 Found 874 hits with Last Name = 'kamiya' and Initial = 'h'
Found 874 hits with Last Name = 'kamiya' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor 3
(RAT) | BDBM50252103
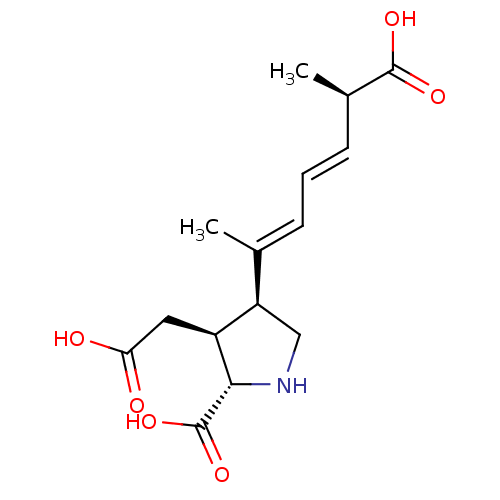
((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50002369
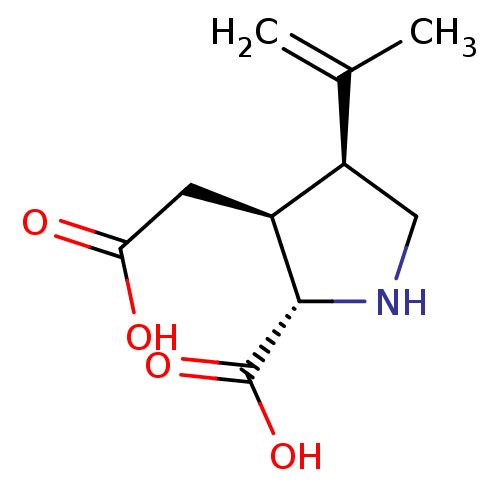
((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor 3
(RAT) | BDBM85740
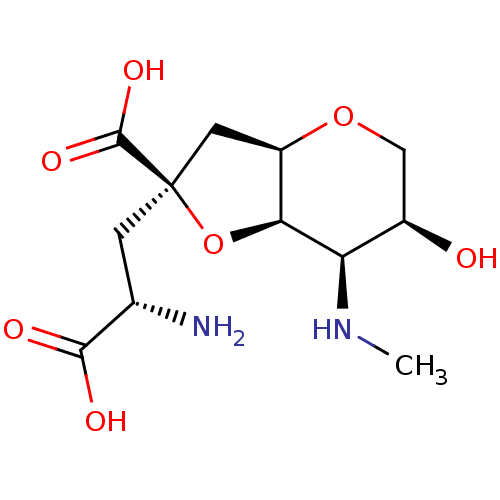
(Dysiherbaine)Show SMILES CN[C@H]1[C@H]2O[C@](C[C@H](N)C(O)=O)(C[C@H]2OC[C@H]1O)C(O)=O Show InChI InChI=1S/C12H20N2O7/c1-14-8-6(15)4-20-7-3-12(11(18)19,21-9(7)8)2-5(13)10(16)17/h5-9,14-15H,2-4,13H2,1H3,(H,16,17)(H,18,19)/t5-,6+,7+,8+,9-,12+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85740

(Dysiherbaine)Show SMILES CN[C@H]1[C@H]2O[C@](C[C@H](N)C(O)=O)(C[C@H]2OC[C@H]1O)C(O)=O Show InChI InChI=1S/C12H20N2O7/c1-14-8-6(15)4-20-7-3-12(11(18)19,21-9(7)8)2-5(13)10(16)17/h5-9,14-15H,2-4,13H2,1H3,(H,16,17)(H,18,19)/t5-,6+,7+,8+,9-,12+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 927 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50002343
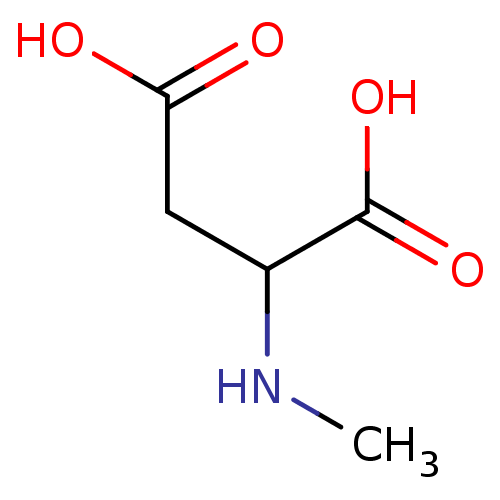
(2-Methylamino-succinic acid | CHEMBL275325 | NMDA)Show InChI InChI=1S/C5H9NO4/c1-6-3(5(9)10)2-4(7)8/h3,6H,2H2,1H3,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85740

