t34-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426413

(CHEMBL2322021)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1nc(Cl)nc2CN(CCc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H48Cl2F3N7O4S3/c1-54(2)22-18-33(29-61-35-9-4-3-5-10-35)50-39-17-16-36(26-41(39)62(57,58)44(47,48)49)63(59,60)53-42-38-21-25-56(28-40(38)51-43(46)52-42)34-19-23-55(24-20-34)27-31-8-6-7-11-37(31)30-12-14-32(45)15-13-30/h3-17,26,33-34,50H,18-25,27-29H2,1-2H3,(H,51,52,53)/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426416
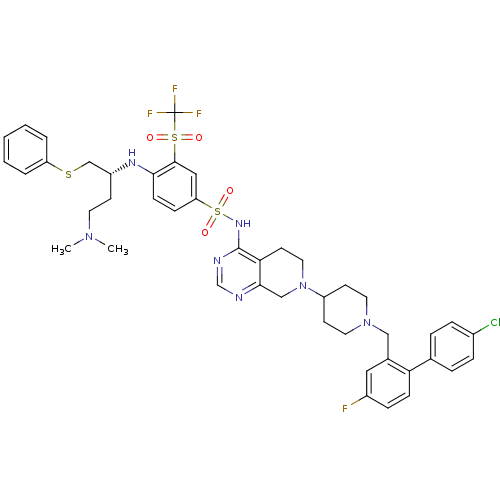
(CHEMBL2326746)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1ncnc2CN(CCc12)C1CCN(Cc2cc(F)ccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H48ClF4N7O4S3/c1-54(2)20-16-34(28-61-36-6-4-3-5-7-36)52-40-15-13-37(25-42(40)62(57,58)44(47,48)49)63(59,60)53-43-39-19-23-56(27-41(39)50-29-51-43)35-17-21-55(22-18-35)26-31-24-33(46)12-14-38(31)30-8-10-32(45)11-9-30/h3-15,24-25,29,34-35,52H,16-23,26-28H2,1-2H3,(H,50,51,53)/t34-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426415
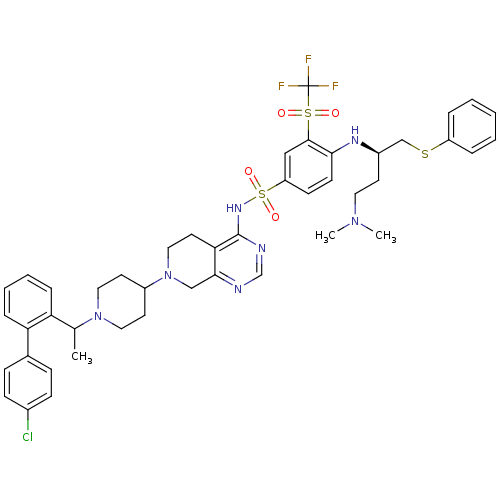
(CHEMBL2326747)Show SMILES CC(N1CCC(CC1)N1CCc2c(C1)ncnc2NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)c1ccccc1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C45H51ClF3N7O4S3/c1-31(38-11-7-8-12-39(38)32-13-15-33(46)16-14-32)55-24-20-35(21-25-55)56-26-22-40-42(28-56)50-30-51-44(40)53-63(59,60)37-17-18-41(43(27-37)62(57,58)45(47,48)49)52-34(19-23-54(2)3)29-61-36-9-5-4-6-10-36/h4-18,27,30-31,34-35,52H,19-26,28-29H2,1-3H3,(H,50,51,53)/t31?,34-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426412

(CHEMBL2322022)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1nc(nc2CN(CCc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1)C(F)(F)F |r| Show InChI InChI=1S/C45H48ClF6N7O4S3/c1-57(2)22-18-33(29-64-35-9-4-3-5-10-35)53-39-17-16-36(26-41(39)65(60,61)45(50,51)52)66(62,63)56-42-38-21-25-59(28-40(38)54-43(55-42)44(47,48)49)34-19-23-58(24-20-34)27-31-8-6-7-11-37(31)30-12-14-32(46)15-13-30/h3-17,26,33-34,53H,18-25,27-29H2,1-2H3,(H,54,55,56)/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50426412

