

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4690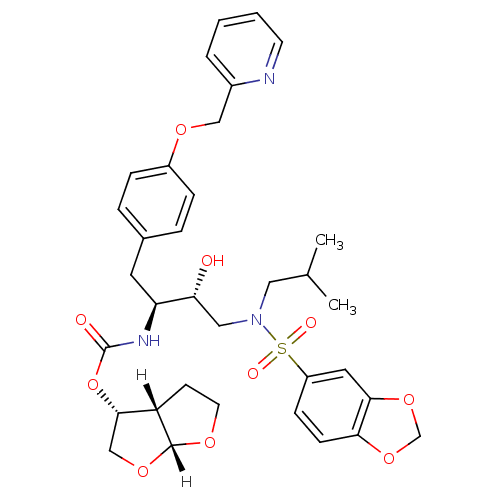 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00000600 | -82.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4690 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW0385 from HIV1 protease | Bioorg Med Chem Lett 16: 1788-94 (2006) Article DOI: 10.1016/j.bmcl.2006.01.035 BindingDB Entry DOI: 10.7270/Q2WS8V1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4689 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000130 | -80.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000150 | -80.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Antimicrob Agents Chemother 51: 3147-54 (2007) Article DOI: 10.1128/aac.00401-07 BindingDB Entry DOI: 10.7270/Q23R0WPV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW0385 from HIV1 protease | Bioorg Med Chem Lett 16: 1788-94 (2006) Article DOI: 10.1016/j.bmcl.2006.01.035 BindingDB Entry DOI: 10.7270/Q2WS8V1F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by fluorescent peptide substrate based assay | Antimicrob Agents Chemother 51: 3147-54 (2007) Article DOI: 10.1128/aac.00401-07 BindingDB Entry DOI: 10.7270/Q23R0WPV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4688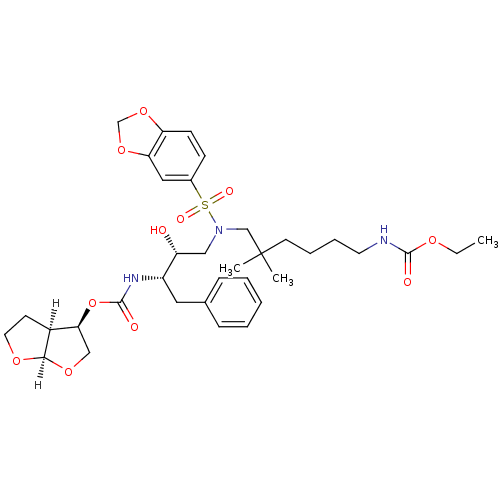 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000165 | -74.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4689 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000220 | -73.