(Dysiherbaine)Show SMILES CN[C@H]1[C@H]2O[C@](C[C@H](N)C(O)=O)(C[C@H]2OC[C@H]1O)C(O)=O Show InChI InChI=1S/C12H20N2O7/c1-14-8-6(15)4-20-7-3-12(11(18)19,21-9(7)8)2-5(13)10(16)17/h5-9,14-15H,2-4,13H2,1H3,(H,16,17)(H,18,19)/t5-,6+,7+,8+,9-,12+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Mus musculus) | BDBM50566781
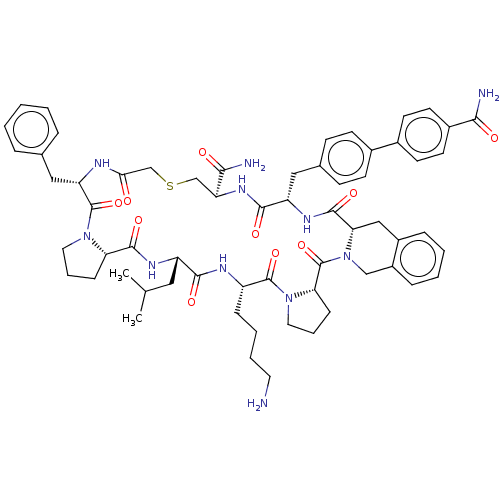
(CHEMBL4867273)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)-c2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50566781

(CHEMBL4867273)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)-c2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593249
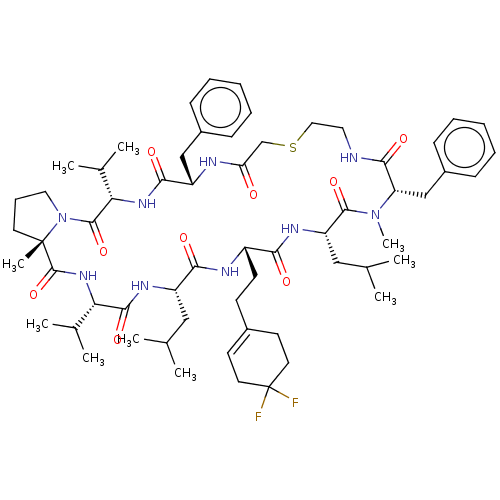
(CHEMBL5199870)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC2=CCC(F)(F)CC2)NC1=O)C(C)C)C(C)C |r,t:68| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593251
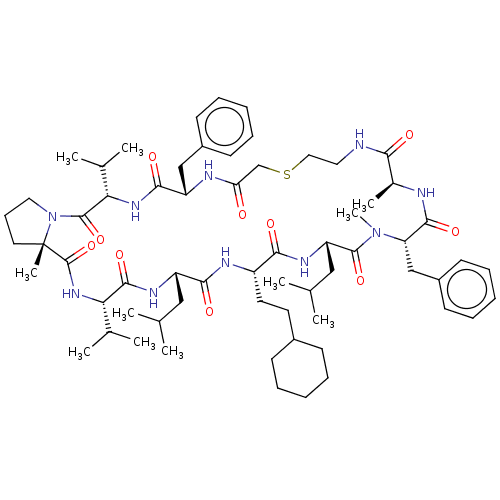
(CHEMBL5199920)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)[C@H](C)NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC2CCCCC2)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593248
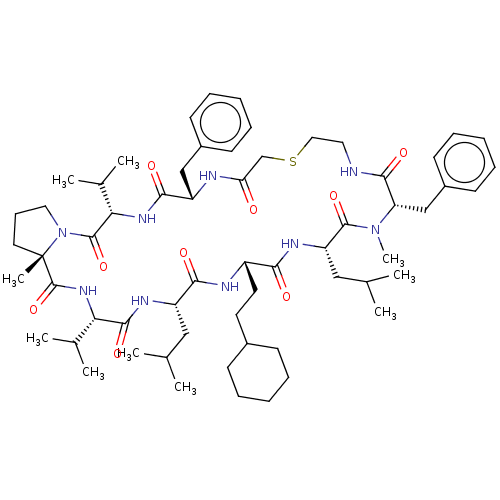
(CHEMBL5208859)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC2CCCCC2)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593250