(CHEMBL2322022)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1nc(nc2CN(CCc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1)C(F)(F)F |r| Show InChI InChI=1S/C45H48ClF6N7O4S3/c1-57(2)22-18-33(29-64-35-9-4-3-5-10-35)53-39-17-16-36(26-41(39)65(60,61)45(50,51)52)66(62,63)56-42-38-21-25-59(28-40(38)54-43(55-42)44(47,48)49)34-19-23-58(24-20-34)27-31-8-6-7-11-37(31)30-12-14-32(46)15-13-30/h3-17,26,33-34,53H,18-25,27-29H2,1-2H3,(H,54,55,56)/t33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL/BAK (unknown origin) interaction |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426423

(CHEMBL2322024)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)Nc1cccc2cc(cnc12)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H46ClN7O4S2/c1-49(2)22-21-36(31-57-38-11-4-3-5-12-38)47-41-20-19-39(28-43(41)52(53)54)58(55,56)48-42-14-8-10-33-27-37(29-46-44(33)42)51-25-23-50(24-26-51)30-34-9-6-7-13-40(34)32-15-17-35(45)18-16-32/h3-20,27-29,36,47-48H,21-26,30-31H2,1-2H3/t36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50426416

(CHEMBL2326746)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1ncnc2CN(CCc12)C1CCN(Cc2cc(F)ccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H48ClF4N7O4S3/c1-54(2)20-16-34(28-61-36-6-4-3-5-7-36)52-40-15-13-37(25-42(40)62(57,58)44(47,48)49)63(59,60)53-43-39-19-23-56(27-41(39)50-29-51-43)35-17-21-55(22-18-35)26-31-24-33(46)12-14-38(31)30-8-10-32(45)11-9-30/h3-15,24-25,29,34-35,52H,16-23,26-28H2,1-2H3,(H,50,51,53)/t34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL/BAK (unknown origin) interaction |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50426415

(CHEMBL2326747)Show SMILES CC(N1CCC(CC1)N1CCc2c(C1)ncnc2NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)c1ccccc1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C45H51ClF3N7O4S3/c1-31(38-11-7-8-12-39(38)32-13-15-33(46)16-14-32)55-24-20-35(21-25-55)56-26-22-40-42(28-56)50-30-51-44(40)53-63(59,60)37-17-18-41(43(27-37)62(57,58)45(47,48)49)52-34(19-23-54(2)3)29-61-36-9-5-4-6-10-36/h4-18,27,30-31,34-35,52H,19-26,28-29H2,1-3H3,(H,50,51,53)/t31?,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL/BAK (unknown origin) interaction |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235403

(CHEMBL4065944)Show SMILES CNc1ncc(-c2ccc(cc2)C(=O)NCC(=O)Nc2ccccc2)c(OCC2CCNCC2)n1 Show InChI InChI=1S/C26H30N6O3/c1-27-26-30-15-22(25(32-26)35-17-18-11-13-28-14-12-18)19-7-9-20(10-8-19)24(34)29-16-23(33)31-21-5-3-2-4-6-21/h2-10,15,18,28H,11-14,16-17H2,1H3,(H,29,34)(H,31,33)(H,27,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426414