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4687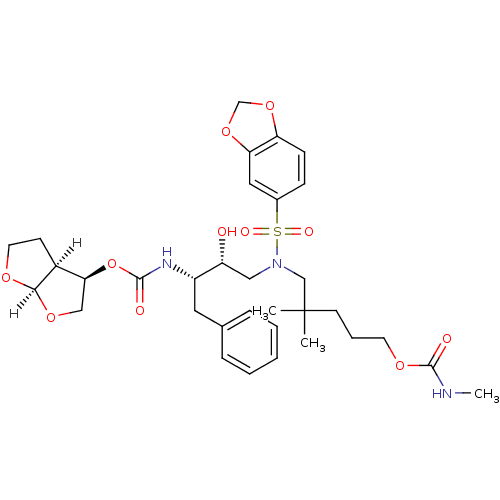 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000240 | -73.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4690 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000420 | -71.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.000750 | -70.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4688 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00120 | -69.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Antimicrob Agents Chemother 51: 3147-54 (2007) Article DOI: 10.1128/aac.00401-07 BindingDB Entry DOI: 10.7270/Q23R0WPV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4690 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00170 | -68.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00200 | -67.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4689 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00240 | -67.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4690 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00260 | -67.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00340 | -66.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4689 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00390 | -66.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4688 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00430 | -66.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4688 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00460 | -65.