(CHEMBL5180391)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)CNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC2CCCCC2)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50566783
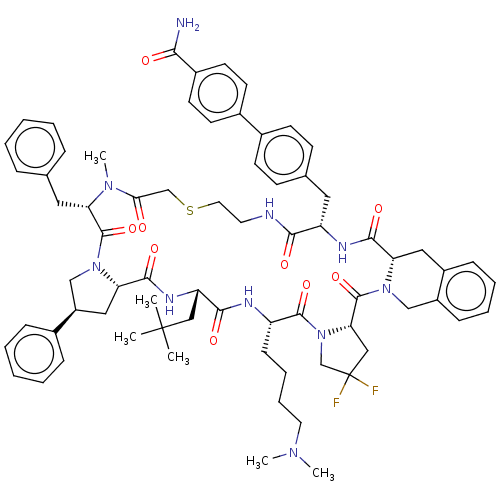
(CHEMBL4859806)Show SMILES CN(C)CCCC[C@@H]1NC(=O)[C@H](CC(C)(C)C)NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)CSCCNC(=O)[C@H](Cc2ccc(cc2)-c2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CC(F)(F)CN2C1=O)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593258
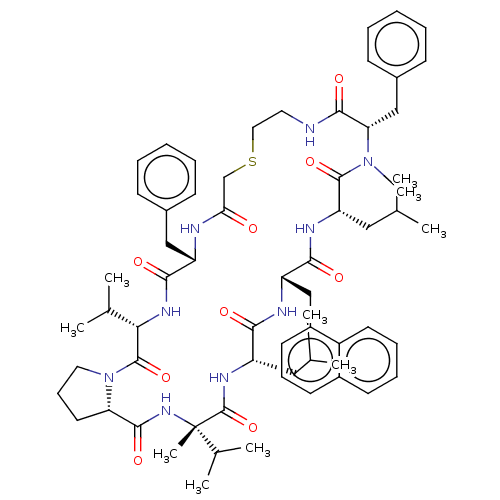
(CHEMBL5175402)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@](C)(NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2cccc3ccccc23)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593247
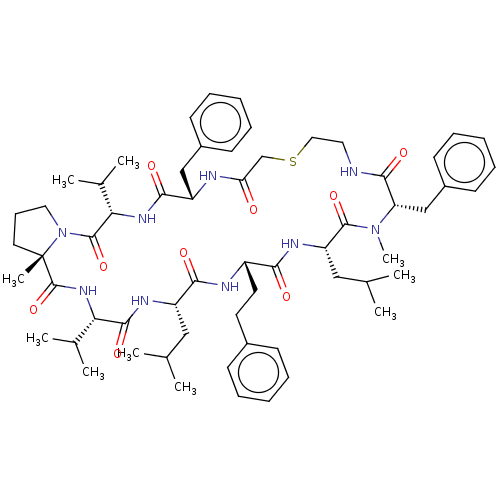
(CHEMBL5194642)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCc2ccccc2)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50566776
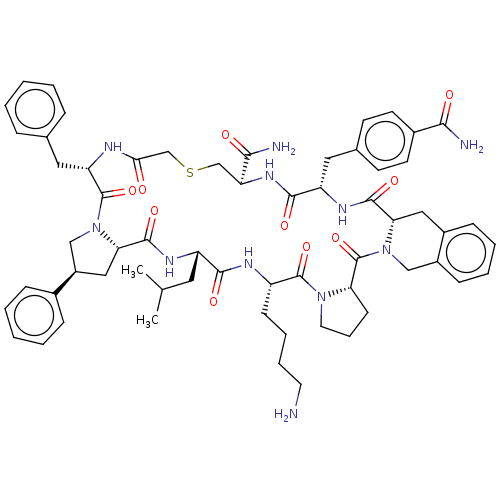
(CHEMBL4847740)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@H](Cc2ccccc2)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC1=O)C(N)=O)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Mus musculus) | BDBM50566783

(CHEMBL4859806)Show SMILES CN(C)CCCC[C@@H]1NC(=O)[C@H](CC(C)(C)C)NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)CSCCNC(=O)[C@H](Cc2ccc(cc2)-c2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CC(F)(F)CN2C1=O)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593255