(CHEMBL2322020)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1ncnc2C(=O)N(CCc12)C1CCN(Cc2cc(F)ccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H46ClF4N7O5S3/c1-54(2)20-16-33(27-62-35-6-4-3-5-7-35)52-39-15-13-36(25-40(39)63(58,59)44(47,48)49)64(60,61)53-42-38-19-23-56(43(57)41(38)50-28-51-42)34-17-21-55(22-18-34)26-30-24-32(46)12-14-37(30)29-8-10-31(45)11-9-29/h3-15,24-25,28,33-34,52H,16-23,26-27H2,1-2H3,(H,50,51,53)/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50235400
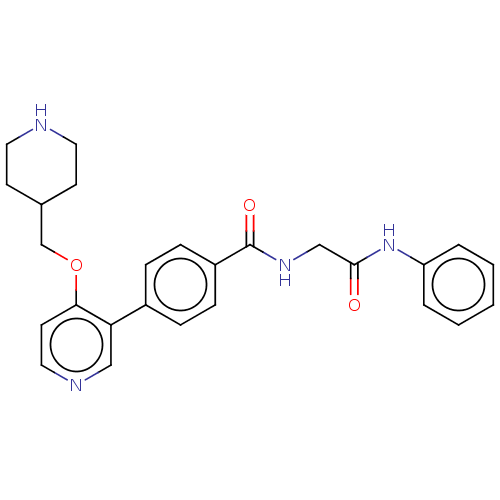
(CHEMBL4093545)Show SMILES O=C(CNC(=O)c1ccc(cc1)-c1cnccc1OCC1CCNCC1)Nc1ccccc1 Show InChI InChI=1S/C26H28N4O3/c31-25(30-22-4-2-1-3-5-22)17-29-26(32)21-8-6-20(7-9-21)23-16-28-15-12-24(23)33-18-19-10-13-27-14-11-19/h1-9,12,15-16,19,27H,10-11,13-14,17-18H2,(H,29,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235400

(CHEMBL4093545)Show SMILES O=C(CNC(=O)c1ccc(cc1)-c1cnccc1OCC1CCNCC1)Nc1ccccc1 Show InChI InChI=1S/C26H28N4O3/c31-25(30-22-4-2-1-3-5-22)17-29-26(32)21-8-6-20(7-9-21)23-16-28-15-12-24(23)33-18-19-10-13-27-14-11-19/h1-9,12,15-16,19,27H,10-11,13-14,17-18H2,(H,29,32)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426419
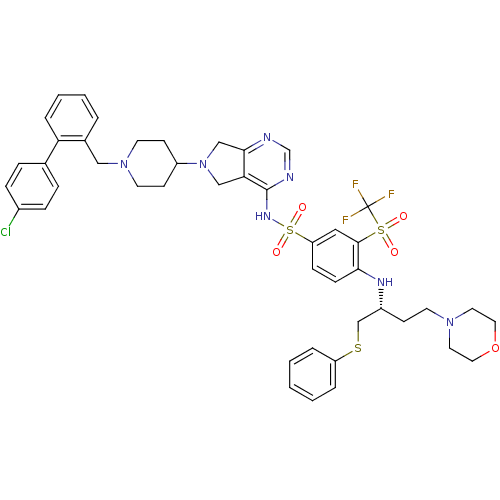
(CHEMBL2322029)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)Nc1ncnc2CN(Cc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C45H49ClF3N7O5S3/c46-34-12-10-32(11-13-34)39-9-5-4-6-33(39)27-55-20-17-36(18-21-55)56-28-40-42(29-56)50-31-51-44(40)53-64(59,60)38-14-15-41(43(26-38)63(57,58)45(47,48)49)52-35(16-19-54-22-24-61-25-23-54)30-62-37-7-2-1-3-8-37/h1-15,26,31,35-36,52H,16-25,27-30H2,(H,50,51,53)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426424

(CHEMBL2322023)Show SMILES [O-][N+](=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)Nc1cccc2cc(ccc12)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C47H49ClN6O5S2/c48-38-15-13-35(14-16-38)43-11-5-4-7-37(43)33-52-23-25-53(26-24-52)40-17-19-44-36(31-40)8-6-12-45(44)50-61(57,58)42-18-20-46(47(32-42)54(55)56)49-39(21-22-51-27-29-59-30-28-51)34-60-41-9-2-1-3-10-41/h1-20,31-32,39,49-50H,21-30,33-34H2/t39-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50235401

