 Found 298 hits with Last Name = 'kachur' and Initial = 'jf'
Found 298 hits with Last Name = 'kachur' and Initial = 'jf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50403547

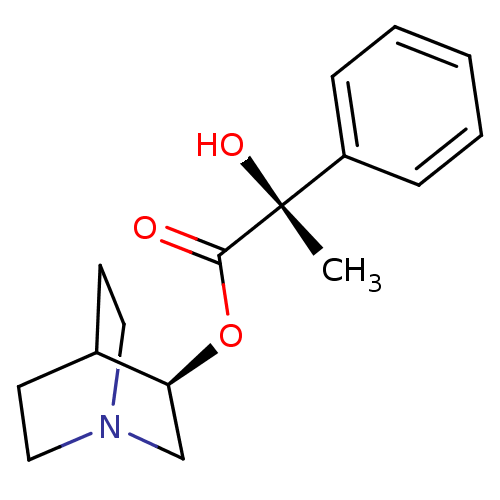
(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50405720
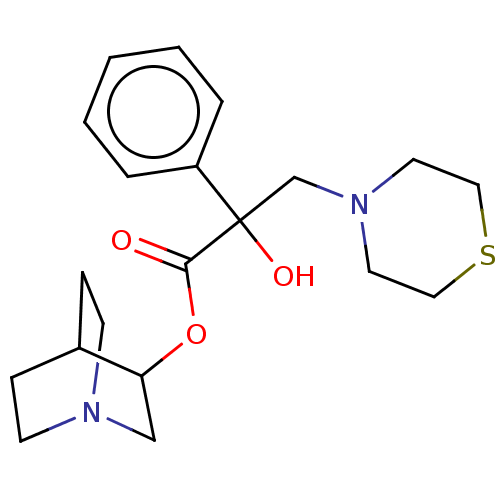
(CHEMBL2115342)Show SMILES C[C@](O)(C(=O)O[C@H]1CN2CCC1CC2)c1ccccc1 |wU:1.2,wD:6.5,1.1,THB:5:6:10.9:12.13,(-.9,-11.74,;-.13,-10.4,;.64,-9.06,;1.2,-11.18,;1.2,-12.72,;2.53,-10.4,;4.08,-10.4,;4.03,-11.69,;5.38,-10.64,;6.78,-11.57,;6.74,-10.39,;5.41,-9.62,;5.49,-8.49,;4.44,-9.01,;-1.47,-9.63,;-2.81,-10.4,;-4.14,-9.63,;-4.14,-8.07,;-2.81,-7.3,;-1.47,-8.07,)| Show InChI InChI=1S/C16H21NO3/c1-16(19,13-5-3-2-4-6-13)15(18)20-14-11-17-9-7-12(14)8-10-17/h2-6,12,14,19H,7-11H2,1H3/t14-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228362
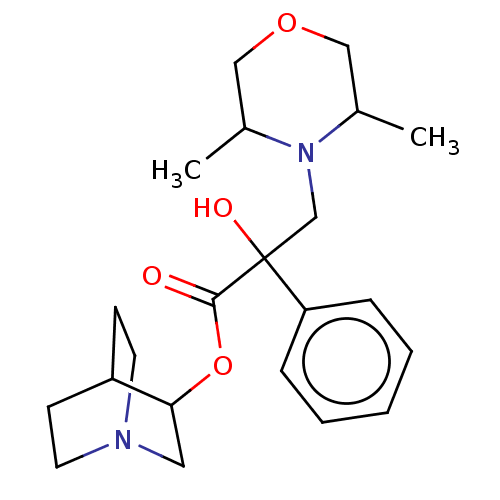
(CHEMBL1202960)Show SMILES Cl.Cl.OC(CN1CCSCC1)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |(10.34,-1.66,;7.78,-1.66,;-1.56,-.37,;-1.57,-1.6,;-2.91,-2.36,;-2.93,-3.9,;-4.27,-4.66,;-4.28,-6.2,;-2.96,-6.98,;-1.62,-6.22,;-1.6,-4.68,;-.25,-2.38,;-.26,-3.62,;1.1,-1.63,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;-2.9,-.82,;-4.24,-1.57,;-5.57,-.79,;-5.55,.75,;-4.21,1.5,;-2.88,.72,)| Show InChI InChI=1S/C20H28N2O3S.2ClH/c23-19(25-18-14-21-8-6-16(18)7-9-21)20(24,17-4-2-1-3-5-17)15-22-10-12-26-13-11-22;;/h1-5,16,18,24H,6-15H2;2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228375

(CHEMBL1202955)Show SMILES Cl.Cl.OC(CN1CCOCC1)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |(10.34,-1.66,;7.78,-1.66,;-1.56,-.37,;-1.57,-1.6,;-2.91,-2.36,;-2.93,-3.9,;-4.27,-4.66,;-4.28,-6.2,;-2.96,-6.98,;-1.62,-6.22,;-1.6,-4.68,;-.25,-2.38,;-.26,-3.62,;1.1,-1.63,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;-2.9,-.82,;-4.24,-1.57,;-5.57,-.79,;-5.55,.75,;-4.21,1.5,;-2.88,.72,)| Show InChI InChI=1S/C20H28N2O4.2ClH/c23-19(26-18-14-21-8-6-16(18)7-9-21)20(24,17-4-2-1-3-5-17)15-22-10-12-25-13-11-22;;/h1-5,16,18,24H,6-15H2;2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228357
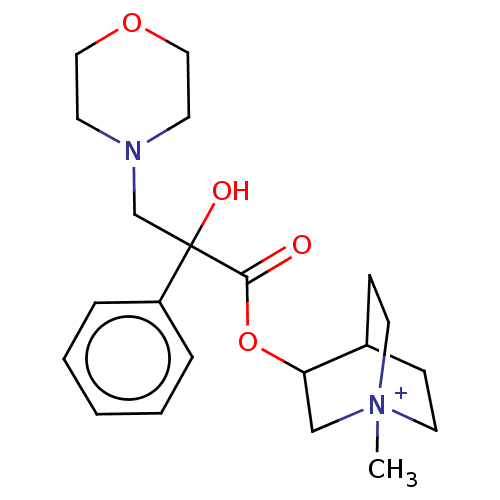
(CHEMBL1202954)Show SMILES Cl.Cl.CC1COCC(C)N1CC(O)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |(10.34,-1.66,;7.78,-1.66,;-.53,-4.07,;-1.6,-4.68,;-1.62,-6.22,;-2.96,-6.98,;-4.28,-6.2,;-4.27,-4.66,;-5.33,-4.03,;-2.93,-3.9,;-2.91,-2.36,;-1.57,-1.6,;-1.56,-.37,;-.25,-2.38,;-.26,-3.62,;1.1,-1.63,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;-2.9,-.82,;-4.24,-1.57,;-5.57,-.79,;-5.55,.75,;-4.21,1.5,;-2.88,.72,)| Show InChI InChI=1S/C22H32N2O4.2ClH/c1-16-13-27-14-17(2)24(16)15-22(26,19-6-4-3-5-7-19)21(25)28-20-12-23-10-8-18(20)9-11-23;;/h3-7,16-18,20,26H,8-15H2,1-2H3;2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228377