(CHEMBL5201455)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSC(C)(C)CNC(=O)[C@H](C)NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC2CCCCC2)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593245
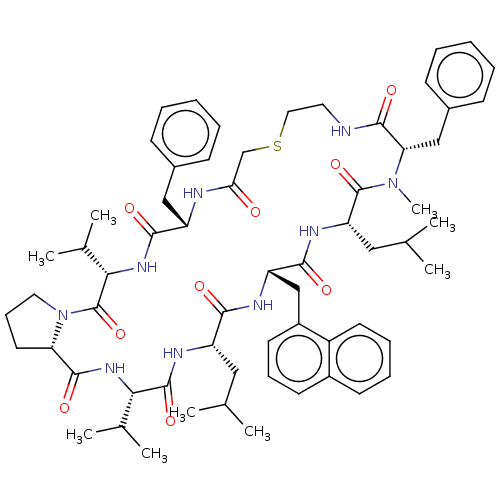
(CHEMBL5195759)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2cccc3ccccc23)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593246
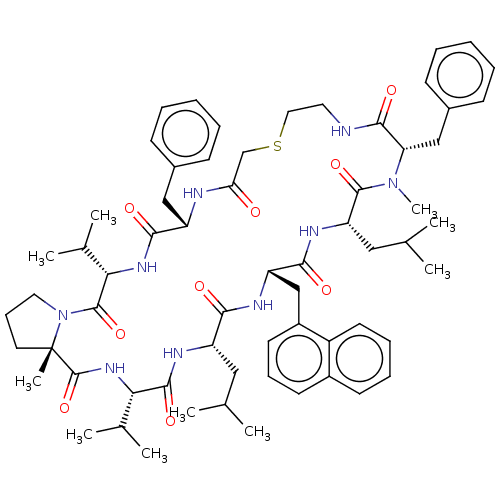
(CHEMBL5183416)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2cccc3ccccc23)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50566782
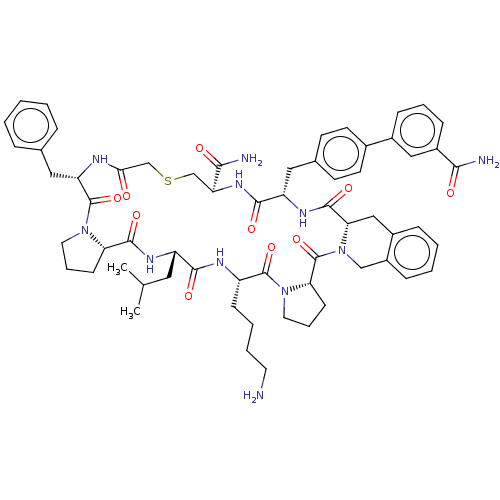
(CHEMBL4857394)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)-c2cccc(c2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Mus musculus) | BDBM50566782

(CHEMBL4857394)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)-c2cccc(c2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593257
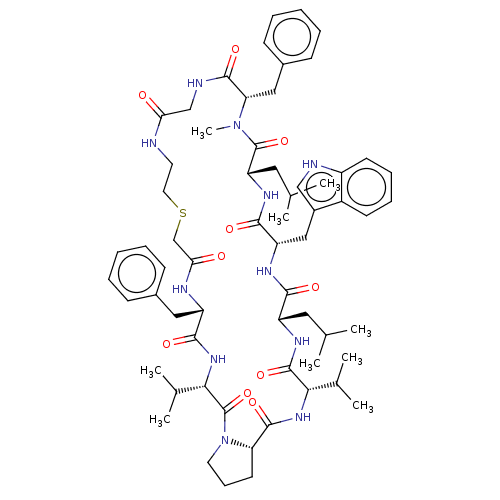
(CHEMBL5203523)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)CNC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50566772

(CHEMBL4878738)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCCN)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Mus musculus) | BDBM50566776

(CHEMBL4847740)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@H](Cc2ccccc2)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC1=O)C(N)=O)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Mus musculus) | BDBM50566772

(CHEMBL4878738)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCCN)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50566784

(CHEMBL4872872)Show SMILES CN1[C@@H](Cc2ccccc2)C(=O)N2C[C@@H](C[C@H]2C(=O)N[C@@H](CC(C)(C)C)C(=O)N2CCCC[C@H]2C(=O)N2CC(F)(F)C[C@H]2C(=O)N2Cc3ccccc3C[C@H]2C(=O)N[C@@H](Cc2ccc(cc2)-c2ccc(cc2)C(N)=O)C(=O)NCCSCC1=O)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Mus musculus) | BDBM50566784