(CHEMBL4071823)Show SMILES COc1ccc2nc(CNC(=O)c3ccc(cc3)-c3cnccc3OCC3CCNCC3)[nH]c2c1 Show InChI InChI=1S/C27H29N5O3/c1-34-21-6-7-23-24(14-21)32-26(31-23)16-30-27(33)20-4-2-19(3-5-20)22-15-29-13-10-25(22)35-17-18-8-11-28-12-9-18/h2-7,10,13-15,18,28H,8-9,11-12,16-17H2,1H3,(H,30,33)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426422

(CHEMBL2322025)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)Nc1nn(C)c2nc(ncc12)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C41H45ClN10O4S2/c1-48(2)20-19-32(28-57-33-10-5-4-6-11-33)44-37-18-17-34(25-38(37)52(53)54)58(55,56)47-39-36-26-43-41(45-40(36)49(3)46-39)51-23-21-50(22-24-51)27-30-9-7-8-12-35(30)29-13-15-31(42)16-14-29/h4-18,25-26,32,44H,19-24,27-28H2,1-3H3,(H,46,47)/t32-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2/biotinylated BAK (unknown origin) interaction incubated for 1 hr by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485626
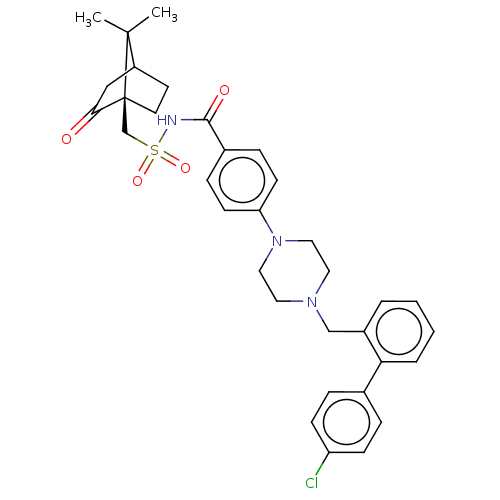
(CHEMBL2089285)Show SMILES CC1(C)C2CC[C@]1(CS(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc3ccccc3-c3ccc(Cl)cc3)CC1)C(=O)C2 |r| Show InChI InChI=1S/C34H38ClN3O4S/c1-33(2)27-15-16-34(33,31(39)21-27)23-43(41,42)36-32(40)25-9-13-29(14-10-25)38-19-17-37(18-20-38)22-26-5-3-4-6-30(26)24-7-11-28(35)12-8-24/h3-14,27H,15-23H2,1-2H3,(H,36,40)/t27?,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485627

(CHEMBL2089283)Show SMILES CC(C)(C)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C28H32ClN3O3S/c1-28(2,3)36(34,35)30-27(33)22-10-14-25(15-11-22)32-18-16-31(17-19-32)20-23-6-4-5-7-26(23)21-8-12-24(29)13-9-21/h4-15H,16-20H2,1-3H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485625

