

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solute carrier family 22 member 8 (Homo sapiens (Human)) | BDBM50206509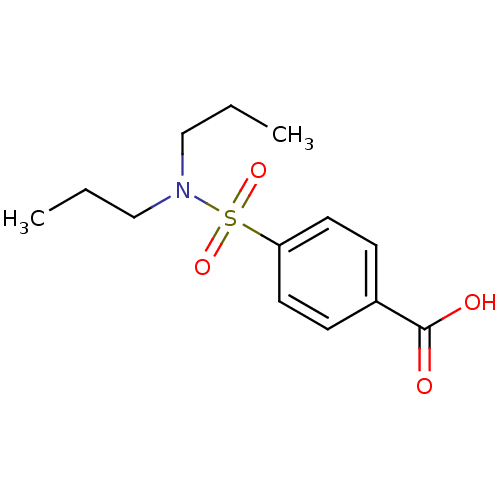 (4-Dipropylsulfamoyl-benzoic acid | 4-Dipropylsulfa...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT3-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM50206509 (4-Dipropylsulfamoyl-benzoic acid | 4-Dipropylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT1-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Solute carrier family 22 member 8 (Homo sapiens (Human)) | BDBM85245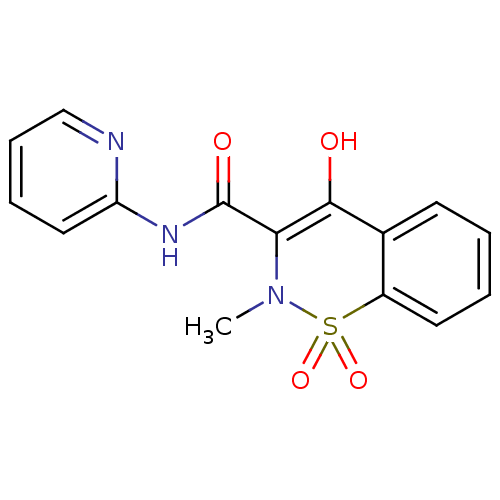 (CAS_36322-90-4 | NSC_4856 | Piroxicam) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT3-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM50485608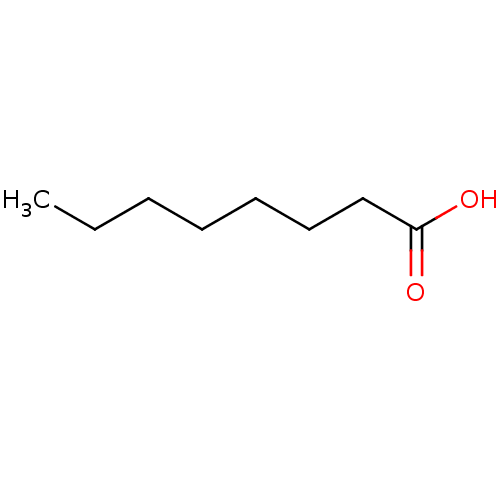 (CHEBI:28837 | Caprylic acid | EDENOR C 8-98-100 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 5.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT1-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM50240008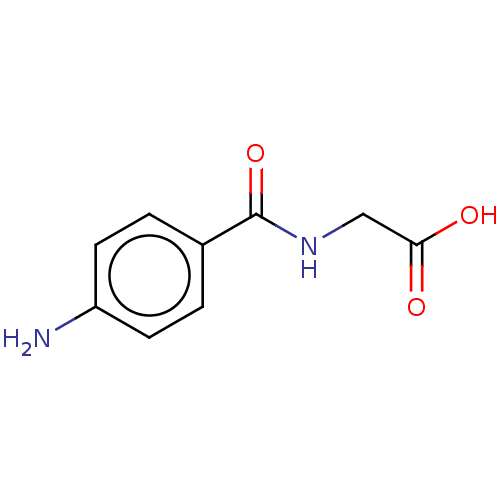 (Aminohippuric Acid | CHEBI:104011 | PAHA | Para-Am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT1-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Solute carrier family 22 member 8 (Homo sapiens (Human)) | BDBM50485608 (CHEBI:28837 | Caprylic acid | EDENOR C 8-98-100 | ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT3-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Homo sapiens (Human)) | BDBM50344961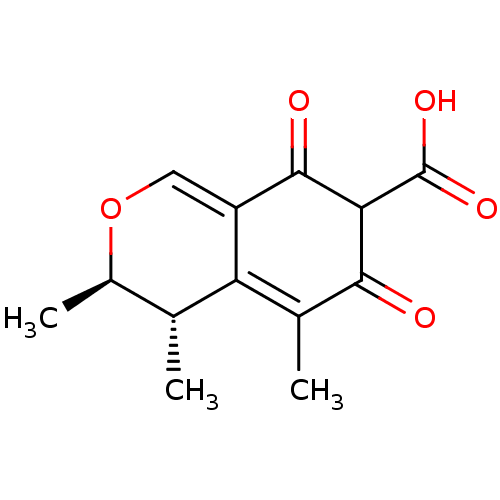 (CHEMBL510139 | citrinin) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT3-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Homo sapiens (Human)) | BDBM50240008 (Aminohippuric Acid | CHEBI:104011 | PAHA | Para-Am...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT3-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM85245 (CAS_36322-90-4 | NSC_4856 | Piroxicam) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT1-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Rattus norvegicus) | BDBM82898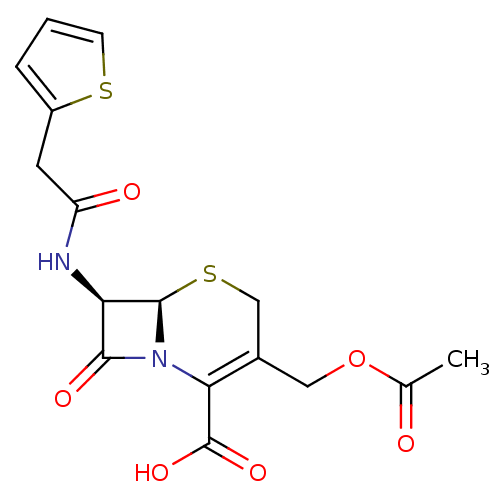 ((6R,7R)-3-(acetoxymethyl)-8-keto-7-[[2-(2-thienyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Estrone sulfate uptake in OAT3-expressing S2 cells | Life Sci 70: 1861-74 (2002) Article DOI: 10.1016/s0024-3205(02)01500-x BindingDB Entry DOI: 10.7270/Q2SN0CV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Rattus norvegicus) | BDBM50390999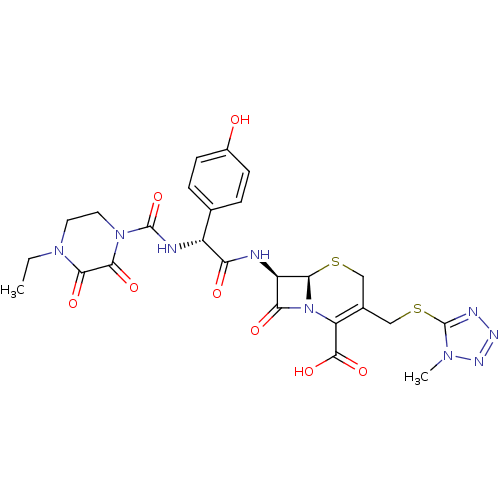 (CEFOPERAZONE) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in OAT1-expressing S2 cells | Life Sci 70: 1861-74 (2002) Article DOI: 10.1016/s0024-3205(02)01500-x BindingDB Entry DOI: 10.7270/Q2SN0CV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Rattus norvegicus) | BDBM82898 ((6R,7R)-3-(acetoxymethyl)-8-keto-7-[[2-(2-thienyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 5.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in OAT1-expressing S2 cells | Life Sci 70: 1861-74 (2002) Article DOI: 10.1016/s0024-3205(02)01500-x BindingDB Entry DOI: 10.7270/Q2SN0CV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Rattus norvegicus) | BDBM50370587 (CEFAZOLIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in OAT1-expressing S2 cells | Life Sci 70: 1861-74 (2002) Article DOI: 10.1016/s0024-3205(02)01500-x BindingDB Entry DOI: 10.7270/Q2SN0CV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Rattus norvegicus) | BDBM50390999 (CEFOPERAZONE) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Estrone sulfate uptake in OAT3-expressing S2 cells | Life Sci 70: 1861-74 (2002) Article DOI: 10.1016/s0024-3205(02)01500-x BindingDB Entry DOI: 10.7270/Q2SN0CV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Rattus norvegicus) | BDBM50370587 (CEFAZOLIN) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Estrone sulfate uptake in OAT3-expressing S2 cells | Life Sci 70: 1861-74 (2002) Article DOI: 10.1016/s0024-3205(02)01500-x BindingDB Entry DOI: 10.7270/Q2SN0CV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Rattus norvegicus) | BDBM50103624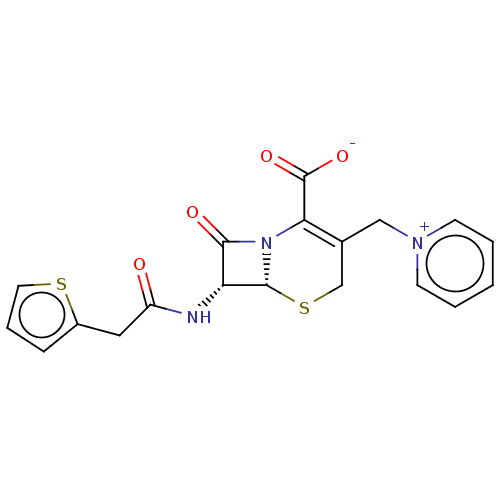 (40602 | CHEBI:3537 | Cefaloridine | Cephaloridine ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.14E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Estrone