(CHEMBL4872872)Show SMILES CN1[C@@H](Cc2ccccc2)C(=O)N2C[C@@H](C[C@H]2C(=O)N[C@@H](CC(C)(C)C)C(=O)N2CCCC[C@H]2C(=O)N2CC(F)(F)C[C@H]2C(=O)N2Cc3ccccc3C[C@H]2C(=O)N[C@@H](Cc2ccc(cc2)-c2ccc(cc2)C(N)=O)C(=O)NCCSCC1=O)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593252
(CHEMBL5205912)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC2CCCCC2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593253

(CHEMBL5189547)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC2CCCCC2)NC1=O)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346986
(US10202379, Reference Example 730)Show SMILES C[C@@H](N1CN([C@@H]2c3ccccc3SCc3cccc(C#C)c23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C26H20F3N3O3S/c1-3-16-7-6-8-17-13-36-20-10-5-4-9-18(20)22(21(16)17)32-14-30(15(2)26(27,28)29)25(35)23-24(34)19(33)11-12-31(23)32/h1,4-12,15,22,34H,13-14H2,2H3/t15-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.52 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346979
(US10202379, Reference Example 719)Show SMILES C[C@@H](N1CN([C@@H]2c3ccccc3SCc3c(Cl)cccc23)n2cc(CO)c(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C25H21ClF3N3O4S/c1-13(25(27,28)29)30-12-32(31-9-14(10-33)22(34)23(35)21(31)24(30)36)20-15-6-4-7-18(26)17(15)11-37-19-8-3-2-5-16(19)20/h2-9,13,20,33,35H,10-12H2,1H3/t13-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346964
(US10202379, Reference Example 666)Show SMILES C[C@@H](N1CN([C@@H]2c3ccccc3SCc3cccc(Cl)c23)n2cc(CO)c(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C25H21ClF3N3O4S/c1-13(25(27,28)29)30-12-32(31-9-15(10-33)22(34)23(35)21(31)24(30)36)20-16-6-2-3-8-18(16)37-11-14-5-4-7-17(26)19(14)20/h2-9,13,20,33,35H,10-12H2,1H3/t13-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.36 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50593256
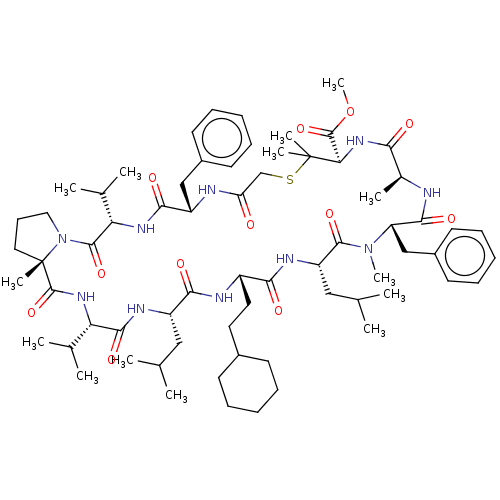
(CHEMBL5186053)Show SMILES COC(=O)[C@H]1NC(=O)[C@H](C)NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC2CCCCC2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@]2(C)CCCN2C(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CSC1(C)C)C(C)C)C(C)C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00310
BindingDB Entry DOI: 10.7270/Q2CF9V42 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346968
