 Found 152 hits with Last Name = 'kane' and Initial = 'jm'
Found 152 hits with Last Name = 'kane' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the antagonistic activity against NK1 receptor |
Bioorg Med Chem Lett 7: 2819-2824 (1997)
Article DOI: 10.1016/S0960-894X(97)10097-X
BindingDB Entry DOI: 10.7270/Q29S1RJ0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonism of the guinea pig tachykinin NK1 receptor |
Bioorg Med Chem Lett 7: 2819-2824 (1997)
Article DOI: 10.1016/S0960-894X(97)10097-X
BindingDB Entry DOI: 10.7270/Q29S1RJ0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50175494
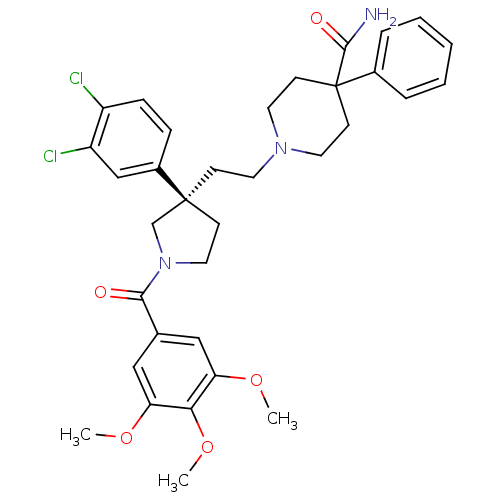
(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H39Cl2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41)/t33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the antagonistic activity against NK1 receptor |
Bioorg Med Chem Lett 7: 2819-2824 (1997)
Article DOI: 10.1016/S0960-894X(97)10097-X
BindingDB Entry DOI: 10.7270/Q29S1RJ0 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290703

(4-[2-(1-{2-[3-Phenyl-1-(3,4,5-trimethoxy-benzoyl)-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(cc2)C(O)=O)(C1)c1ccccc1 Show InChI InChI=1S/C43H46N4O7/c1-52-36-25-32(26-37(53-2)39(36)54-3)41(49)46-24-20-43(28-46,33-9-5-4-6-10-33)19-23-45-21-17-30(18-22-45)38(48)40-44-34-11-7-8-12-35(34)47(40)27-29-13-15-31(16-14-29)42(50)51/h4-16,25-26,30H,17-24,27-28H2,1-3H3,(H,50,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290742
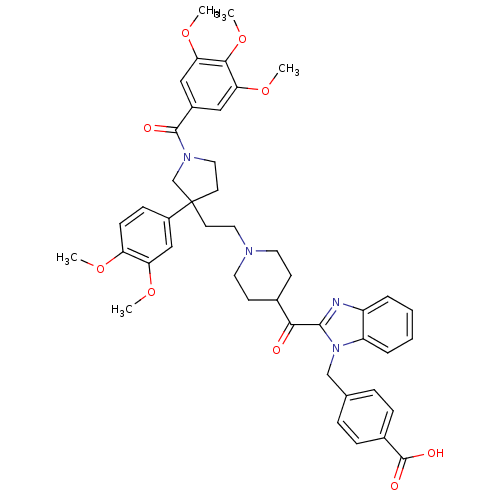
(4-[2-(1-{2-[3-(3,4-Dimethoxy-phenyl)-1-(3,4,5-trim...)Show SMILES COc1ccc(cc1OC)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(cc2)C(O)=O)CCN(C1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C45H50N4O9/c1-54-36-15-14-33(26-37(36)55-2)45(19-23-48(28-45)43(51)32-24-38(56-3)41(58-5)39(25-32)57-4)18-22-47-20-16-30(17-21-47)40(50)42-46-34-8-6-7-9-35(34)49(42)27-29-10-12-31(13-11-29)44(52)53/h6-15,24-26,30H,16-23,27-28H2,1-5H3,(H,52,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290731

(CHEMBL96466 | [3-Benzo[1,3]dioxol-5-yl-3-(2-{4-[1-...)Show SMILES Fc1ccc(Cn2c(nc3ccccc23)C(=O)C2CCN(CCC3(CCN(C3)C(=O)c3ccc(Cl)cc3Cl)c3ccc4OCOc4c3)CC2)cc1 Show InChI InChI=1S/C40H37Cl2FN4O4/c41-29-8-11-31(32(42)22-29)39(49)46-20-16-40(24-46,28-7-12-35-36(21-28)51-25-50-35)15-19-45-17-13-27(14-18-45)37(48)38-44-33-3-1-2-4-34(33)47(38)23-26-5-9-30(43)10-6-26/h1-12,21-22,27H,13-20,23-25H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290746