sulfate uptake in OAT3-expressing S2 cells | Life Sci 70: 1861-74 (2002) Article DOI: 10.1016/s0024-3205(02)01500-x BindingDB Entry DOI: 10.7270/Q2SN0CV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Rattus norvegicus) | BDBM50103624 (40602 | CHEBI:3537 | Cefaloridine | Cephaloridine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.32E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in OAT1-expressing S2 cells | Life Sci 70: 1861-74 (2002) Article DOI: 10.1016/s0024-3205(02)01500-x BindingDB Entry DOI: 10.7270/Q2SN0CV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM50344961 (CHEMBL510139 | citrinin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.08E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Ochratoxin A uptake in OAT1-expressing S2 cells | Life Sci 69: 2123-35 (2001) Article DOI: 10.1016/s0024-3205(01)01296-6 BindingDB Entry DOI: 10.7270/Q2XD14JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50219120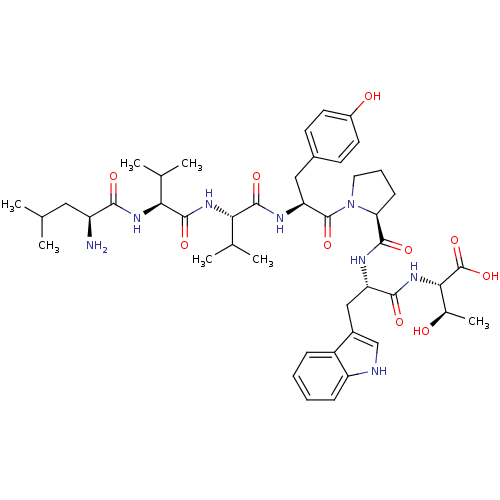 (CHEMBL395493 | LVVYPWT) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00830 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current by two electrode voltage cl... | J Med Chem 50: 4543-7 (2007) Article DOI: 10.1021/jm070114m BindingDB Entry DOI: 10.7270/Q25X29QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50219120 (CHEMBL395493 | LVVYPWT) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0481 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2X3 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current in presence of ... | J Med Chem 50: 4543-7 (2007) Article DOI: 10.1021/jm070114m BindingDB Entry DOI: 10.7270/Q25X29QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537922 (CHEMBL4645319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537922 (CHEMBL4645319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537919 (CHEMBL4633146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537918 (CHEMBL3951168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM167444 (US9073906, 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537919 (CHEMBL4633146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537918 (CHEMBL3951168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537921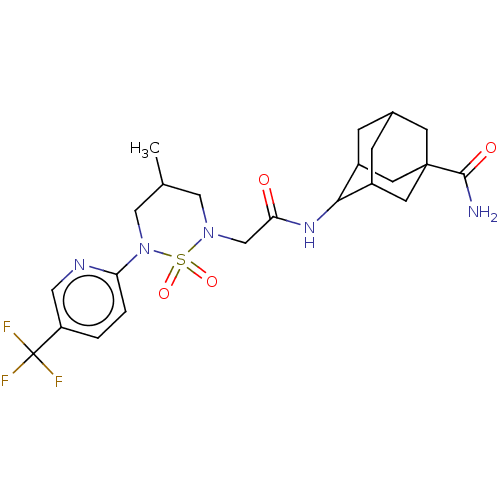 (CHEMBL4649416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM167444 (US9073906, 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537917 (CHEMBL4648389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50317662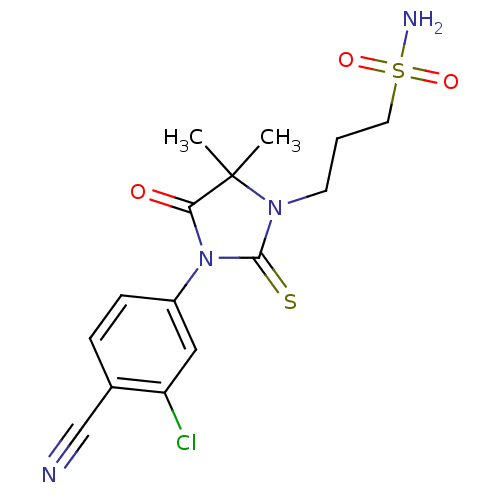 (3-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at AR in bicalutamide-resistant human LNCAP cells assessed as effect on cell proliferation after 6 days | Bioorg Med Chem 18: 3159-68 (2010) Article DOI: 10.1016/j.bmc.2010.03.036 BindingDB Entry DOI: 10.7270/Q2DJ5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537917 (CHEMBL4648389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50219121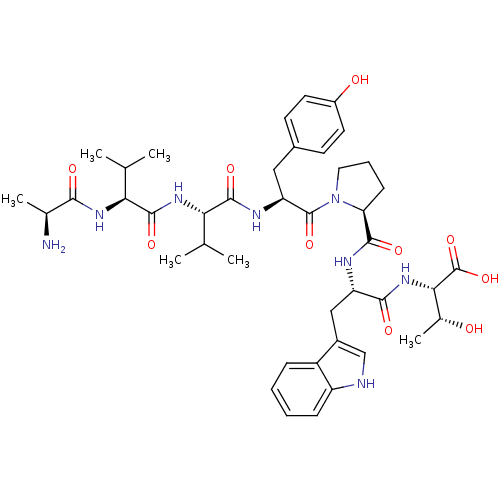 (AVVYPWT | CHEMBL238310) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current by two electrode voltage cl... | J Med Chem 50: 4543-7 (2007) Article DOI: 10.1021/jm070114m BindingDB Entry DOI: 10.7270/Q25X29QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50317664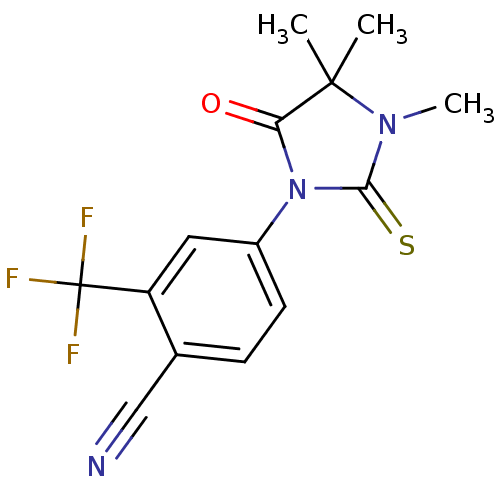 (2-(trifluoromethyl)-4-(3,4,4-trimethyl-5-oxo-2-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from human AR expressed in CHO-K1 cells after 2 hrs by scintillation counting | Bioorg Med Chem 18: 3159-68 (2010) Article DOI: 10.1016/j.bmc.2010.03.036 BindingDB Entry DOI: 10.7270/Q2DJ5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537920 (CHEMBL4632806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50317655 (3-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at AR in bicalutamide-resistant human LNCAP cells assessed as effect on cell proliferation after 6 days | Bioorg Med Chem 18: 3159-68 (2010) Article DOI: 10.1016/j.bmc.2010.03.036 BindingDB Entry DOI: 10.7270/Q2DJ5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (Mus musculus) | BDBM50102295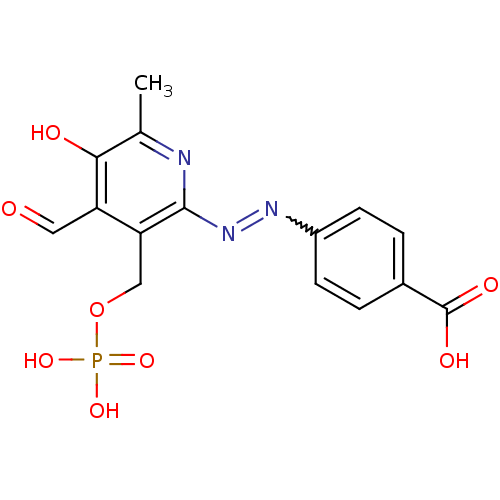 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at recombinant mouse P2X1 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current