(CHEMBL1202958)Show SMILES Cl.OC(CN1CCNCC1)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |(7.78,-1.66,;-1.56,-.37,;-1.57,-1.6,;-2.91,-2.36,;-2.93,-3.9,;-4.27,-4.66,;-4.28,-6.2,;-2.96,-6.98,;-1.62,-6.22,;-1.6,-4.68,;-.25,-2.38,;-.26,-3.62,;1.1,-1.63,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;-2.9,-.82,;-4.24,-1.57,;-5.57,-.79,;-5.55,.75,;-4.21,1.5,;-2.88,.72,)| Show InChI InChI=1S/C20H29N3O3.ClH/c24-19(26-18-14-22-10-6-16(18)7-11-22)20(25,17-4-2-1-3-5-17)15-23-12-8-21-9-13-23;/h1-5,16,18,21,25H,6-15H2;1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228354
(CHEMBL557216)Show SMILES [I-].C[N+]12CCC(CC1)C(C2)OC(=O)C(O)(CN1CCOCC1)c1ccccc1 |TLB:10:8:3.4:7.6,(16.62,-7.46,;13.88,-5.4,;13.9,-3.88,;15.47,-4.83,;16.27,-3.69,;14.73,-2.76,;14.72,-1.69,;13.9,-2.51,;12.54,-3.36,;12.25,-4.76,;11.5,-4.15,;10.01,-3.78,;9.57,-2.3,;8.93,-4.89,;7.84,-6.01,;10.05,-5.96,;10.13,-7.51,;11.5,-8.18,;11.58,-9.73,;10.29,-10.57,;8.93,-9.87,;8.85,-8.34,;7.81,-3.81,;6.33,-4.25,;5.22,-3.18,;5.59,-1.68,;7.07,-1.25,;8.18,-2.32,)| Show InChI InChI=1S/C21H31N2O4.HI/c1-23-11-7-17(8-12-23)19(15-23)27-20(24)21(25,18-5-3-2-4-6-18)16-22-9-13-26-14-10-22;/h2-6,17,19,25H,7-16H2,1H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228376

(CHEMBL320755)Show SMILES OC(CN1CCC2(CC1)OCCO2)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |THB:15:16:22.23:20.19,(6.52,-6.31,;7.62,-5.19,;8.74,-6.26,;8.83,-7.8,;7.54,-8.64,;7.61,-10.16,;8.98,-10.87,;10.27,-10.02,;10.18,-8.48,;7.66,-11.62,;7.63,-13.15,;10.29,-13.16,;10.3,-11.62,;8.69,-4.07,;8.26,-2.6,;10.18,-4.45,;11.22,-3.66,;10.94,-5.06,;12.58,-4.19,;12.58,-2.82,;13.41,-1.99,;13.41,-3.08,;14.95,-4,;14.15,-5.13,;6.5,-4.11,;5.03,-4.54,;3.9,-3.48,;4.28,-1.99,;5.76,-1.56,;6.87,-2.62,)| Show InChI InChI=1S/C23H32N2O5/c26-21(30-20-16-24-10-6-18(20)7-11-24)23(27,19-4-2-1-3-5-19)17-25-12-8-22(9-13-25)28-14-15-29-22/h1-5,18,20,27H,6-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228355
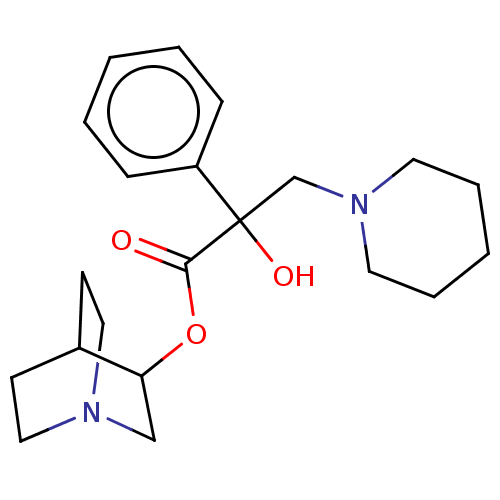
(CHEMBL1202956)Show SMILES Cl.Cl.OC(CN1CCCCC1)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |(10.34,-1.66,;7.78,-1.66,;-1.56,-.37,;-1.57,-1.6,;-2.91,-2.36,;-2.93,-3.9,;-4.27,-4.66,;-4.28,-6.2,;-2.96,-6.98,;-1.62,-6.22,;-1.6,-4.68,;-.25,-2.38,;-.26,-3.62,;1.1,-1.63,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;-2.9,-.82,;-4.24,-1.57,;-5.57,-.79,;-5.55,.75,;-4.21,1.5,;-2.88,.72,)| Show InChI InChI=1S/C21H30N2O3.2ClH/c24-20(26-19-15-22-13-9-17(19)10-14-22)21(25,18-7-3-1-4-8-18)16-23-11-5-2-6-12-23;;/h1,3-4,7-8,17,19,25H,2,5-6,9-16H2;2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228356