(CHEMBL2089288)Show SMILES C[C@]12C[C@](C)([C@H](CS(=O)(=O)NC(=O)c3ccc(cc3)N3CCN(Cc4ccccc4-c4ccc(Cl)cc4)CC3)CC1)C2(C)C |r| Show InChI InChI=1S/C36H44ClN3O3S/c1-34(2)35(3)18-17-29(36(34,4)25-35)24-44(42,43)38-33(41)27-11-15-31(16-12-27)40-21-19-39(20-22-40)23-28-7-5-6-8-32(28)26-9-13-30(37)14-10-26/h5-16,29H,17-25H2,1-4H3,(H,38,41)/t29-,35+,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485624
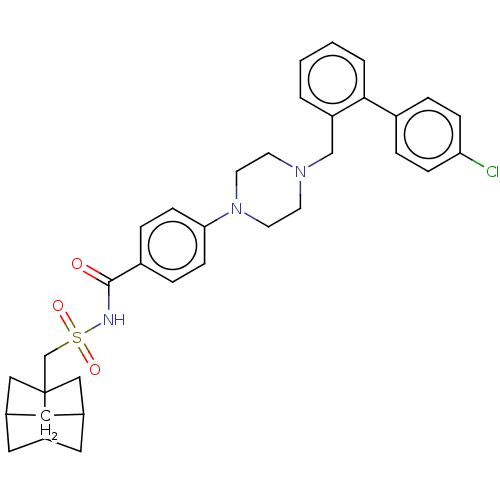
(CHEMBL2089292)Show SMILES Clc1ccc(cc1)-c1ccccc1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)CC12CC3CC1CC(C2)C3 |THB:38:39:34:36.37,38:37:34:40.41.39| Show InChI InChI=1S/C34H38ClN3O3S/c35-30-9-5-26(6-10-30)32-4-2-1-3-28(32)22-37-13-15-38(16-14-37)31-11-7-27(8-12-31)33(39)36-42(40,41)23-34-20-24-17-25(21-34)19-29(34)18-24/h1-12,24-25,29H,13-23H2,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485623
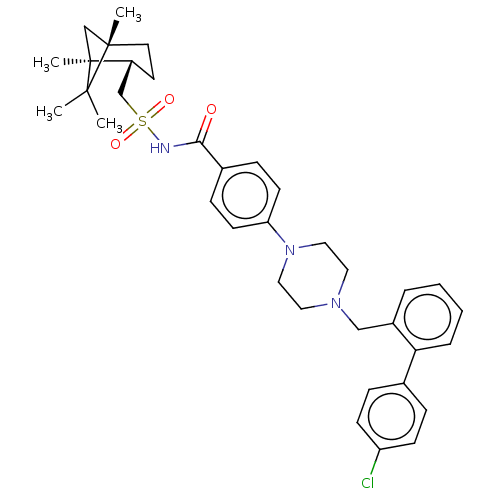
(CHEMBL2089287)Show SMILES C[C@@]12C[C@@](C)([C@@H](CS(=O)(=O)NC(=O)c3ccc(cc3)N3CCN(Cc4ccccc4-c4ccc(Cl)cc4)CC3)CC1)C2(C)C |r| Show InChI InChI=1S/C36H44ClN3O3S/c1-34(2)35(3)18-17-29(36(34,4)25-35)24-44(42,43)38-33(41)27-11-15-31(16-12-27)40-21-19-39(20-22-40)23-28-7-5-6-8-32(28)26-9-13-30(37)14-10-26/h5-16,29H,17-25H2,1-4H3,(H,38,41)/t29-,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
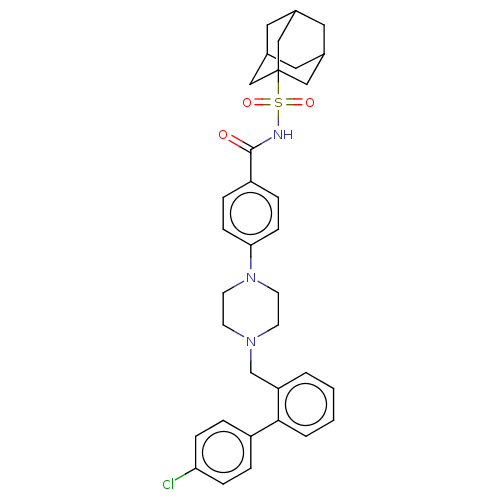
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485622
