

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50050776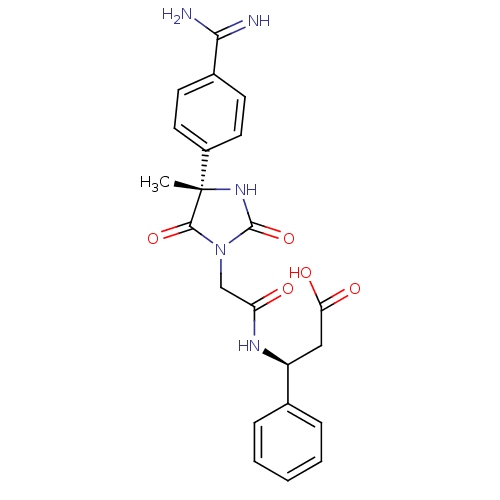 ((S)-3-{2-[(S)-4-(4-Carbamimidoyl-phenyl)-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Inhibition of [125I]-fibrinogen binding to isolated GPIIb/IIIa | J Med Chem 39: 2118-22 (1996) Article DOI: 10.1021/jm960210f BindingDB Entry DOI: 10.7270/Q2KW5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397165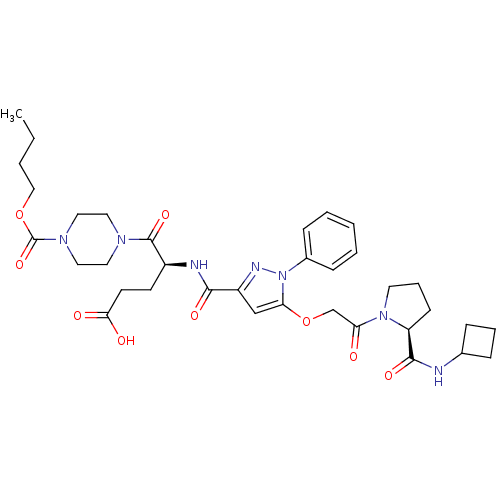 (CHEMBL2172149) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397156 (CHEMBL2172129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397166 (CHEMBL2172148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397164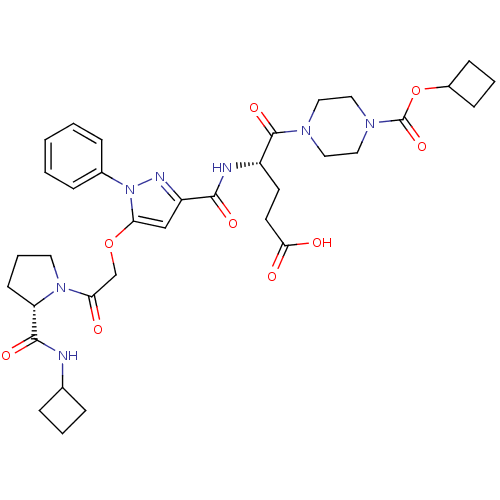 (CHEMBL2172150) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397157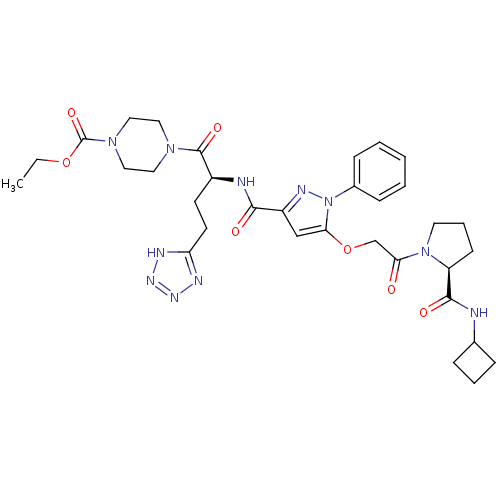 (CHEMBL2172128) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397168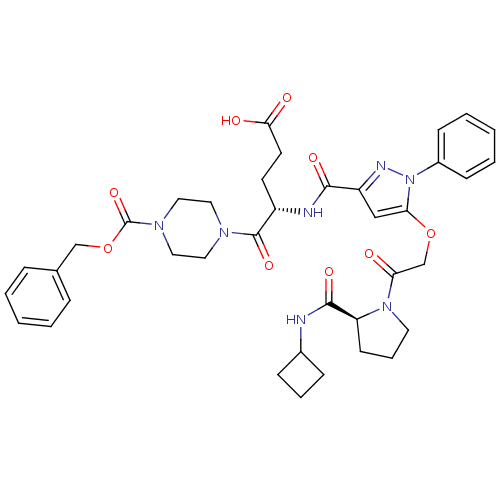 (CHEMBL2172146) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397152 (CHEMBL2172133) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397144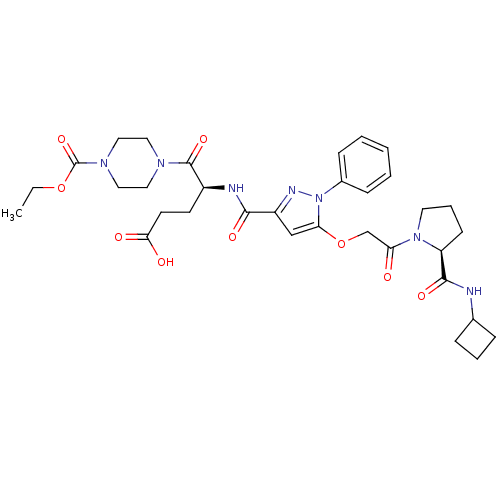 (CHEMBL2172272) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397143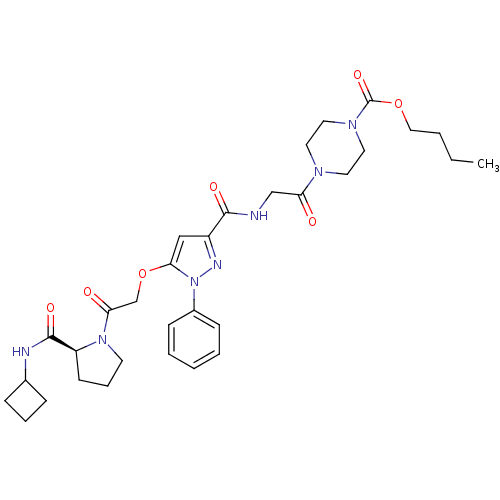 (CHEMBL2172140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397163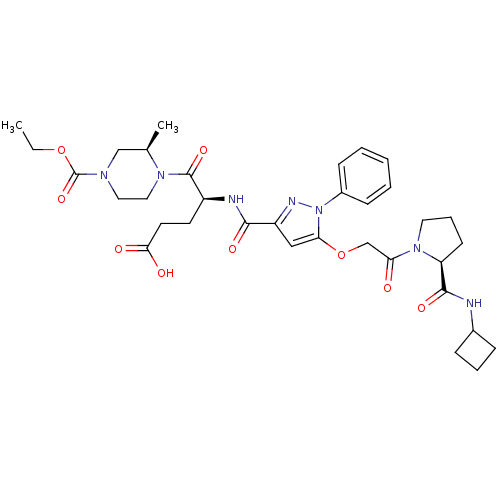 (CHEMBL2172151) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50050776 ((S)-3-{2-[(S)-4-(4-Carbamimidoyl-phenyl)-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Inhibition of [125I]-fibrinogen binding to ADP-activated human gel