(CHEMBL1202957)Show SMILES Cl.CN1CCN(CC(O)(C(=O)OC2CN3CCC2CC3)c2ccccc2)CC1 |(7.78,-1.66,;-2.97,-8.21,;-2.96,-6.98,;-4.28,-6.2,;-4.27,-4.66,;-2.93,-3.9,;-2.91,-2.36,;-1.57,-1.6,;-1.56,-.37,;-.25,-2.38,;-.26,-3.62,;1.1,-1.63,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;-2.9,-.82,;-4.24,-1.57,;-5.57,-.79,;-5.55,.75,;-4.21,1.5,;-2.88,.72,;-1.6,-4.68,;-1.62,-6.22,)| Show InChI InChI=1S/C21H31N3O3.ClH/c1-22-11-13-24(14-12-22)16-21(26,18-5-3-2-4-6-18)20(25)27-19-15-23-9-7-17(19)8-10-23;/h2-6,17,19,26H,7-16H2,1H3;1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228353

(CHEMBL1202959)Show SMILES Cl.OC(CN1CCCC1)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |(7.78,-1.66,;-1.56,-.37,;-1.57,-1.6,;-2.91,-2.36,;-2.93,-3.9,;-4.18,-4.77,;-3.72,-6.24,;-2.18,-6.25,;-1.69,-4.79,;-.25,-2.38,;-.26,-3.62,;1.1,-1.63,;2.43,-2.41,;2.41,-4.03,;3.81,-4.83,;5.21,-4.01,;5.21,-2.38,;3.81,-1.57,;3.26,-2.74,;4.33,-3.38,;-2.9,-.82,;-4.24,-1.57,;-5.57,-.79,;-5.55,.75,;-4.21,1.5,;-2.88,.72,)| Show InChI InChI=1S/C20H28N2O3.ClH/c23-19(25-18-14-21-12-8-16(18)9-13-21)20(24,15-22-10-4-5-11-22)17-6-2-1-3-7-17;/h1-3,6-7,16,18,24H,4-5,8-15H2;1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50228378
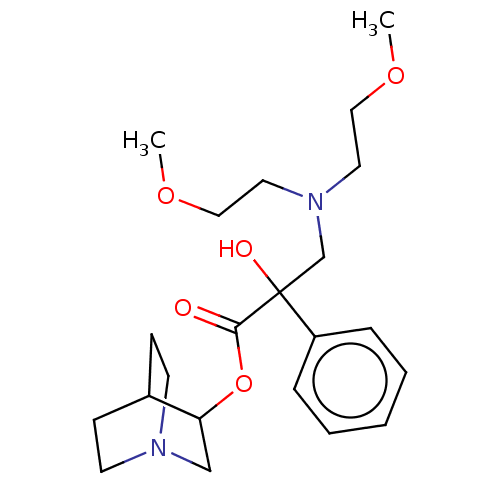
(CHEMBL1202961)Show SMILES Cl.Cl.COCCN(CCOC)CC(O)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |(10.34,.26,;7.77,.26,;3.73,-5.95,;3.74,-4.72,;2.41,-3.94,;2.42,-2.4,;1.1,-1.62,;1.11,-.08,;2.44,.68,;2.45,2.22,;3.35,2.73,;-.24,-2.38,;-1.57,-1.6,;-.5,-.99,;-1.56,-.06,;-2.62,.57,;-.21,.7,;-.2,2.24,;-1.59,3.06,;-1.55,4.67,;-.13,5.45,;1.26,4.61,;1.24,2.99,;-.05,3.12,;-.04,4.37,;-2.91,-2.36,;-2.93,-3.9,;-4.27,-4.65,;-5.59,-3.87,;-5.58,-2.33,;-4.24,-1.57,)| Show InChI InChI=1S/C22H34N2O5.2ClH/c1-27-14-12-24(13-15-28-2)17-22(26,19-6-4-3-5-7-19)21(25)29-20-16-23-10-8-18(20)9-11-23;;/h3-7,18,20,26H,8-17H2,1-2H3;2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB from rat ileum Muscarinic acetylcholine receptor |
J Med Chem 33: 307-10 (1990)
BindingDB Entry DOI: 10.7270/Q2CJ8GQS |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50213060
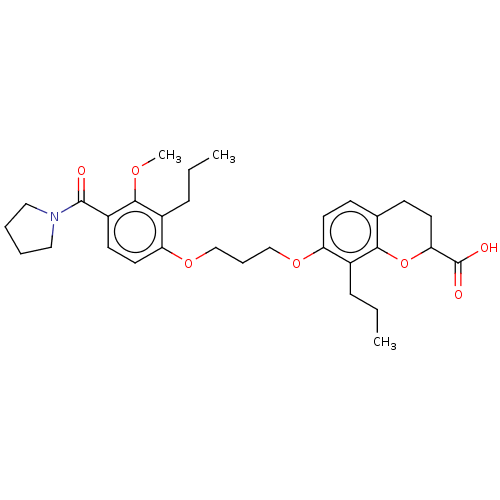
(CHEMBL355401 | SC-50135)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(=O)N2CCCC2)c1OC Show InChI InChI=1S/C31H41NO7/c1-4-9-22-25(14-11-21-12-15-27(31(34)35)39-28(21)22)37-19-8-20-38-26-16-13-24(29(36-3)23(26)10-5-2)30(33)32-17-6-7-18-32/h11,13-14,16,27H,4-10,12,15,17-20H2,1-3H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50284125