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479982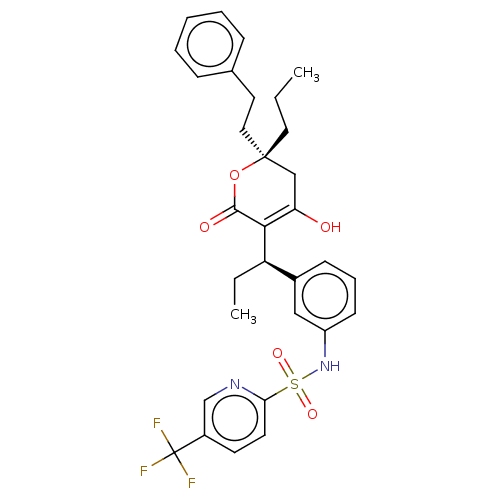 (Aptivus | CHEBI:63628 | Tipranavir | U-140690 | US...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by fluorescent peptide substrate based assay | Antimicrob Agents Chemother 51: 3147-54 (2007) Article DOI: 10.1128/aac.00401-07 BindingDB Entry DOI: 10.7270/Q23R0WPV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM4687 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | -61.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM4687 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0270 | -61.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Antimicrob Agents Chemother 51: 3147-54 (2007) Article DOI: 10.1128/aac.00401-07 BindingDB Entry DOI: 10.7270/Q23R0WPV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM4687 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0544 | -59.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0570 | -59.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50086433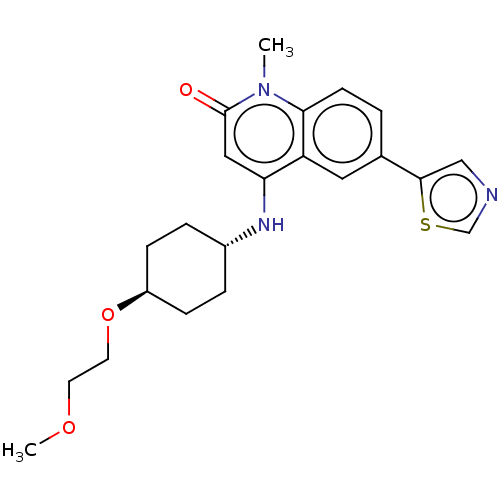 (CHEMBL3426034) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis | J Med Chem 58: 3548-71 (2015) Article DOI: 10.1021/jm502009h BindingDB Entry DOI: 10.7270/Q2NV9M00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50086438 (CHEMBL3426039) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis | J Med Chem 58: 3548-71 (2015) Article DOI: 10.1021/jm502009h BindingDB Entry DOI: 10.7270/Q2NV9M00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50086434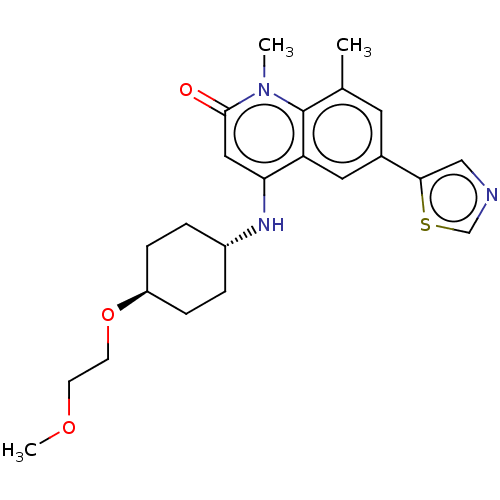 (CHEMBL3426035) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis | J Med Chem 58: 3548-71 (2015) Article DOI: 10.1021/jm502009h BindingDB Entry DOI: 10.7270/Q2NV9M00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085044 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418564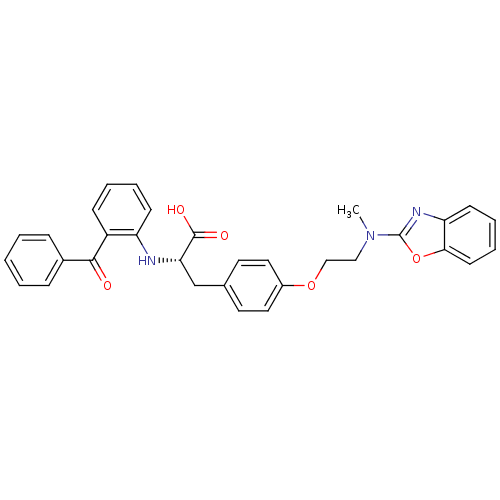 (CHEMBL423026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM21363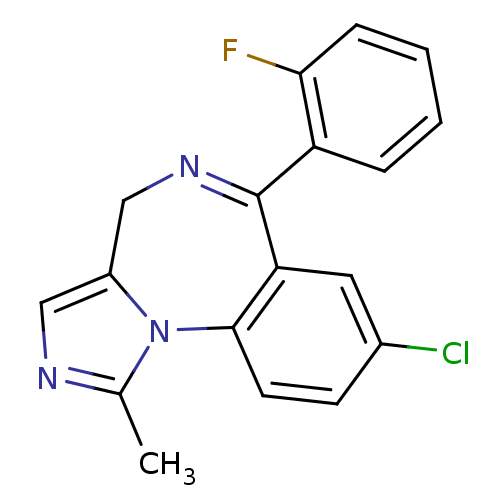 (12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,8-triaza...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30 | -50.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418568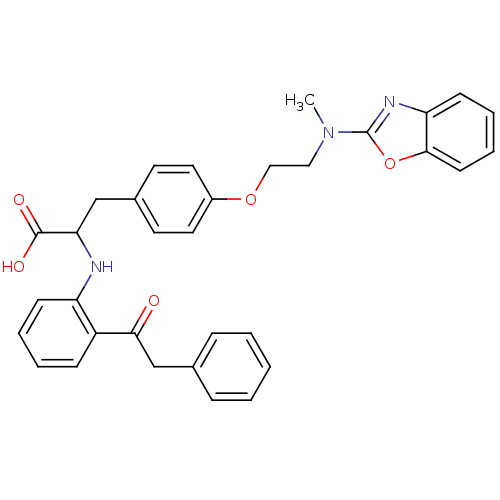 (CHEMBL146822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50000012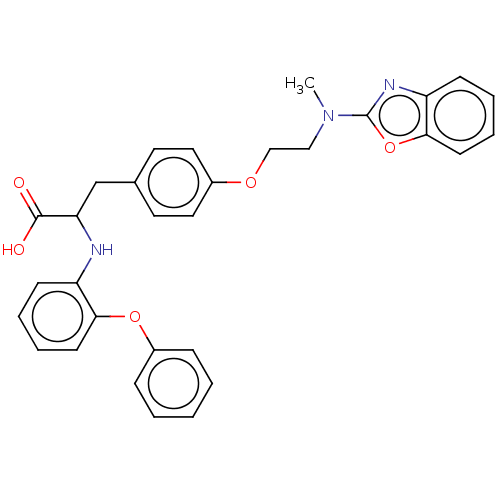 (CHEMBL147826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50064451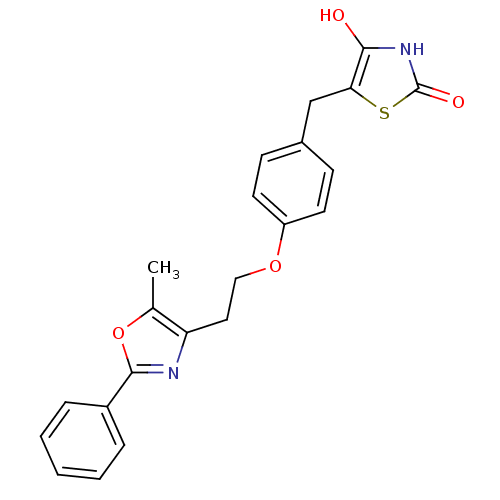 (5-{4-[2-(5-Methyl-2-phenyl-oxazol-4-yl)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539V] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4.90 | -48.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of... | Biochemistry 43: 14500-7 (2004) Article DOI: 10.1021/bi0488799 BindingDB Entry DOI: 10.7270/Q25M63WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418575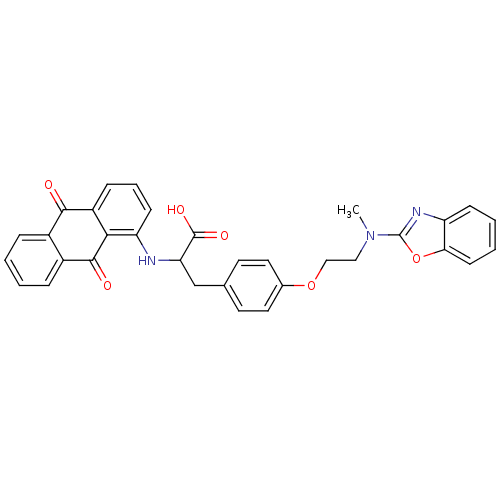 (CHEMBL358137) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418563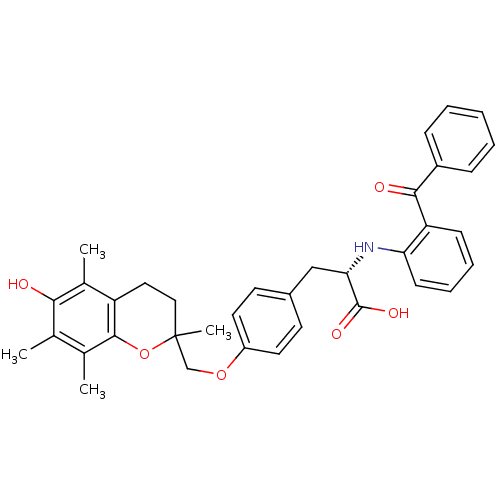 (CHEMBL148301) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418571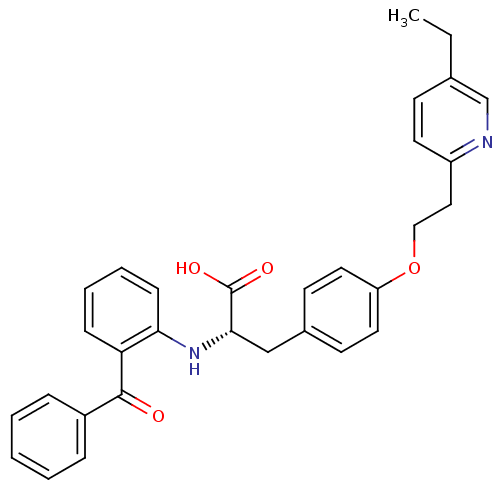 (CHEMBL346219) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120353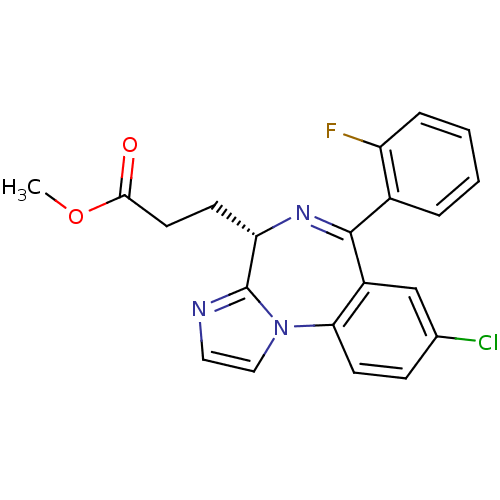 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-4H-3,5,10b-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120340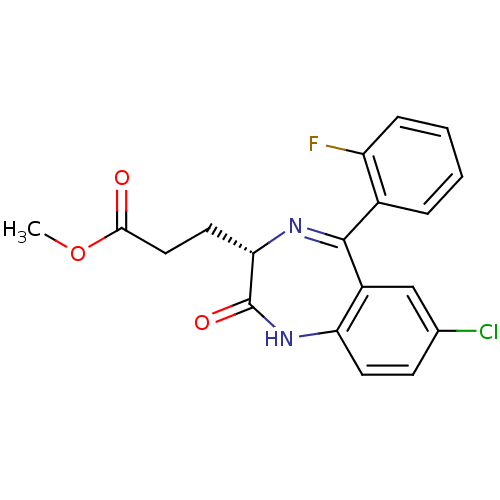 (3-[(S)-7-Chloro-5-(2-fluoro-phenyl)-2-oxo-2,3-dihy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120343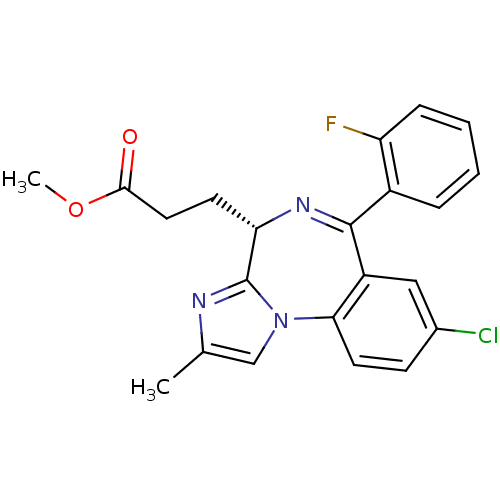 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-2-methyl-4H-3,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120360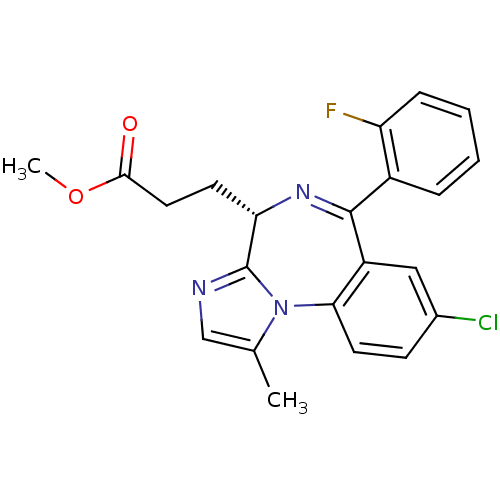 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-1-methyl-4H-3,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120344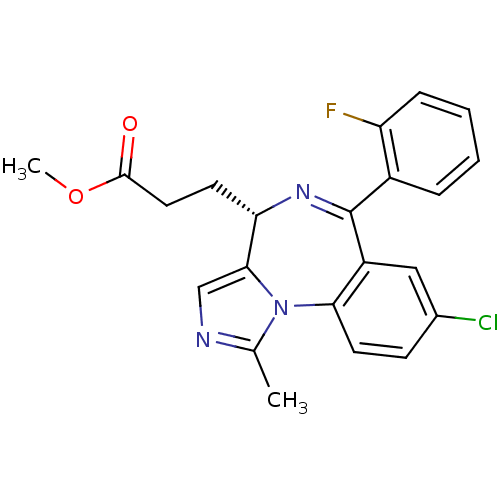 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-1-methyl-4H-2,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418557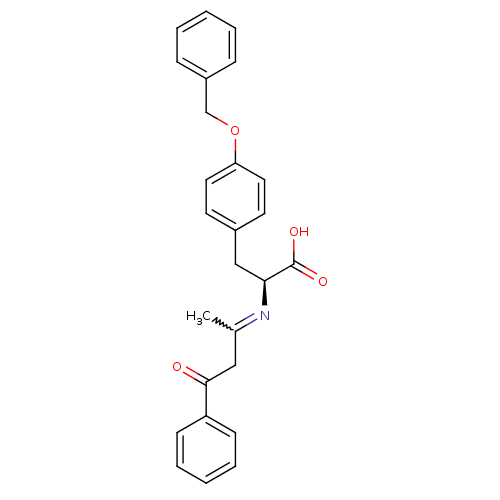 (CHEMBL1785028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418557 (CHEMBL1785028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Homo sapiens (Human)) | BDBM50086425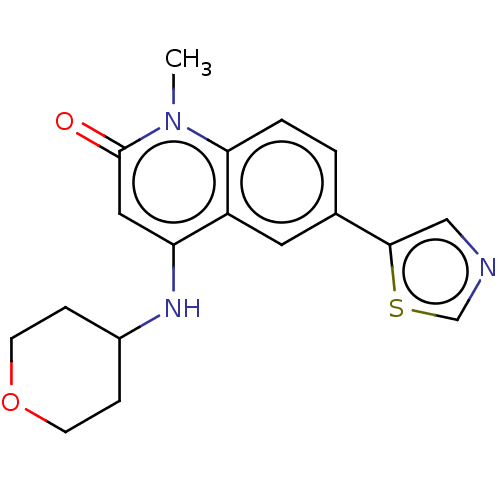 (CHEMBL3426030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis | J Med Chem 58: 3548-71 (2015) Article DOI: 10.1021/jm502009h BindingDB Entry DOI: 10.7270/Q2NV9M00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 489 total ) | Next | Last >> |