filtered platelets | J Med Chem 39: 2118-22 (1996) Article DOI: 10.1021/jm960210f BindingDB Entry DOI: 10.7270/Q2KW5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397205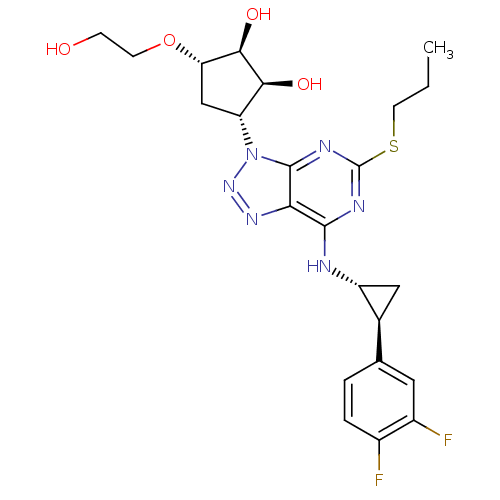 (AR-C126532XX | AZD-6140 | AZD6140 | BRILINTA | TIC...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397183 (CHEMBL2172156) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397185 (CHEMBL2172273) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397174 (CHEMBL2172165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397158 (CHEMBL2172127) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397153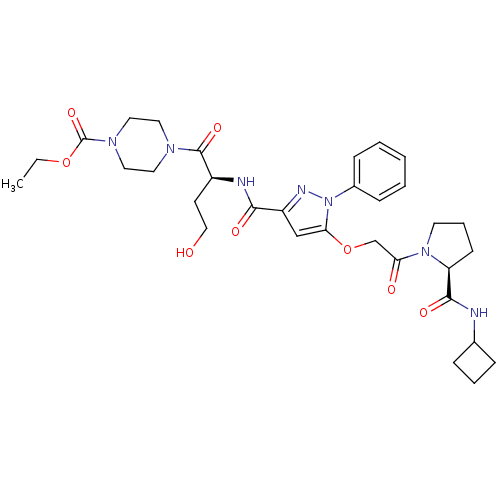 (CHEMBL2172132) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13122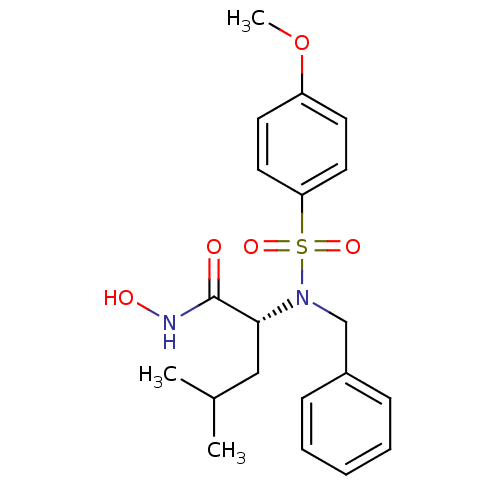 