(7-[3-(4-Isopropylcarbamoyl-3-methoxy-2-propyl-phen...)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(=O)NC(C)C)c1OC Show InChI InChI=1S/C30H41NO7/c1-6-9-21-24(14-11-20-12-15-26(30(33)34)38-27(20)21)36-17-8-18-37-25-16-13-23(29(32)31-19(3)4)28(35-5)22(25)10-7-2/h11,13-14,16,19,26H,6-10,12,15,17-18H2,1-5H3,(H,31,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50284122
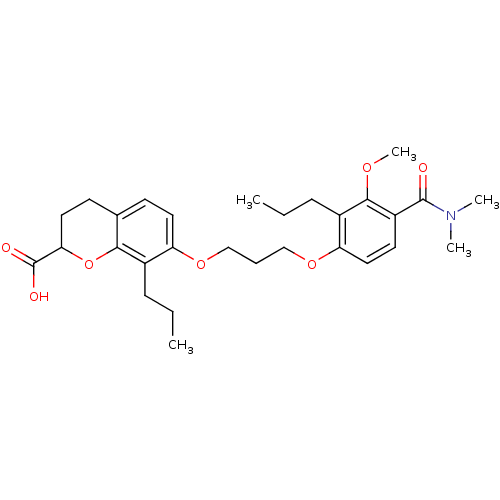
(7-[3-(4-Dimethylcarbamoyl-3-methoxy-2-propyl-pheno...)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(=O)N(C)C)c1OC Show InChI InChI=1S/C29H39NO7/c1-6-9-20-23(14-11-19-12-15-25(29(32)33)37-26(19)20)35-17-8-18-36-24-16-13-22(28(31)30(3)4)27(34-5)21(24)10-7-2/h11,13-14,16,25H,6-10,12,15,17-18H2,1-5H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116537
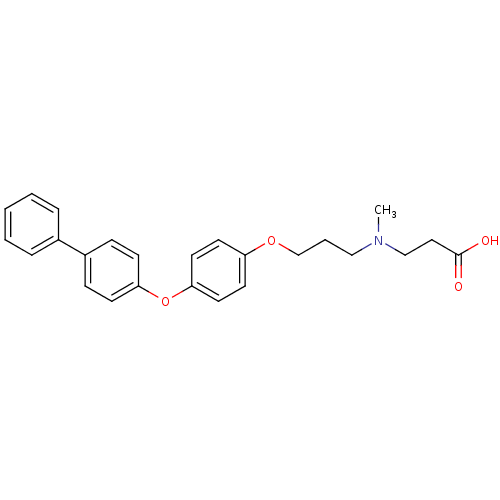
(3-({3-[4-(Biphenyl-4-yloxy)-phenoxy]-propyl}-methy...)Show SMILES CN(CCCOc1ccc(Oc2ccc(cc2)-c2ccccc2)cc1)CCC(O)=O Show InChI InChI=1S/C25H27NO4/c1-26(18-16-25(27)28)17-5-19-29-22-12-14-24(15-13-22)30-23-10-8-21(9-11-23)20-6-3-2-4-7-20/h2-4,6-15H,5,16-19H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human whole blood LTB-4 production (Leukotriene B-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125433
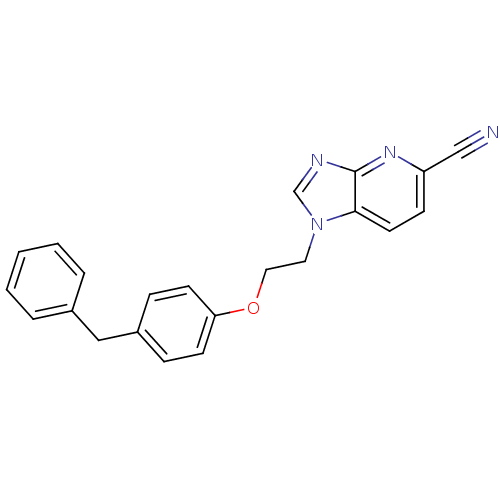
(1-[2-(4-Benzyl-phenoxy)-ethyl]-1H-imidazo[4,5-b]py...)Show InChI InChI=1S/C22H18N4O/c23-15-19-8-11-21-22(25-19)24-16-26(21)12-13-27-20-9-6-18(7-10-20)14-17-4-2-1-3-5-17/h1-11,16H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50284123
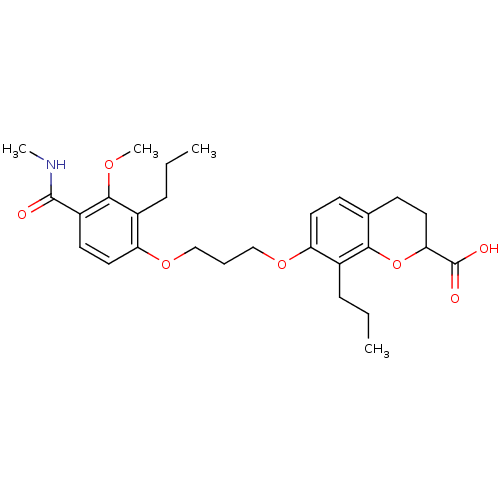
(7-[3-(3-Methoxy-4-methylcarbamoyl-2-propyl-phenoxy...)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(=O)NC)c1OC Show InChI InChI=1S/C28H37NO7/c1-5-8-19-22(13-10-18-11-14-24(28(31)32)36-25(18)19)34-16-7-17-35-23-15-12-21(27(30)29-3)26(33-4)20(23)9-6-2/h10,12-13,15,24H,5-9,11,14,16-17H2,1-4H3,(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116563
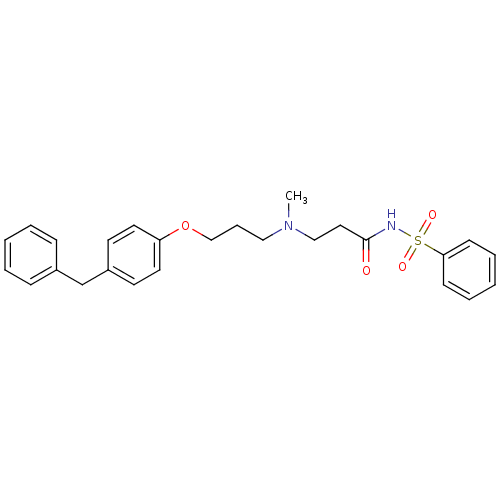
(CHEMBL323686 | N-(3-{[3-(4-Benzyl-phenoxy)-propyl]...)Show SMILES CN(CCCOc1ccc(Cc2ccccc2)cc1)CCC(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C26H30N2O4S/c1-28(19-17-26(29)27-33(30,31)25-11-6-3-7-12-25)18-8-20-32-24-15-13-23(14-16-24)21-22-9-4-2-5-10-22/h2-7,9-16H,8,17-21H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50001610