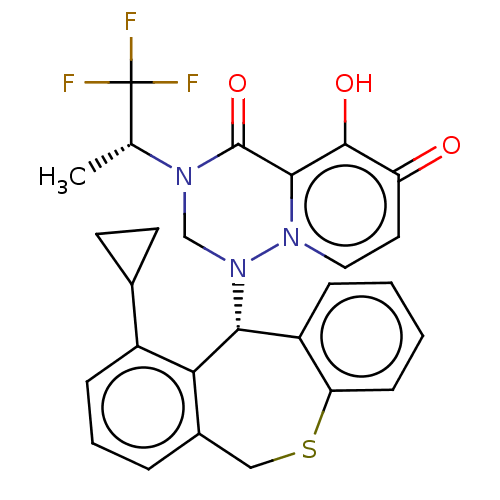
(US10202379, Reference Example 691)Show SMILES C[C@@H](N1CN([C@@H]2c3ccccc3SCc3cccc(C4CC4)c23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C27H24F3N3O3S/c1-15(27(28,29)30)31-14-33(32-12-11-20(34)25(35)24(32)26(31)36)23-19-6-2-3-8-21(19)37-13-17-5-4-7-18(22(17)23)16-9-10-16/h2-8,11-12,15-16,23,35H,9-10,13-14H2,1H3/t15-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.67 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346984
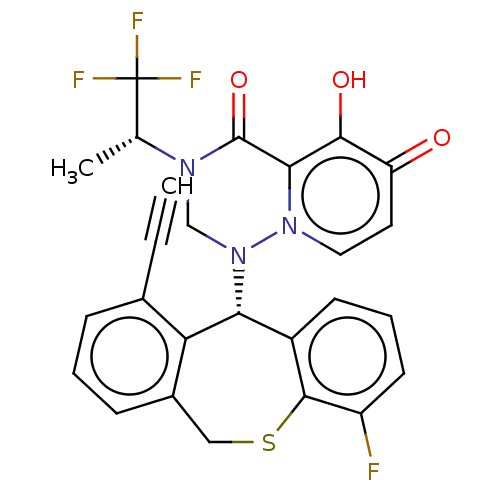
(US10202379, Reference Example 726)Show SMILES C[C@@H](N1CN([C@@H]2c3cccc(F)c3SCc3cccc(C#C)c23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C26H19F4N3O3S/c1-3-15-6-4-7-16-12-37-24-17(8-5-9-18(24)27)21(20(15)16)33-13-31(14(2)26(28,29)30)25(36)22-23(35)19(34)10-11-32(22)33/h1,4-11,14,21,35H,12-13H2,2H3/t14-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.74 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346923
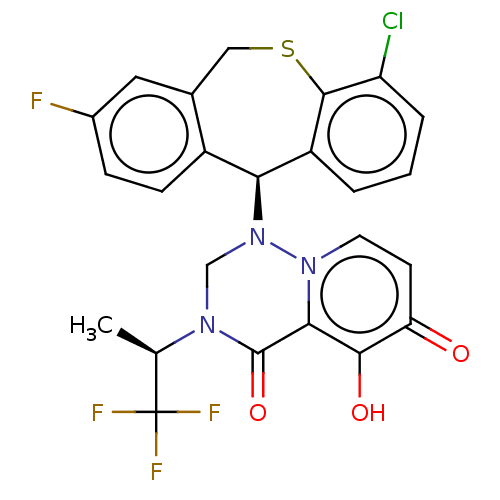
(US10202379, Reference Example 602)Show SMILES C[C@@H](N1CN([C@H]2c3ccc(F)cc3CSc3c(Cl)cccc23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C24H18ClF4N3O3S/c1-12(24(27,28)29)30-11-32(31-8-7-18(33)21(34)20(31)23(30)35)19-15-6-5-14(26)9-13(15)10-36-22-16(19)3-2-4-17(22)25/h2-9,12,19,34H,10-11H2,1H3/t12-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346931
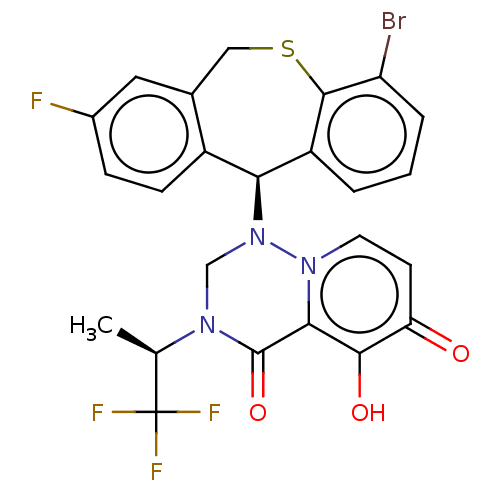
(US10202379, Reference Example 619)Show SMILES C[C@@H](N1CN([C@H]2c3ccc(F)cc3CSc3c(Br)cccc23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C24H18BrF4N3O3S/c1-12(24(27,28)29)30-11-32(31-8-7-18(33)21(34)20(31)23(30)35)19-15-6-5-14(26)9-13(15)10-36-22-16(19)3-2-4-17(22)25/h2-9,12,19,34H,10-11H2,1H3/t12-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
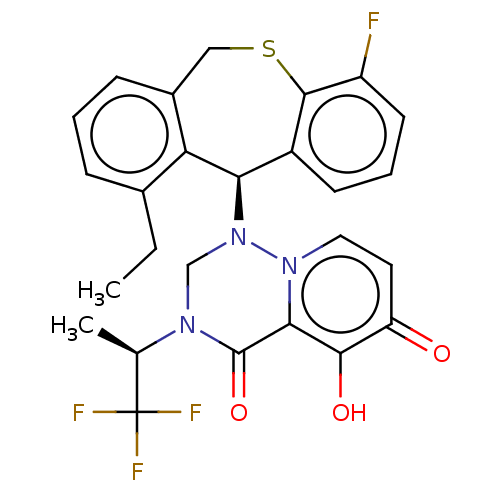
(Homo sapiens (Human)) | BDBM346983
(US10202379, Reference Example 724)Show SMILES CCc1cccc2CSc3c(F)cccc3[C@@H](N3CN([C@H](C)C(F)(F)F)C(=O)c4c(O)c(=O)ccn34)c12 |r| Show InChI InChI=1S/C26H23F4N3O3S/c1-3-15-6-4-7-16-12-37-24-17(8-5-9-18(24)27)21(20(15)16)33-13-31(14(2)26(28,29)30)25(36)22-23(35)19(34)10-11-32(22)33/h4-11,14,21,35H,3,12-13H2,1-2H3/t14-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.07 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346995