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397204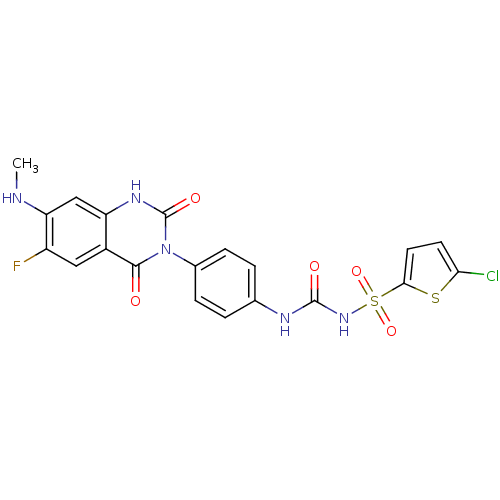 (ELINOGREL) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397184 (CHEMBL2172274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13133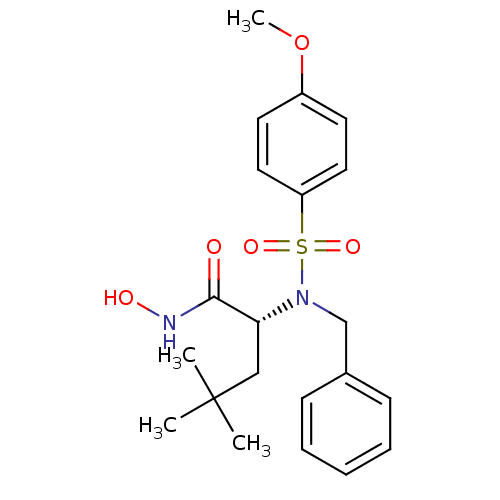 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13121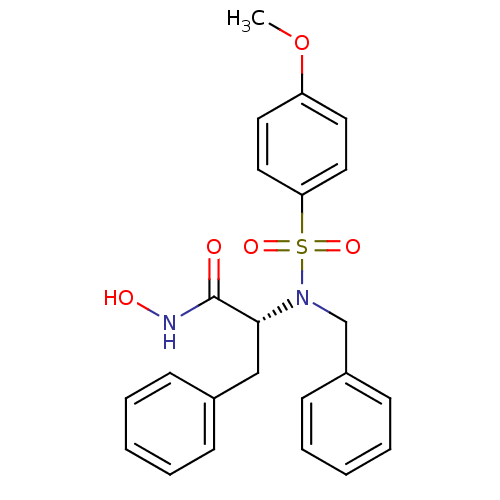 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098711 (CHEMBL17675 | N-(Carboxy-phenyl-methyl)-3-{2-[4-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma AG Curated by ChEMBL | Assay Description Inhibition of 125 I-fibrinogen binding to glycoprotein IIb/IIIa receptor | J Med Chem 44: 1158-76 (2001) BindingDB Entry DOI: 10.7270/Q2BG2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397171 (CHEMBL2172143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13127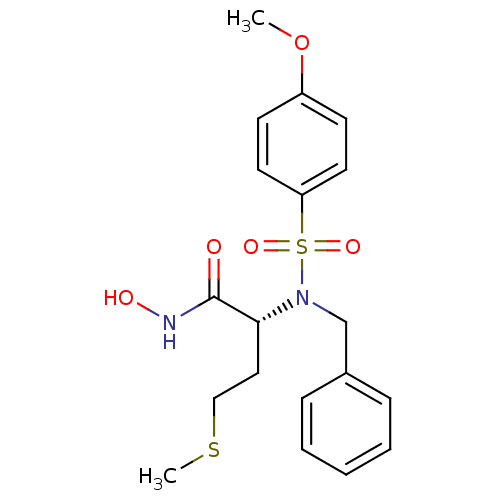 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397176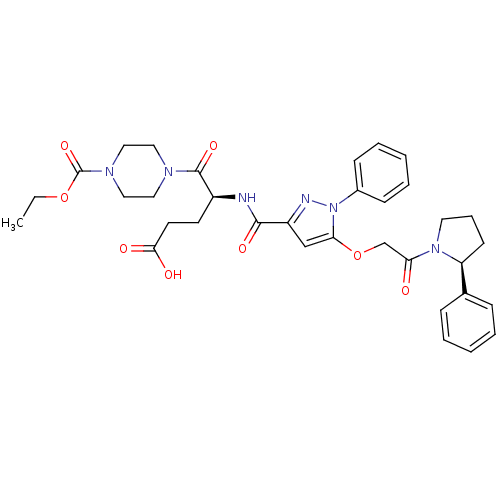 (CHEMBL2172163) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13126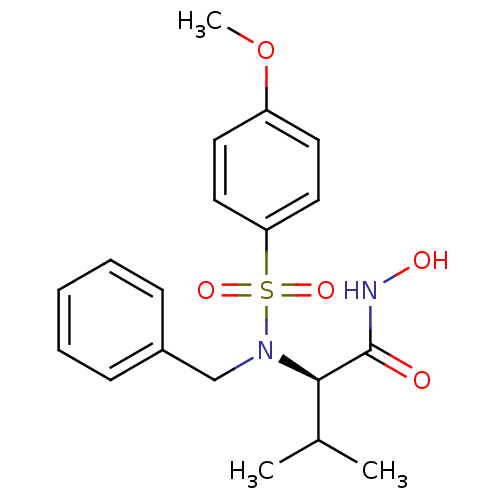 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13119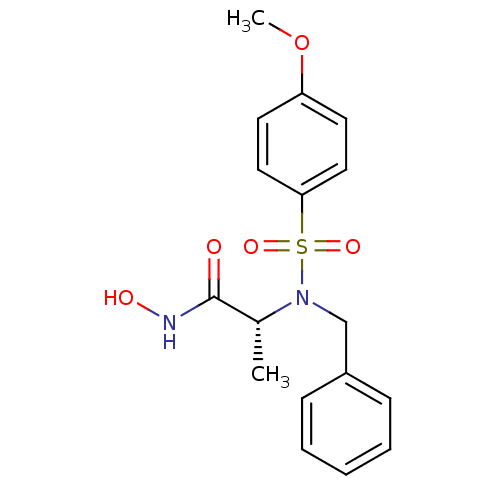 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13100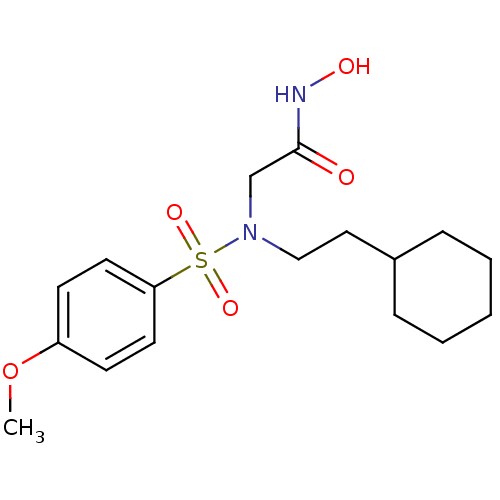 (2-[(2-cyclohexylethyl)(4-methoxybenzene)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13135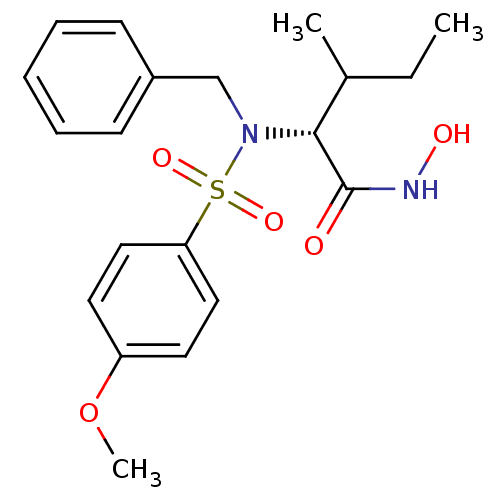 ((2R,3S)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397170 (CHEMBL2172144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397188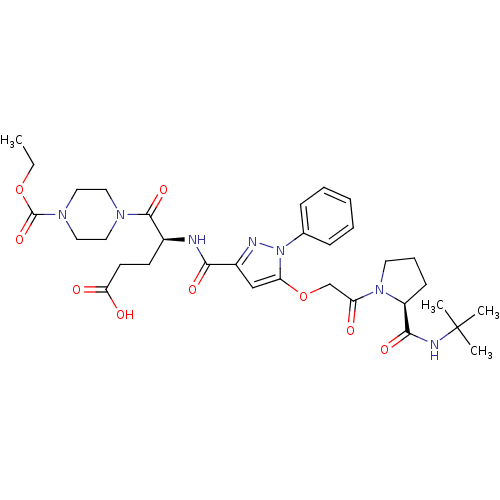 (CHEMBL2172269) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397182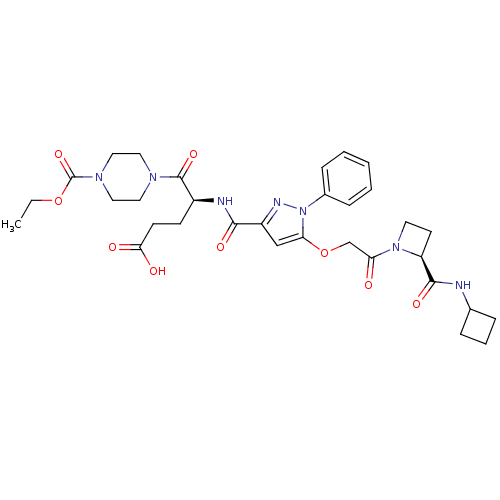 (CHEMBL2172157) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397154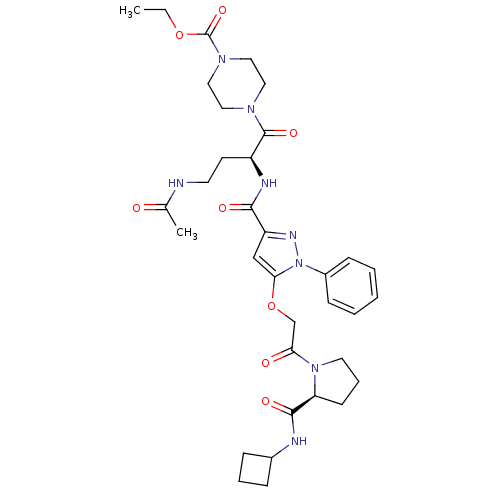 (CHEMBL2172131) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13141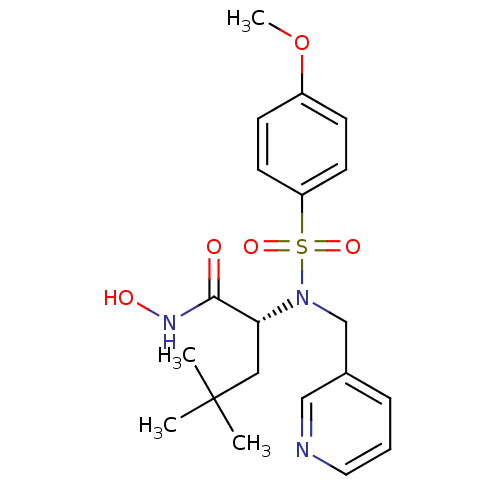 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13134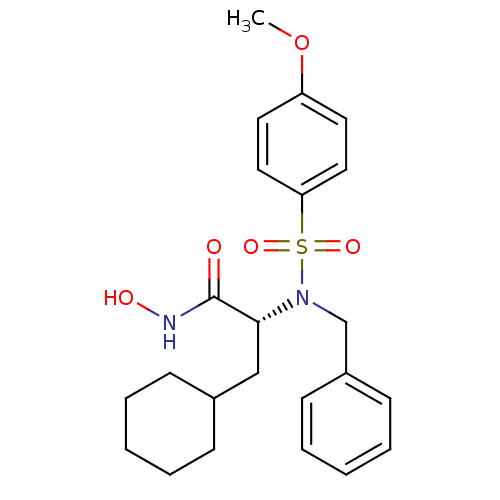 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13137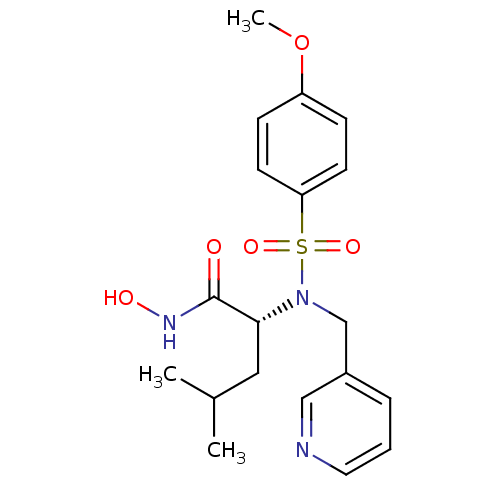 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397159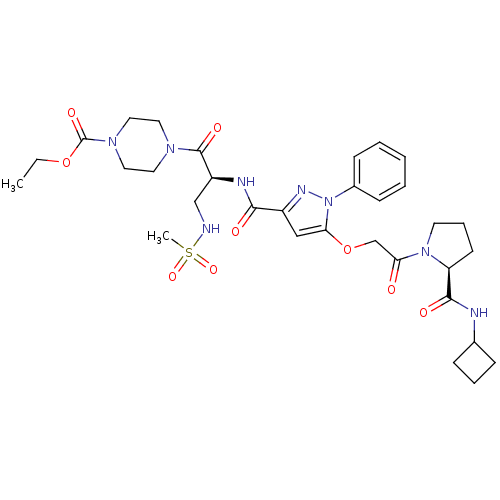 (CHEMBL2172155) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13131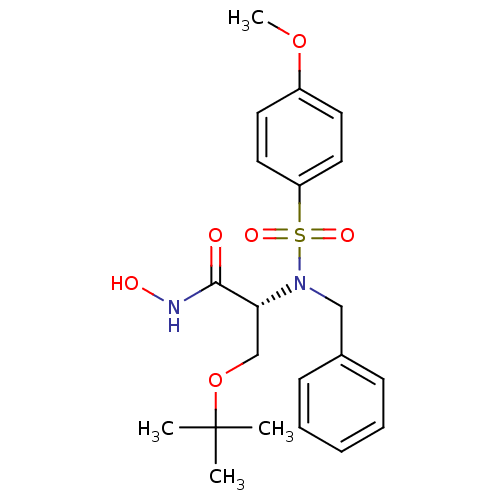 