(7-[3-(4-Acetyl-3-methoxy-2-propyl-phenoxy)-propoxy...)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(C)=O)c1OC Show InChI InChI=1S/C28H36O7/c1-5-8-21-23(13-10-19-11-14-25(28(30)31)35-26(19)21)33-16-7-17-34-24-15-12-20(18(3)29)27(32-4)22(24)9-6-2/h10,12-13,15,25H,5-9,11,14,16-17H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116554
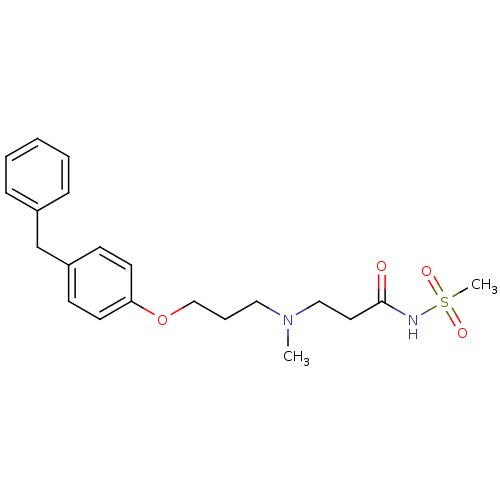
(CHEMBL119683 | N-(3-{[3-(4-Benzyl-phenoxy)-propyl]...)Show SMILES CN(CCCOc1ccc(Cc2ccccc2)cc1)CCC(=O)NS(C)(=O)=O Show InChI InChI=1S/C21H28N2O4S/c1-23(15-13-21(24)22-28(2,25)26)14-6-16-27-20-11-9-19(10-12-20)17-18-7-4-3-5-8-18/h3-5,7-12H,6,13-17H2,1-2H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116562
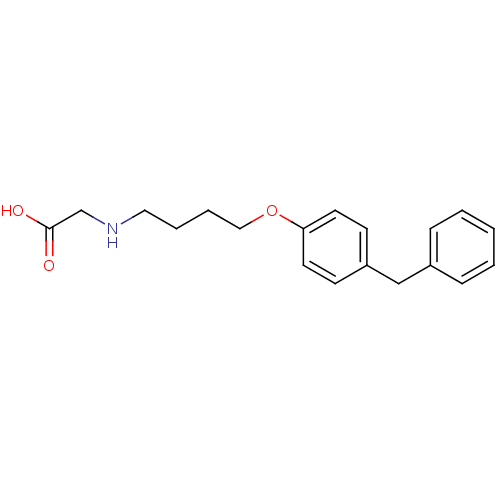
(CHEMBL117549 | [4-(4-Benzyl-phenoxy)-butylamino]-a...)Show InChI InChI=1S/C19H23NO3/c21-19(22)15-20-12-4-5-13-23-18-10-8-17(9-11-18)14-16-6-2-1-3-7-16/h1-3,6-11,20H,4-5,12-15H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116560

(3-{Methyl-[3-(4-thiophen-3-ylmethyl-phenoxy)-propy...)Show InChI InChI=1S/C18H23NO3S/c1-19(10-7-18(20)21)9-2-11-22-17-5-3-15(4-6-17)13-16-8-12-23-14-16/h3-6,8,12,14H,2,7,9-11,13H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50033746
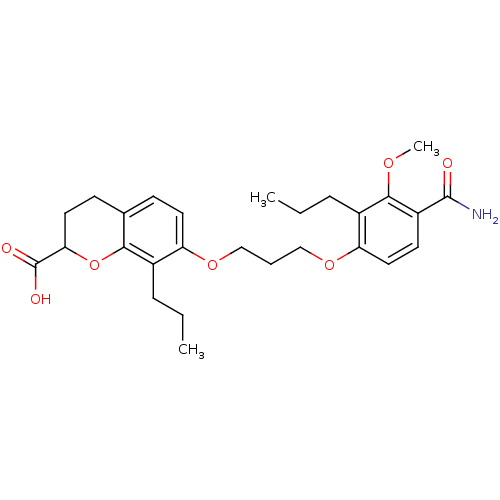
(7-[3-(4-Carbamoyl-3-methoxy-2-propyl-phenoxy)-prop...)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(N)=O)c1OC Show InChI InChI=1S/C27H35NO7/c1-4-7-18-21(12-9-17-10-13-23(27(30)31)35-24(17)18)33-15-6-16-34-22-14-11-20(26(28)29)25(32-3)19(22)8-5-2/h9,11-12,14,23H,4-8,10,13,15-16H2,1-3H3,(H2,28,29)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538
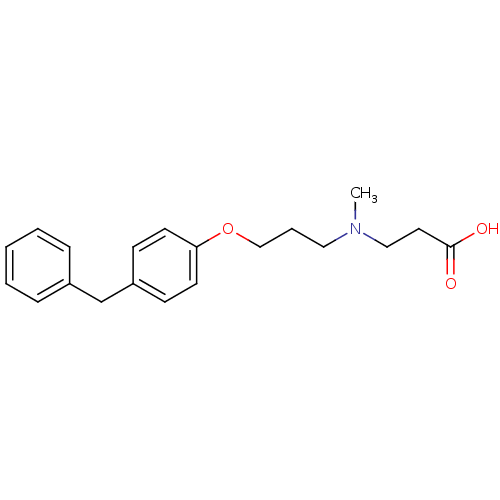
(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538

(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50033743
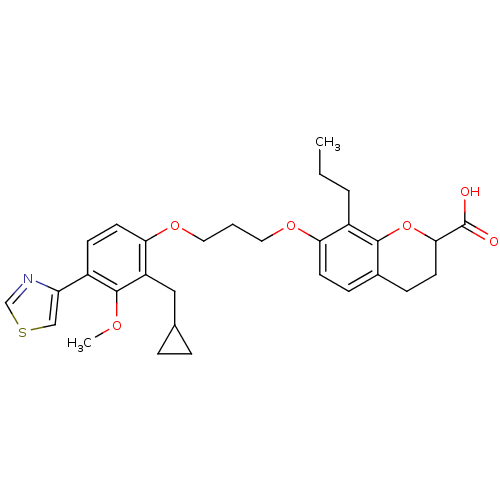
(7-[3-(2-Cyclopropylmethyl-3-methoxy-4-thiazol-4-yl...)Show SMILES CCCc1c(OCCCOc2ccc(-c3cscn3)c(OC)c2CC2CC2)ccc2CCC(Oc12)C(O)=O Show InChI InChI=1S/C30H35NO6S/c1-3-5-22-25(11-8-20-9-12-27(30(32)33)37-28(20)22)35-14-4-15-36-26-13-10-21(24-17-38-18-31-24)29(34-2)23(26)16-19-6-7-19/h8,10-11,13,17-19,27H,3-7,9,12,14-16H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA. |
J Med Chem 38: 858-68 (1995)
BindingDB Entry DOI: 10.7270/Q22806N0 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116544
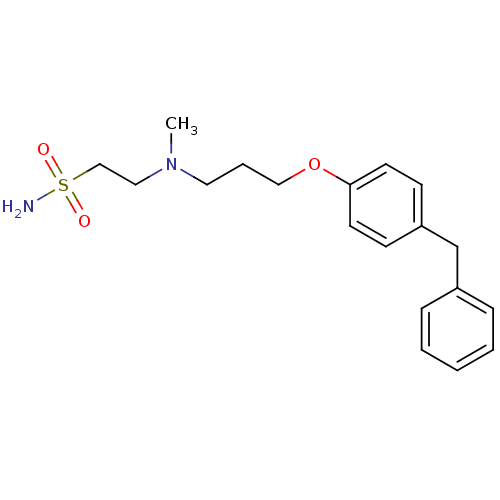
(2-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-eth...)Show InChI InChI=1S/C19H26N2O3S/c1-21(13-15-25(20,22)23)12-5-14-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116542