(US10202379, Reference Example 772)Show SMILES C[C@@H](N1CN([C@@H]2c3ccc(F)cc3SCc3cccc(Cl)c23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C24H18ClF4N3O3S/c1-12(24(27,28)29)30-11-32(31-8-7-17(33)22(34)21(31)23(30)35)20-15-6-5-14(26)9-18(15)36-10-13-3-2-4-16(25)19(13)20/h2-9,12,20,34H,10-11H2,1H3/t12-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.22 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
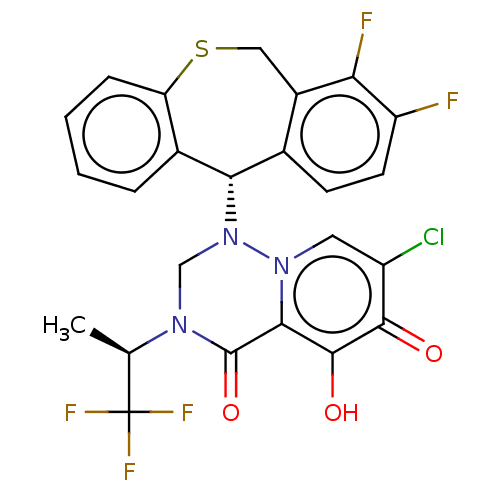
(Homo sapiens (Human)) | BDBM346978
(US10202379, Reference Example 716)Show SMILES C[C@@H](N1CN([C@@H]2c3ccccc3SCc3c(F)c(F)ccc23)n2cc(Cl)c(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C24H17ClF5N3O3S/c1-11(24(28,29)30)31-10-33(32-8-15(25)21(34)22(35)20(32)23(31)36)19-12-6-7-16(26)18(27)14(12)9-37-17-5-3-2-4-13(17)19/h2-8,11,19,35H,9-10H2,1H3/t11-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.54 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
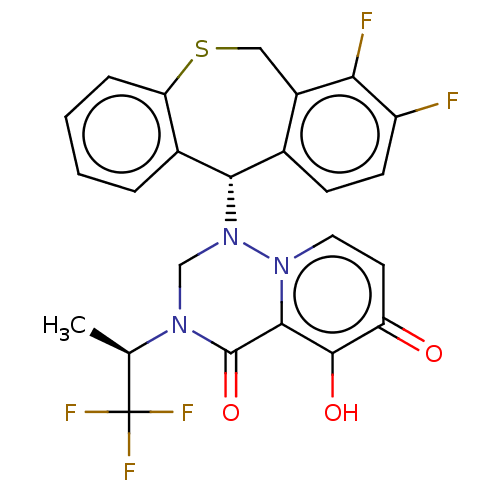
(Homo sapiens (Human)) | BDBM346976
(US10202379, Reference Example 711)Show SMILES C[C@@H](N1CN([C@@H]2c3ccccc3SCc3c(F)c(F)ccc23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C24H18F5N3O3S/c1-12(24(27,28)29)30-11-32(31-9-8-17(33)22(34)21(31)23(30)35)20-13-6-7-16(25)19(26)15(13)10-36-18-5-3-2-4-14(18)20/h2-9,12,20,34H,10-11H2,1H3/t12-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.57 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346987
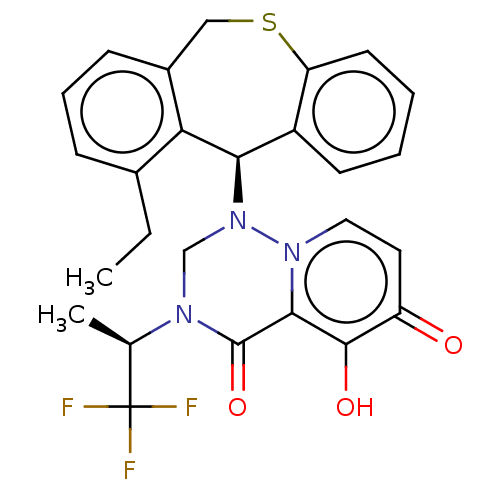
(US10202379, Reference Example 731)Show SMILES CCc1cccc2CSc3ccccc3[C@@H](N3CN([C@H](C)C(F)(F)F)C(=O)c4c(O)c(=O)ccn34)c12 |r| Show InChI InChI=1S/C26H24F3N3O3S/c1-3-16-7-6-8-17-13-36-20-10-5-4-9-18(20)22(21(16)17)32-14-30(15(2)26(27,28)29)25(35)23-24(34)19(33)11-12-31(23)32/h4-12,15,22,34H,3,13-14H2,1-2H3/t15-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.76 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50566775
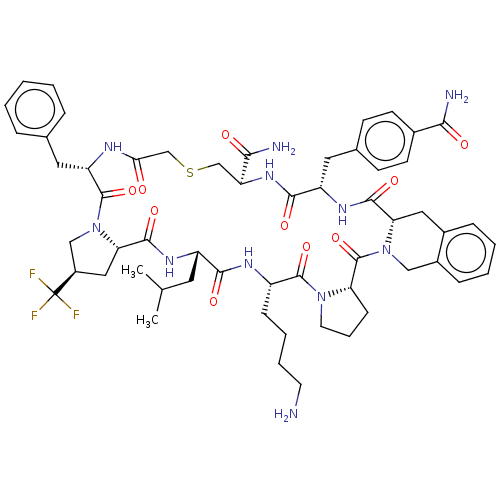
(CHEMBL4856854)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@H](Cc2ccccc2)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(cc2)C(N)=O)NC(=O)[C@@H]2Cc3ccccc3CN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC1=O)C(N)=O)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00134
BindingDB Entry DOI: 10.7270/Q21J9FJD |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346994