(CHEMBL2089284)Show SMILES Clc1ccc(cc1)-c1ccccc1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)C12CC3CC(CC(C3)C1)C2 |TLB:29:32:35:39.37.38,THB:37:36:33:39.38.40,37:38:35.36.41:33,40:38:35:41.32.33,40:32:35:39.37.38| Show InChI InChI=1S/C34H38ClN3O3S/c35-30-9-5-27(6-10-30)32-4-2-1-3-29(32)23-37-13-15-38(16-14-37)31-11-7-28(8-12-31)33(39)36-42(40,41)34-20-24-17-25(21-34)19-26(18-24)22-34/h1-12,24-26H,13-23H2,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
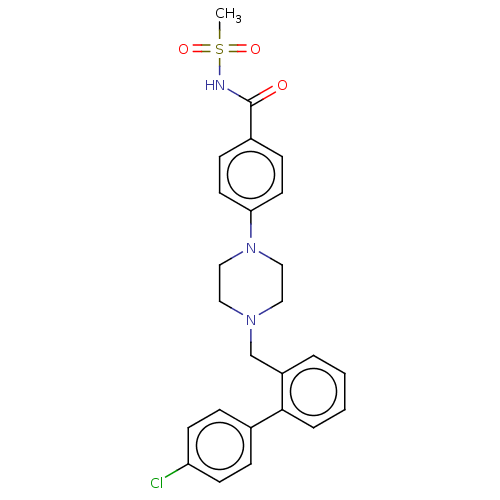
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485621
(CHEMBL2089281)Show SMILES CS(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C25H26ClN3O3S/c1-33(31,32)27-25(30)20-8-12-23(13-9-20)29-16-14-28(15-17-29)18-21-4-2-3-5-24(21)19-6-10-22(26)11-7-19/h2-13H,14-18H2,1H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
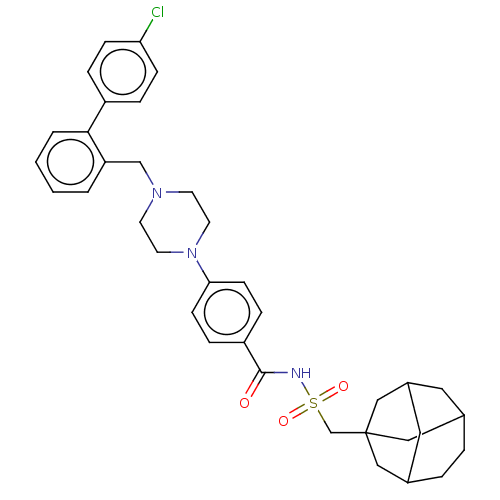
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485620
(CHEMBL2089293)Show SMILES Clc1ccc(cc1)-c1ccccc1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)CC12CC3CC(C1)CCC(C3)C2 |TLB:36:35:43:38.37.40.39,36:37:34.35.42:43,THB:40:41:34:38.36.37,39:37:34:42.41.43| Show InChI InChI=1S/C36H42ClN3O3S/c37-32-11-7-29(8-12-32)34-4-2-1-3-31(34)24-39-15-17-40(18-16-39)33-13-9-30(10-14-33)35(41)38-44(42,43)25-36-21-26-5-6-27(22-36)20-28(19-26)23-36/h1-4,7-14,26-28H,5-6,15-25H2,(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485619