(3-{Methyl-[3-(4-thiophen-2-ylmethyl-phenoxy)-propy...)Show InChI InChI=1S/C18H23NO3S/c1-19(11-9-18(20)21)10-3-12-22-16-7-5-15(6-8-16)14-17-4-2-13-23-17/h2,4-8,13H,3,9-12,14H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50105264
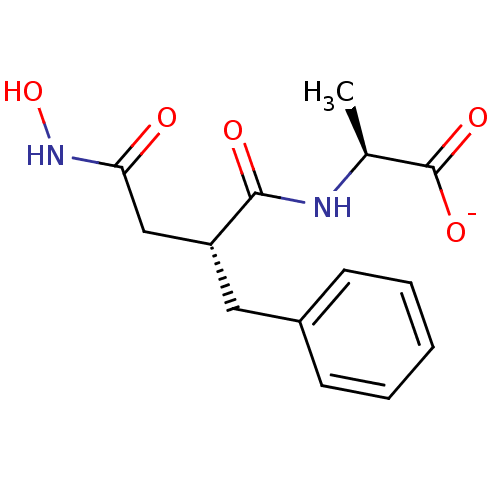
(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50033744

(7-{3-[3-Methoxy-2-propyl-4-(3H-[1,2,3]triazol-4-yl...)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(-c2c[nH]nn2)c1OC Show InChI InChI=1S/C28H35N3O6/c1-4-7-20-23(12-9-18-10-13-25(28(32)33)37-26(18)20)35-15-6-16-36-24-14-11-19(22-17-29-31-30-22)27(34-3)21(24)8-5-2/h9,11-12,14,17,25H,4-8,10,13,15-16H2,1-3H3,(H,32,33)(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA. |
J Med Chem 38: 858-68 (1995)
BindingDB Entry DOI: 10.7270/Q22806N0 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50284121
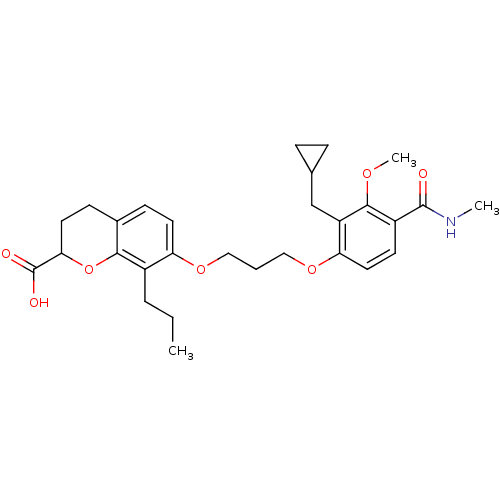
(7-[3-(2-Cyclopropylmethyl-3-methoxy-4-methylcarbam...)Show SMILES CCCc1c(OCCCOc2ccc(C(=O)NC)c(OC)c2CC2CC2)ccc2CCC(Oc12)C(O)=O Show InChI InChI=1S/C29H37NO7/c1-4-6-20-23(12-9-19-10-13-25(29(32)33)37-26(19)20)35-15-5-16-36-24-14-11-21(28(31)30-2)27(34-3)22(24)17-18-7-8-18/h9,11-12,14,18,25H,4-8,10,13,15-17H2,1-3H3,(H,30,31)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085261

(CHEMBL163478 | [2-(4-Benzyl-phenoxy)-ethyl]-dimeth...)Show InChI InChI=1S/C17H21NO/c1-18(2)12-13-19-17-10-8-16(9-11-17)14-15-6-4-3-5-7-15/h3-11H,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085288
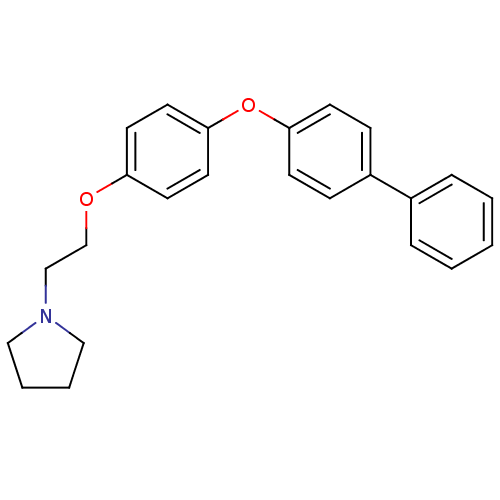
(1-{2-[4-(Biphenyl-4-yloxy)-phenoxy]-ethyl}-pyrroli...)Show InChI InChI=1S/C24H25NO2/c1-2-6-20(7-3-1)21-8-10-23(11-9-21)27-24-14-12-22(13-15-24)26-19-18-25-16-4-5-17-25/h1-3,6-15H,4-5,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116548
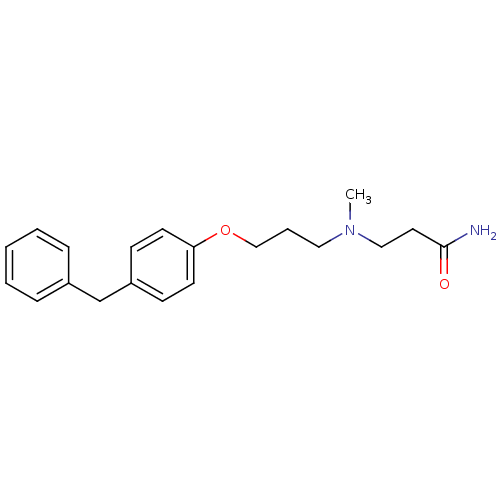
(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H26N2O2/c1-22(14-12-20(21)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H2,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085282

