

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112660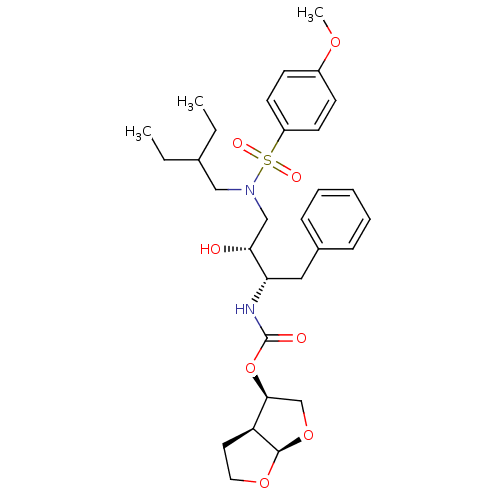 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000200 | -72.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112661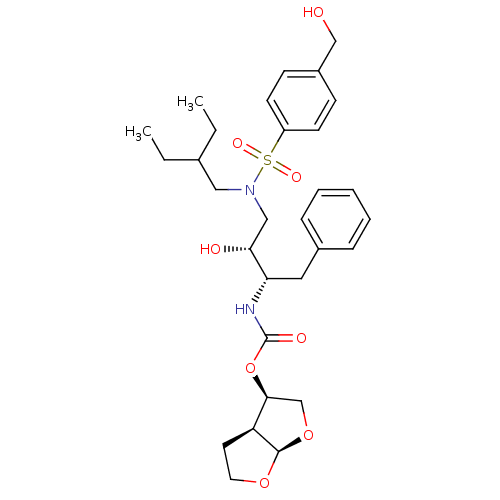 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000500 | -70.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000500 | -70.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q496K] (Human immunodeficiency virus type 1) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.000800 | -70.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
University of Massachusetts Medical School | Assay Description HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an... | J Med Chem 49: 7342-56 (2006) Article DOI: 10.1021/jm060666p BindingDB Entry DOI: 10.7270/Q25T3HQ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112662 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000800 | -69.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112657 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000900 | -68.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112660 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | -68.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112663 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00150 | -67.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483092 (CHEMBL1276087) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12878 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12878 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q496K] (Human immunodeficiency virus type 1) | BDBM12878 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00400 | -66.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