(CHEMBL2089291)Show SMILES Clc1ccc(cc1)-c1ccccc1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:36:37:34.35.40:41,THB:36:35:41:42.37.38,38:37:34:40.39.41,38:39:34:42.36.37| Show InChI InChI=1S/C34H39ClN4O3S/c35-30-9-5-27(6-10-30)32-4-2-1-3-29(32)23-38-13-15-39(16-14-38)31-11-7-28(8-12-31)33(40)36-43(41,42)37-34-20-24-17-25(21-34)19-26(18-24)22-34/h1-12,24-26,37H,13-23H2,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer/Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50485615

(CHEMBL2089282)Show SMILES CCS(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C26H28ClN3O3S/c1-2-34(32,33)28-26(31)21-9-13-24(14-10-21)30-17-15-29(16-18-30)19-22-5-3-4-6-25(22)20-7-11-23(27)12-8-20/h3-14H,2,15-19H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485616
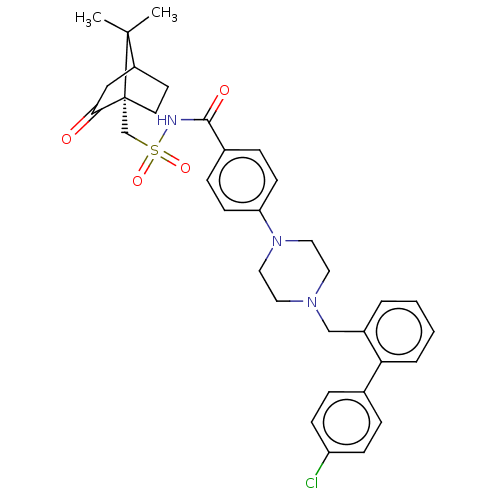
(CHEMBL2089286)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc3ccccc3-c3ccc(Cl)cc3)CC1)C(=O)C2 |r| Show InChI InChI=1S/C34H38ClN3O4S/c1-33(2)27-15-16-34(33,31(39)21-27)23-43(41,42)36-32(40)25-9-13-29(14-10-25)38-19-17-37(18-20-38)22-26-5-3-4-6-30(26)24-7-11-28(35)12-8-24/h3-14,27H,15-23H2,1-2H3,(H,36,40)/t27?,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485617
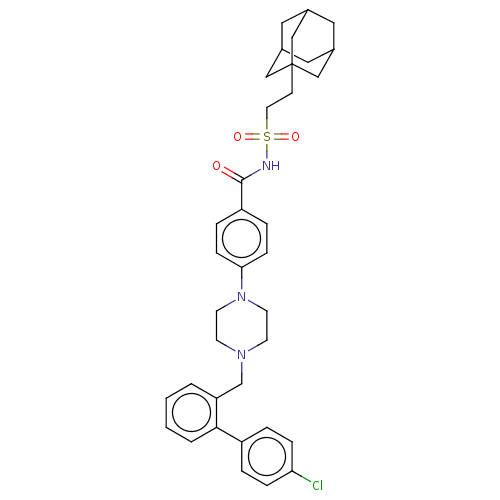
(CHEMBL2089289)Show SMILES Clc1ccc(cc1)-c1ccccc1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)CCC12CC3CC(CC(C3)C1)C2 |TLB:37:38:35.36.41:42,THB:37:36:42:43.38.39,39:38:35:41.40.42,39:40:35:43.37.38| Show InChI InChI=1S/C36H42ClN3O3S/c37-32-9-5-29(6-10-32)34-4-2-1-3-31(34)25-39-14-16-40(17-15-39)33-11-7-30(8-12-33)35(41)38-44(42,43)18-13-36-22-26-19-27(23-36)21-28(20-26)24-36/h1-12,26-28H,13-25H2,(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50485618
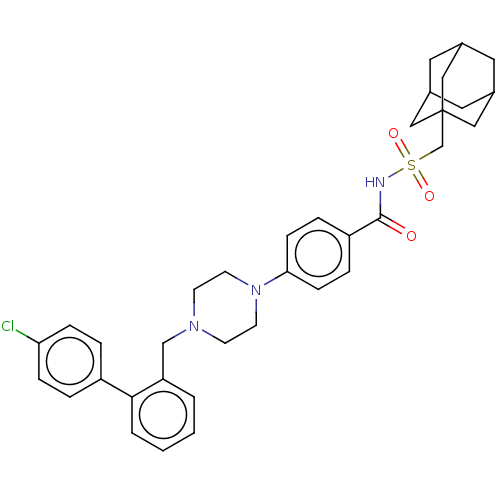
(CHEMBL2089290)Show SMILES Clc1ccc(cc1)-c1ccccc1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)CC12CC3CC(CC(C3)C1)C2 |TLB:36:37:34.35.40:41,THB:36:35:41:42.37.38,38:37:34:40.39.41,38:39:34:42.36.37| Show InChI InChI=1S/C35H40ClN3O3S/c36-31-9-5-28(6-10-31)33-4-2-1-3-30(33)23-38-13-15-39(16-14-38)32-11-7-29(8-12-32)34(40)37-43(41,42)24-35-20-25-17-26(21-35)19-27(18-25)22-35/h1-12,25-27H,13-24H2,(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1-BAK interaction |
ACS Med Chem Lett 3: 579-83 (2012)
Article DOI: 10.1021/ml300095a
BindingDB Entry DOI: 10.7270/Q23X89GW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426412