(5-Benzyl-2-(2-pyrrolidin-1-yl-ethoxy)-pyridine | C...)Show InChI InChI=1S/C18H22N2O/c1-2-6-16(7-3-1)14-17-8-9-18(19-15-17)21-13-12-20-10-4-5-11-20/h1-3,6-9,15H,4-5,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116561

(3-[4-(4-Benzyl-phenoxy)-butylamino]-propionic acid...)Show InChI InChI=1S/C22H29NO3/c1-2-25-22(24)14-16-23-15-6-7-17-26-21-12-10-20(11-13-21)18-19-8-4-3-5-9-19/h3-5,8-13,23H,2,6-7,14-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125435

(1-[2-(4-Benzyl-phenoxy)-ethyl]-1H-imidazo[4,5-b]py...)Show InChI InChI=1S/C21H19N3O/c1-2-5-17(6-3-1)15-18-8-10-19(11-9-18)25-14-13-24-16-23-21-20(24)7-4-12-22-21/h1-12,16H,13-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50284124

(3-{(R)-7-[3-(2-Cyclopropylmethyl-3-methoxy-4-methy...)Show SMILES CCCc1c(OCCCOc2ccc(C(=O)NC)c(OC)c2CC2CC2)ccc2CC[C@H](CCC(O)=O)Oc12 Show InChI InChI=1S/C31H41NO7/c1-4-6-23-26(14-10-21-9-11-22(39-29(21)23)12-16-28(33)34)37-17-5-18-38-27-15-13-24(31(35)32-2)30(36-3)25(27)19-20-7-8-20/h10,13-15,20,22H,4-9,11-12,16-19H2,1-3H3,(H,32,35)(H,33,34)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085290
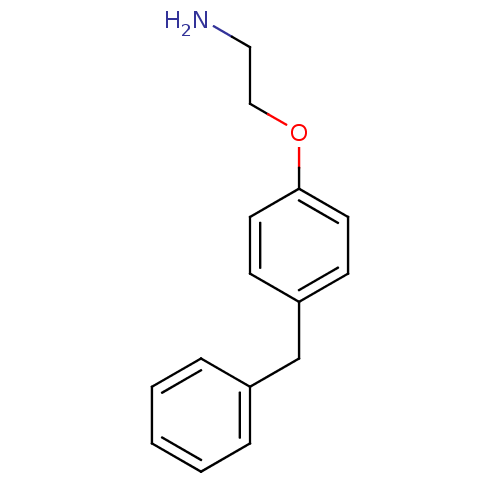
(2-(4-Benzyl-phenoxy)-ethylamine | 2-(4-benzylpheno...)Show InChI InChI=1S/C15H17NO/c16-10-11-17-15-8-6-14(7-9-15)12-13-4-2-1-3-5-13/h1-9H,10-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085277
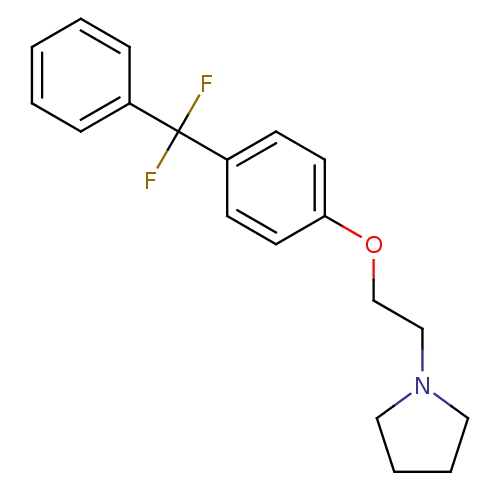
(1-{2-[4-(Difluoro-phenyl-methyl)-phenoxy]-ethyl}-p...)Show InChI InChI=1S/C19H21F2NO/c20-19(21,16-6-2-1-3-7-16)17-8-10-18(11-9-17)23-15-14-22-12-4-5-13-22/h1-3,6-11H,4-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Leukotriene A4 hydrolase |
J Med Chem 43: 721-35 (2000)
BindingDB Entry DOI: 10.7270/Q2CN74MZ |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50052017
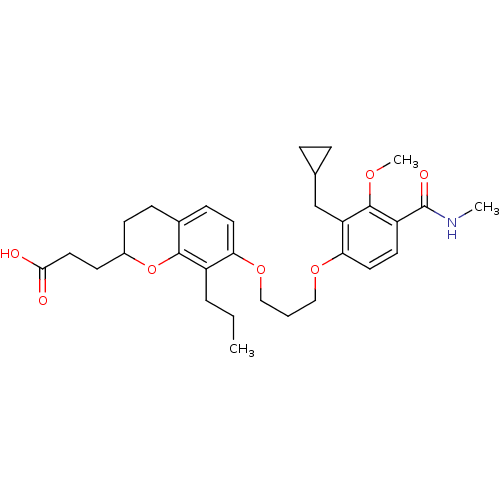
(3-{7-[3-(2-Cyclopropylmethyl-3-methoxy-4-methylcar...)Show SMILES CCCc1c(OCCCOc2ccc(C(=O)NC)c(OC)c2CC2CC2)ccc2CCC(CCC(O)=O)Oc12 Show InChI InChI=1S/C31H41NO7/c1-4-6-23-26(14-10-21-9-11-22(39-29(21)23)12-16-28(33)34)37-17-5-18-38-27-15-13-24(31(35)32-2)30(36-3)25(27)19-20-7-8-20/h10,13-15,20,22H,4-9,11-12,16-19H2,1-3H3,(H,32,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125420