(US10202379, Reference Example 771)Show SMILES C[C@@H](N1CN([C@@H]2c3cccc(F)c3SCc3cccc(Br)c23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C24H18BrF4N3O3S/c1-12(24(27,28)29)30-11-32(31-9-8-17(33)21(34)20(31)23(30)35)19-14-5-3-7-16(26)22(14)36-10-13-4-2-6-15(25)18(13)19/h2-9,12,19,34H,10-11H2,1H3/t12-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.89 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM346932
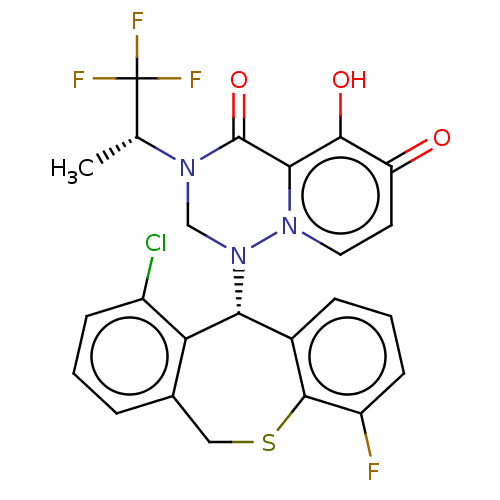
(US10202379, Reference Example 621)Show SMILES C[C@@H](N1CN([C@@H]2c3cccc(F)c3SCc3cccc(Cl)c23)n2ccc(=O)c(O)c2C1=O)C(F)(F)F |r| Show InChI InChI=1S/C24H18ClF4N3O3S/c1-12(24(27,28)29)30-11-32(31-9-8-17(33)21(34)20(31)23(30)35)19-14-5-3-7-16(26)22(14)36-10-13-4-2-6-15(25)18(13)19/h2-9,12,19,34H,10-11H2,1H3/t12-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
A test sample was diluted with a culture medium to an appropriate concentration in advance, and then 2 to 5-fold serial dilution on a 96 well plate (... |
US Patent US10202379 (2019)
BindingDB Entry DOI: 10.7270/Q2XK8HP2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data