(CHEMBL2322022)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1nc(nc2CN(CCc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1)C(F)(F)F |r| Show InChI InChI=1S/C45H48ClF6N7O4S3/c1-57(2)22-18-33(29-64-35-9-4-3-5-10-35)53-39-17-16-36(26-41(39)65(60,61)45(50,51)52)66(62,63)56-42-38-21-25-59(28-40(38)54-43(55-42)44(47,48)49)34-19-23-58(24-20-34)27-31-8-6-7-11-37(31)30-12-14-32(46)15-13-30/h3-17,26,33-34,53H,18-25,27-29H2,1-2H3,(H,54,55,56)/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-2 (unknown origin) by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426413

(CHEMBL2322021)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1nc(Cl)nc2CN(CCc12)C1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H48Cl2F3N7O4S3/c1-54(2)22-18-33(29-61-35-9-4-3-5-10-35)50-39-17-16-36(26-41(39)62(57,58)44(47,48)49)63(59,60)53-42-38-21-25-56(28-40(38)51-43(46)52-42)34-19-23-55(24-20-34)27-31-8-6-7-11-37(31)30-12-14-32(45)15-13-30/h3-17,26,33-34,50H,18-25,27-29H2,1-2H3,(H,51,52,53)/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-2 (unknown origin) by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426414

(CHEMBL2322020)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1S(=O)(=O)C(F)(F)F)S(=O)(=O)Nc1ncnc2C(=O)N(CCc12)C1CCN(Cc2cc(F)ccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H46ClF4N7O5S3/c1-54(2)20-16-33(27-62-35-6-4-3-5-7-35)52-39-15-13-36(25-40(39)63(58,59)44(47,48)49)64(60,61)53-42-38-19-23-56(43(57)41(38)50-28-51-42)34-17-21-55(22-18-34)26-30-24-32(46)12-14-37(30)29-8-10-31(45)11-9-29/h3-15,24-25,28,33-34,52H,16-23,26-27H2,1-2H3,(H,50,51,53)/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-2 (unknown origin) by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50426415

(CHEMBL2326747)Show SMILES CC(N1CCC(CC1)N1CCc2c(C1)ncnc2NS(=O)(=O)c1ccc(N[C@H](CCN(C)C)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)c1ccccc1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C45H51ClF3N7O4S3/c1-31(38-11-7-8-12-39(38)32-13-15-33(46)16-14-32)55-24-20-35(21-25-55)56-26-22-40-42(28-56)50-30-51-44(40)53-63(59,60)37-17-18-41(43(27-37)62(57,58)45(47,48)49)52-34(19-23-54(2)3)29-61-36-9-5-4-6-10-36/h4-18,27,30-31,34-35,52H,19-26,28-29H2,1-3H3,(H,50,51,53)/t31?,34-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-2 (unknown origin) by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-2 (unknown origin) by surface plasmon resonance analysis |
ACS Med Chem Lett 4: 186-90 (2013)
Article DOI: 10.1021/ml300321d
BindingDB Entry DOI: 10.7270/Q2XS5WRF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data