(5-[2-(4-Benzyl-phenoxy)-ethyl]-5H-imidazo[4,5-c]py...)Show InChI InChI=1S/C21H19N3O/c1-2-4-17(5-3-1)14-18-6-8-19(9-7-18)25-13-12-24-11-10-20-21(15-24)23-16-22-20/h1-11,15-16H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human leukotriene A4 hydrolase. |
Bioorg Med Chem Lett 13: 1137-9 (2003)
BindingDB Entry DOI: 10.7270/Q2891587 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50033742
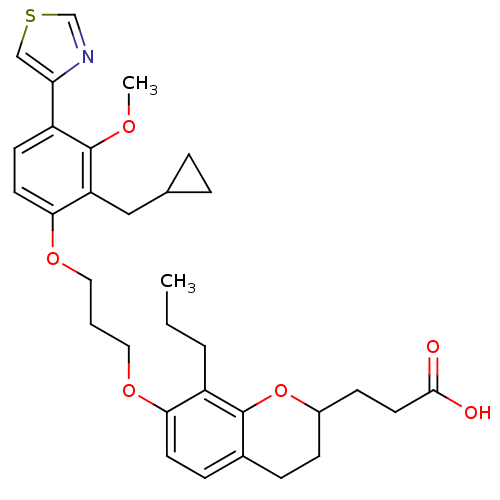
(3-{7-[3-(2-Cyclopropylmethyl-3-methoxy-4-thiazol-4...)Show SMILES CCCc1c(OCCCOc2ccc(-c3cscn3)c(OC)c2CC2CC2)ccc2CCC(CCC(O)=O)Oc12 Show InChI InChI=1S/C32H39NO6S/c1-3-5-25-28(13-9-22-8-10-23(39-31(22)25)11-15-30(34)35)37-16-4-17-38-29-14-12-24(27-19-40-20-33-27)32(36-2)26(29)18-21-6-7-21/h9,12-14,19-21,23H,3-8,10-11,15-18H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA. |
J Med Chem 38: 858-68 (1995)
BindingDB Entry DOI: 10.7270/Q22806N0 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116532
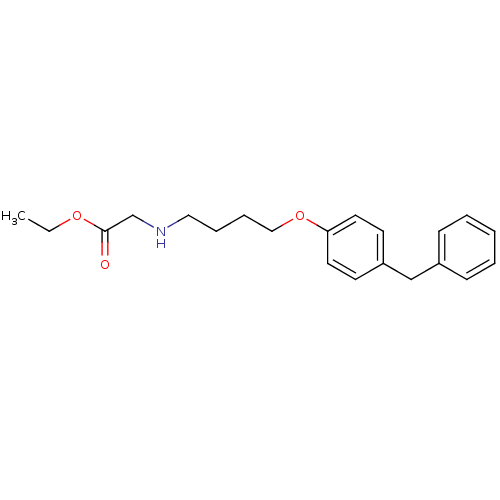
(CHEMBL420502 | [4-(4-Benzyl-phenoxy)-butylamino]-a...)Show InChI InChI=1S/C21H27NO3/c1-2-24-21(23)17-22-14-6-7-15-25-20-12-10-19(11-13-20)16-18-8-4-3-5-9-18/h3-5,8-13,22H,2,6-7,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121000
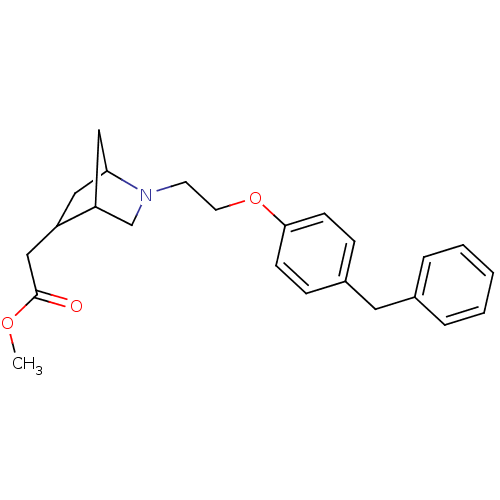
(CHEMBL116174 | {2-[2-(4-Benzyl-phenoxy)-ethyl]-2-a...)Show SMILES COC(=O)CC1CC2CC1CN2CCOc1ccc(Cc2ccccc2)cc1 |THB:4:5:11.10:8| Show InChI InChI=1S/C24H29NO3/c1-27-24(26)16-20-14-22-15-21(20)17-25(22)11-12-28-23-9-7-19(8-10-23)13-18-5-3-2-4-6-18/h2-10,20-22H,11-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50037390
(3-{(S)-2-(2-Carboxy-ethyl)-7-[3-(2-cyclopropylmeth...)Show SMILES CCCc1c(OCCCOc2ccc(C(=O)NC)c(OC)c2CC2CC2)ccc2CC[C@@H](CCC(O)=O)Oc12 Show InChI InChI=1S/C31H41NO7/c1-4-6-23-26(14-10-21-9-11-22(39-29(21)23)12-16-28(33)34)37-17-5-18-38-27-15-13-24(31(35)32-2)30(36-3)25(27)19-20-7-8-20/h10,13-15,20,22H,4-9,11-12,16-19H2,1-3H3,(H,32,35)(H,33,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121000

(CHEMBL116174 | {2-[2-(4-Benzyl-phenoxy)-ethyl]-2-a...)Show SMILES COC(=O)CC1CC2CC1CN2CCOc1ccc(Cc2ccccc2)cc1 |THB:4:5:11.10:8| Show InChI InChI=1S/C24H29NO3/c1-27-24(26)16-20-14-22-15-21(20)17-25(22)11-12-28-23-9-7-19(8-10-23)13-18-5-3-2-4-6-18/h2-10,20-22H,11-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of leukotriene A4 hydrolase in human recombinant assay |
Bioorg Med Chem Lett 12: 3383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2N8795W |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50033753

(4-[3-(2-Cyclopropylmethyl-3-methoxy-4-thiazol-4-yl...)Show SMILES CCCc1c2OC(C(O)=O)c2ccc1OCCCOc1ccc(-c2cscn2)c(OC)c1CC1CC1 Show InChI InChI=1S/C28H31NO6S/c1-3-5-19-23(11-9-20-26(19)35-27(20)28(30)31)33-12-4-13-34-24-10-8-18(22-15-36-16-29-22)25(32-2)21(24)14-17-6-7-17/h8-11,15-17,27H,3-7,12-14H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA. |
J Med Chem 38: 858-68 (1995)
BindingDB Entry DOI: 10.7270/Q22806N0 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116529

(3-{Methyl-[3-(4-thiophen-2-ylmethyl-phenoxy)-propy...)Show InChI InChI=1S/C19H25NO3S/c1-20(12-10-19(21)22-2)11-4-13-23-17-8-6-16(7-9-17)15-18-5-3-14-24-18/h3,5-9,14H,4,10-13,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase (LTA-4). |
J Med Chem 45: 3482-90 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1KMF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data