University of Massachusetts Medical School | Assay Description HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an... | J Med Chem 49: 7342-56 (2006) Article DOI: 10.1021/jm060666p BindingDB Entry DOI: 10.7270/Q25T3HQ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00400 | -65.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland | Assay Description Inhibition assay using HIV protease and Sulfonamide compounds. | Chem Biol Drug Des 69: 298-313 (2007) Article DOI: 10.1111/j.1747-0285.2007.00514.x BindingDB Entry DOI: 10.7270/Q2TQ6011 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112655 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112659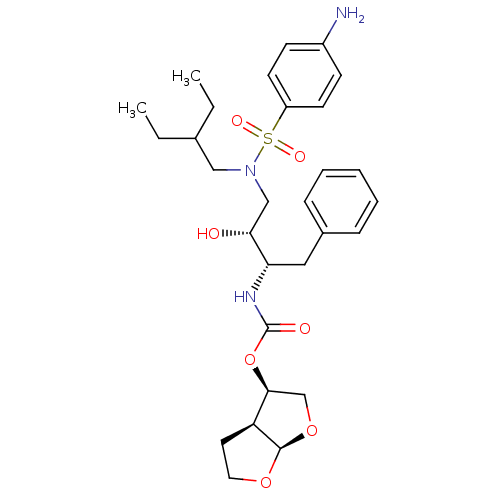 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112661 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q496K] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an... | J Med Chem 49: 7342-56 (2006) Article DOI: 10.1021/jm060666p BindingDB Entry DOI: 10.7270/Q25T3HQ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112662 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00600 | -64.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112654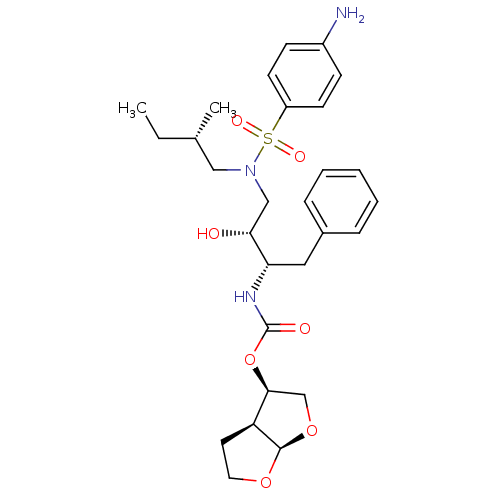 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00600 | -64.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q496K] (Human immunodeficiency virus type 1) | BDBM12885 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an... | J Med Chem 49: 7342-56 (2006) Article DOI: 10.1021/jm060666p BindingDB Entry DOI: 10.7270/Q25T3HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12885 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM12883 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483116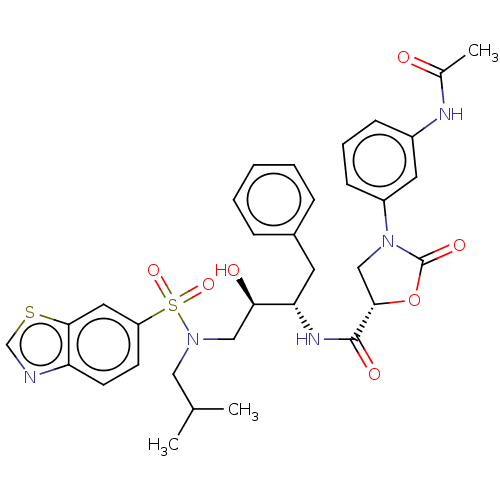 (CHEMBL1276096) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12883 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q496K] (Human immunodeficiency virus type 1) | BDBM12883 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an... | J Med Chem 49: 7342-56 (2006) Article DOI: 10.1021/jm060666p BindingDB Entry DOI: 10.7270/Q25T3HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12885 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12883 