preincubated fo... | Eur J Med Chem 70: 811-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.026 BindingDB Entry DOI: 10.7270/Q2M61MQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50317656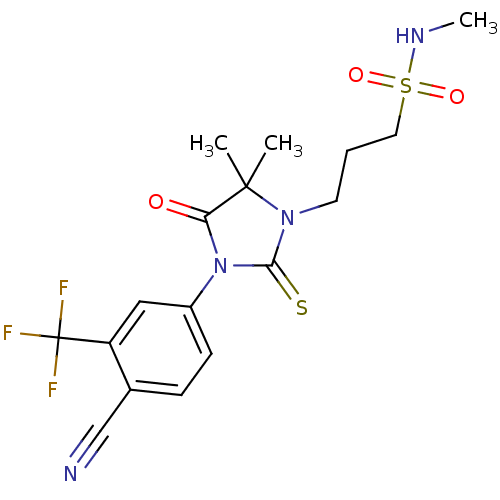 (3-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at AR in bicalutamide-resistant human LNCAP cells assessed as effect on cell proliferation after 6 days | Bioorg Med Chem 18: 3159-68 (2010) Article DOI: 10.1016/j.bmc.2010.03.036 BindingDB Entry DOI: 10.7270/Q2DJ5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (Mus musculus) | BDBM50064800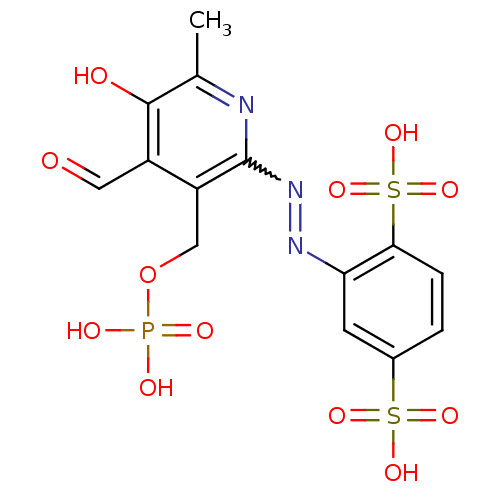 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at mouse recombinant P2X1 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current incubated for 2... | Bioorg Med Chem 21: 2643-50 (2013) Article DOI: 10.1016/j.bmc.2013.01.073 BindingDB Entry DOI: 10.7270/Q2DF6SK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50219123 (CHEMBL395723 | LAVYPWT) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current by two electrode voltage cl... | J Med Chem 50: 4543-7 (2007) Article DOI: 10.1021/jm070114m BindingDB Entry DOI: 10.7270/Q25X29QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM86478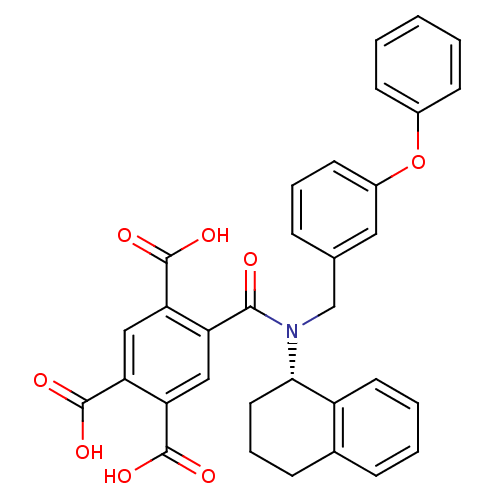 (A-317491) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X3 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current preincubated fo... | Eur J Med Chem 70: 811-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.026 BindingDB Entry DOI: 10.7270/Q2M61MQH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102295 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat P2X1 receptor expressed in Xenopus oocytes assessed as ion flux stimulation | Eur J Med Chem 70: 811-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.026 BindingDB Entry DOI: 10.7270/Q2M61MQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537916 (CHEMBL4646414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50317660 (3-[3-(4-Cyano-3-methylphenyl)-5,5-dimethyl-4-oxo-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at AR