(4-[2-(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trime...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2CCCC(O)=O)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C39H44Cl2N4O7/c1-50-32-21-26(22-33(51-2)36(32)52-3)38(49)44-20-15-39(24-44,27-10-11-28(40)29(41)23-27)14-19-43-17-12-25(13-18-43)35(48)37-42-30-7-4-5-8-31(30)45(37)16-6-9-34(46)47/h4-5,7-8,10-11,21-23,25H,6,9,12-20,24H2,1-3H3,(H,46,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290743

(2-{4-[2-(1-{2-[3-Phenyl-1-(3,4,5-trimethoxy-benzoy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2CCCCOc2ccccc2C(O)=O)(C1)c1ccccc1 Show InChI InChI=1S/C46H52N4O8/c1-55-39-29-33(30-40(56-2)42(39)57-3)44(52)49-27-22-46(31-49,34-13-5-4-6-14-34)21-26-48-24-19-32(20-25-48)41(51)43-47-36-16-8-9-17-37(36)50(43)23-11-12-28-58-38-18-10-7-15-35(38)45(53)54/h4-10,13-18,29-30,32H,11-12,19-28,31H2,1-3H3,(H,53,54) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50175511
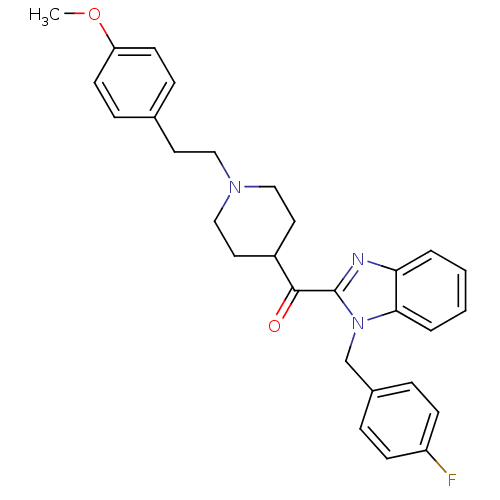
(CHEMBL95051 | MDL-28163 | [1-(4-Fluoro-benzyl)-1H-...)Show SMILES COc1ccc(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C29H30FN3O2/c1-35-25-12-8-21(9-13-25)14-17-32-18-15-23(16-19-32)28(34)29-31-26-4-2-3-5-27(26)33(29)20-22-6-10-24(30)11-7-22/h2-13,23H,14-20H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290728

(CHEMBL96441 | [3-{2-[4-(1H-Benzoimidazole-2-carbon...)Show SMILES COc1ccc(cc1OC)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3[nH]2)CCN(C1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C37H44N4O7/c1-44-29-11-10-26(22-30(29)45-2)37(15-19-41(23-37)36(43)25-20-31(46-3)34(48-5)32(21-25)47-4)14-18-40-16-12-24(13-17-40)33(42)35-38-27-8-6-7-9-28(27)39-35/h6-11,20-22,24H,12-19,23H2,1-5H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290707
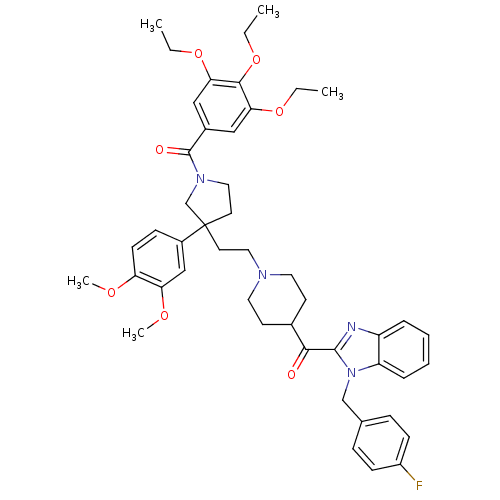
(CHEMBL330367 | CHEMBL330462 | [3-(3,4-Dimethoxy-ph...)Show SMILES CCOc1cc(cc(OCC)c1OCC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C47H55FN4O7/c1-6-57-41-27-34(28-42(58-7-2)44(41)59-8-3)46(54)51-26-22-47(31-51,35-15-18-39(55-4)40(29-35)56-5)21-25-50-23-19-33(20-24-50)43(53)45-49-37-11-9-10-12-38(37)52(45)30-32-13-16-36(48)17-14-32/h9-18,27-29,33H,6-8,19-26,30-31H2,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290720

(4-[2-(1-{2-[3-Phenyl-1-(3,4,5-trimethoxy-benzoyl)-...)Show SMILES COC(=O)c1ccc(Cn2c(nc3ccccc23)C(=O)C2CCN(CCC3(CCN(C3)C(=O)c3cc(OC)c(OC)c(OC)c3)c3ccccc3)CC2)cc1 Show InChI InChI=1S/C44H48N4O7/c1-52-37-26-33(27-38(53-2)40(37)54-3)42(50)47-25-21-44(29-47,34-10-6-5-7-11-34)20-24-46-22-18-31(19-23-46)39(49)41-45-35-12-8-9-13-36(35)48(41)28-30-14-16-32(17-15-30)43(51)55-4/h5-17,26-27,31H,18-25,28-29H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290722