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483066 (CHEMBL1276093) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [491-589,Q498K,D521N,V555I,N579D] (Human immunodeficiency virus type 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an... | J Med Chem 50: 4316-28 (2007) Article DOI: 10.1021/jm070284z BindingDB Entry DOI: 10.7270/Q2W37TKX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112660 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | -62.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112658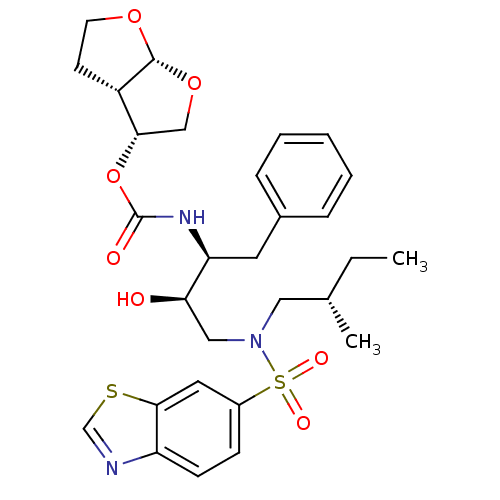 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0120 | -62.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112654 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0130 | -62.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0130 | -62.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50479738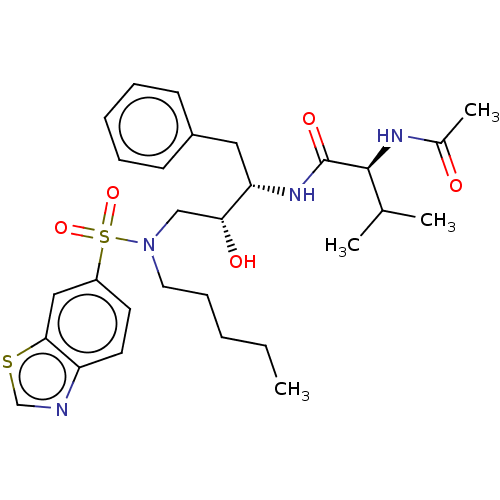 (CHEMBL514810) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50479738 (CHEMBL514810) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112654 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483096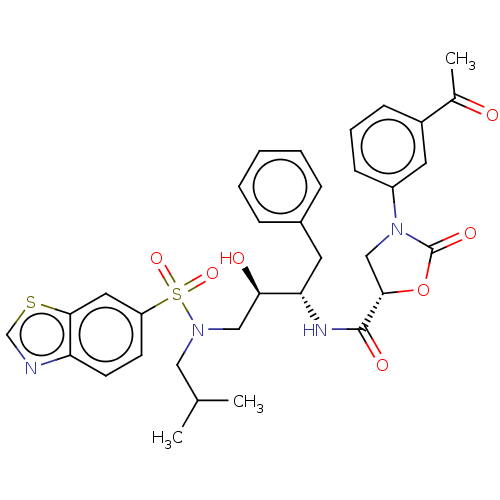 (CHEMBL1276083) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483093 (CHEMBL1276090) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12893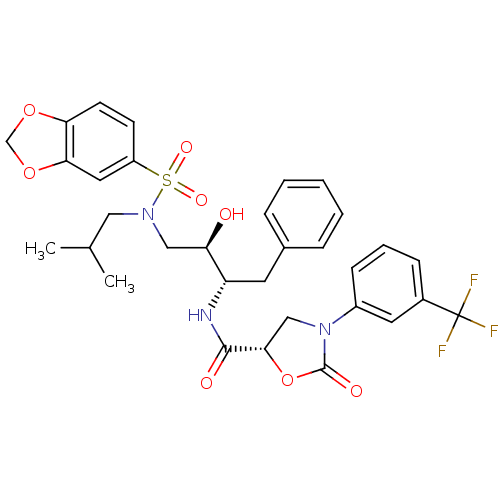 ((5S)-N-[(1S,2R)-3-[[(Benzo[1,3]dioxole-5-sulfonyl)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12894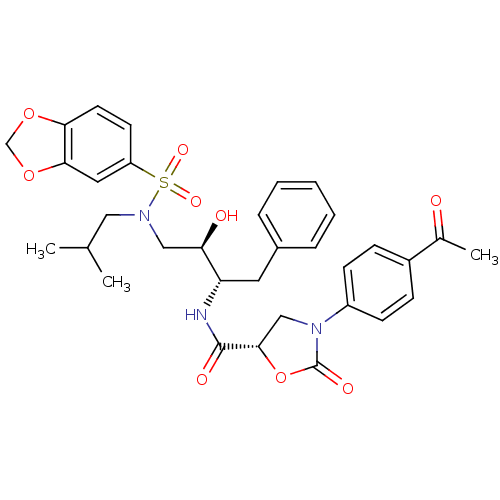 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12893 ((5S)-N-[(1S,2R)-3-[[(Benzo[1,3]dioxole-5-sulfonyl)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483085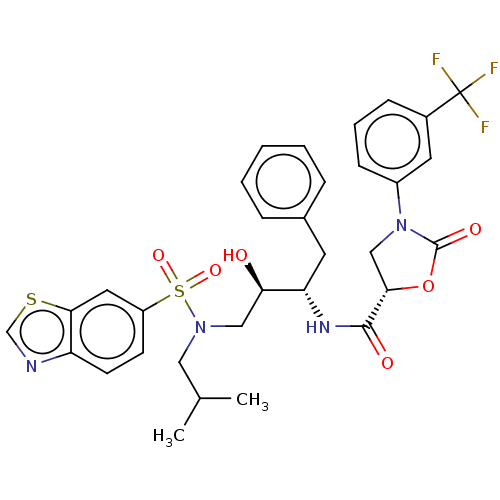 (CHEMBL1276076) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112654 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0160 | -61.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2448 total ) | Next | Last >> |