in bicalutamide-resistant human LNCAP cells assessed as effect on cell proliferation after 6 days | Bioorg Med Chem 18: 3159-68 (2010) Article DOI: 10.1016/j.bmc.2010.03.036 BindingDB Entry DOI: 10.7270/Q2DJ5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50317652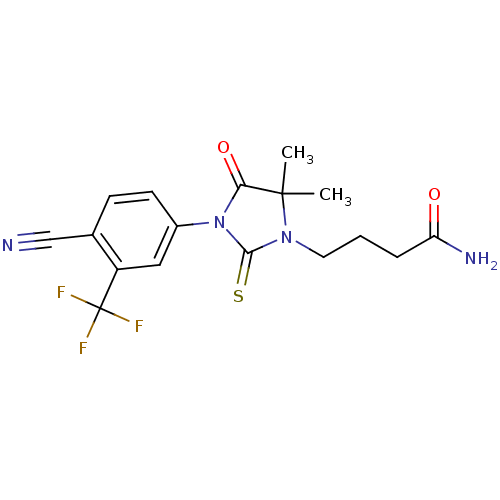 (4-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at AR in bicalutamide-resistant human LNCAP cells assessed as effect on cell proliferation after 6 days | Bioorg Med Chem 18: 3159-68 (2010) Article DOI: 10.1016/j.bmc.2010.03.036 BindingDB Entry DOI: 10.7270/Q2DJ5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50444434 (CHEMBL3091630) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X3 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current preincubated fo... | Eur J Med Chem 70: 811-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.026 BindingDB Entry DOI: 10.7270/Q2M61MQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537916 (CHEMBL4646414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (Mus musculus) | BDBM50433072 (CHEMBL2376126) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at mouse recombinant P2X1 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current incubated for 2... | Bioorg Med Chem 21: 2643-50 (2013) Article DOI: 10.1016/j.bmc.2013.01.073 BindingDB Entry DOI: 10.7270/Q2DF6SK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50317658 (2-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at AR in bicalutamide-resistant human LNCAP cells assessed as effect on cell proliferation after 6 days | Bioorg Med Chem 18: 3159-68 (2010) Article DOI: 10.1016/j.bmc.2010.03.036 BindingDB Entry DOI: 10.7270/Q2DJ5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50433071 (CHEMBL2376118) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2X3 receptor expressed in Xenopus oocytes assessed as inhibition of ATP-induced ion current incubated for 2... | Bioorg Med Chem 21: 2643-50 (2013) Article DOI: 10.1016/j.bmc.2013.01.073 BindingDB Entry DOI: 10.7270/Q2DF6SK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 217 total ) | Next | Last >> |