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-3-(te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13136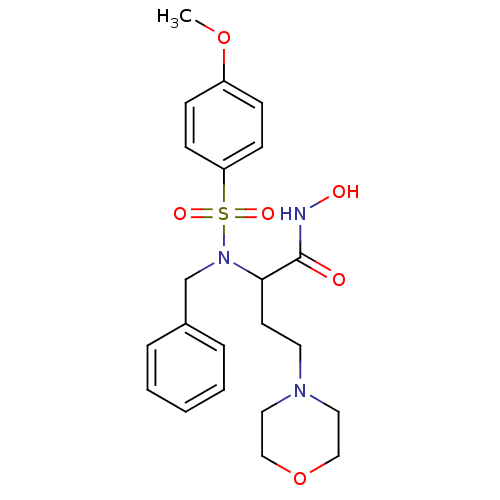 (2-[benzyl(4-methoxybenzene)sulfonamido]-N-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397160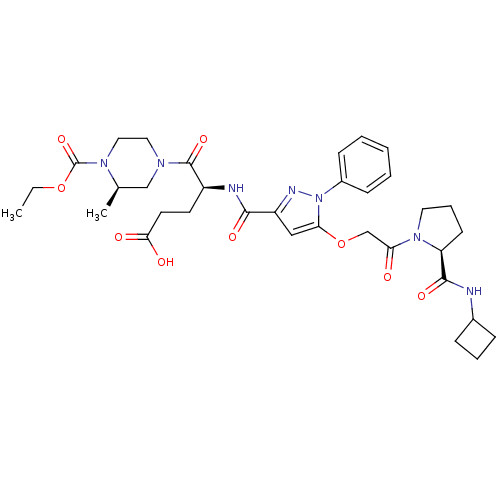 (CHEMBL2172154) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397162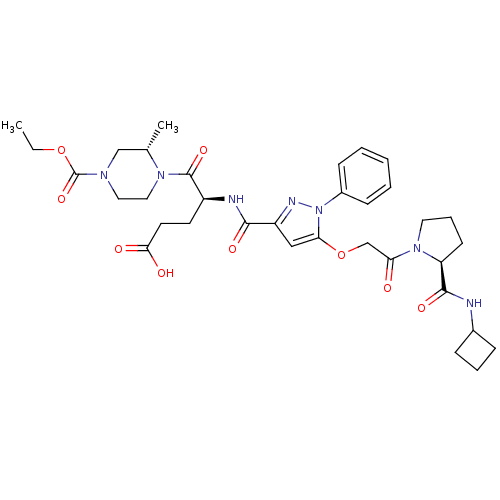 (CHEMBL2172152) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397178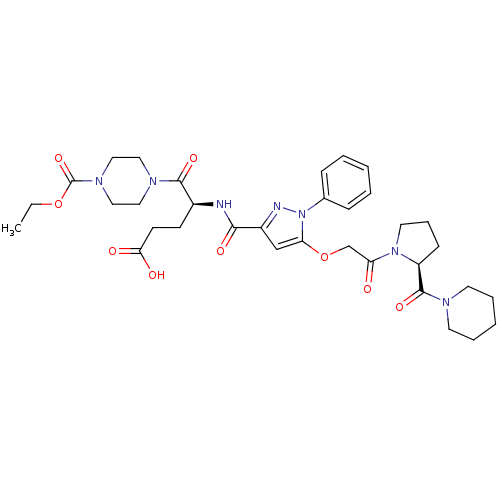 (CHEMBL2172161) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397177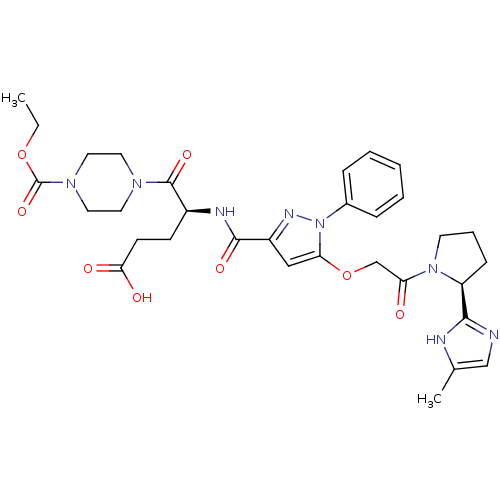 (CHEMBL2172162) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397189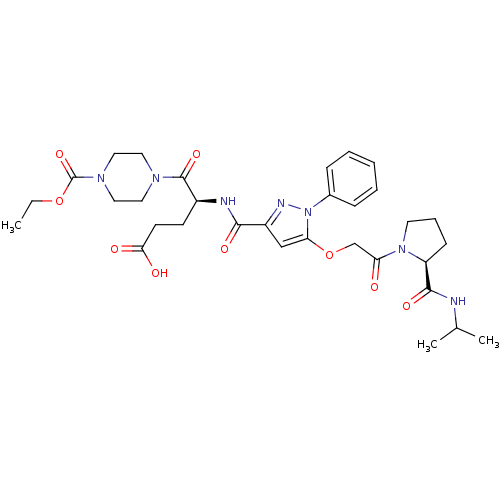 (CHEMBL2172268) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 159 total ) | Next | Last >> |