(CHEMBL98076 | [1-(4-Fluoro-benzyl)-1H-benzoimidazo...)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#7]/c2nc3ccccc3n2-[#6]-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H29FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13H,14-20H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290725

(4-[2-(1-{2-[3-(3,4-Dimethoxy-phenyl)-1-(3,4,5-trim...)Show SMILES COC(=O)c1ccc(Cn2c(nc3ccccc23)C(=O)C2CCN(CCC3(CCN(C3)C(=O)c3cc(OC)c(OC)c(OC)c3)c3ccc(OC)c(OC)c3)CC2)cc1 Show InChI InChI=1S/C46H52N4O9/c1-54-37-16-15-34(27-38(37)55-2)46(20-24-49(29-46)44(52)33-25-39(56-3)42(58-5)40(26-33)57-4)19-23-48-21-17-31(18-22-48)41(51)43-47-35-9-7-8-10-36(35)50(43)28-30-11-13-32(14-12-30)45(53)59-6/h7-16,25-27,31H,17-24,28-29H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290726
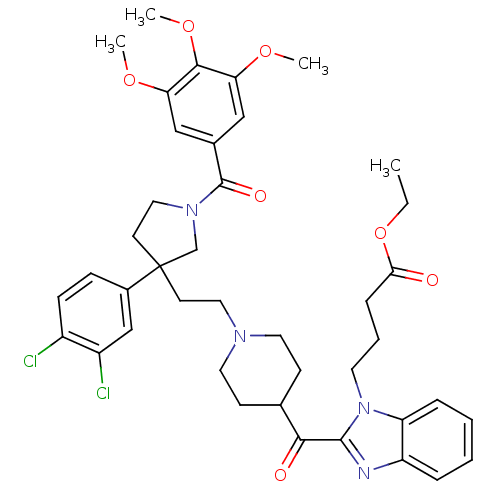
(4-[2-(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trime...)Show SMILES CCOC(=O)CCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C41H48Cl2N4O7/c1-5-54-36(48)11-8-18-47-33-10-7-6-9-32(33)44-39(47)37(49)27-14-19-45(20-15-27)21-16-41(29-12-13-30(42)31(43)25-29)17-22-46(26-41)40(50)28-23-34(51-2)38(53-4)35(24-28)52-3/h6-7,9-10,12-13,23-25,27H,5,8,11,14-22,26H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290727
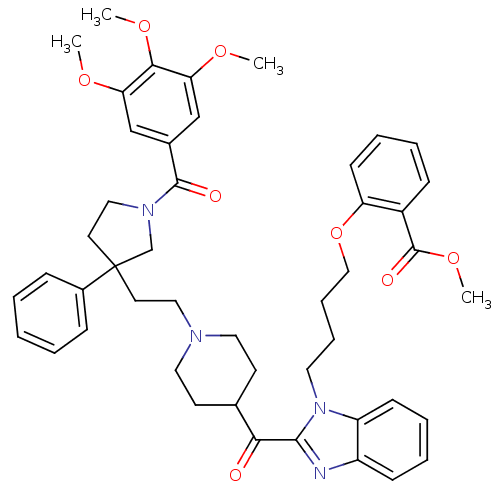
(2-{4-[2-(1-{2-[3-Phenyl-1-(3,4,5-trimethoxy-benzoy...)Show SMILES COC(=O)c1ccccc1OCCCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccccc2)CC1 Show InChI InChI=1S/C47H54N4O8/c1-55-40-30-34(31-41(56-2)43(40)57-3)45(53)50-28-23-47(32-50,35-14-6-5-7-15-35)22-27-49-25-20-33(21-26-49)42(52)44-48-37-17-9-10-18-38(37)51(44)24-12-13-29-59-39-19-11-8-16-36(39)46(54)58-4/h5-11,14-19,30-31,33H,12-13,20-29,32H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290729

(CHEMBL327888 | [3-(3,4-Dimethoxy-phenyl)-3-(2-{4-[...)Show SMILES CCOCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccc(OC)c(OC)c2)CC1 Show InChI InChI=1S/C41H52N4O8/c1-7-53-23-22-45-32-11-9-8-10-31(32)42-39(45)37(46)28-14-18-43(19-15-28)20-16-41(30-12-13-33(48-2)34(26-30)49-3)17-21-44(27-41)40(47)29-24-35(50-4)38(52-6)36(25-29)51-5/h8-13,24-26,28H,7,14-23,27H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290715

((1-{2-[1-Benzoyl-3-(3,4-dimethoxy-phenyl)-pyrrolid...)Show SMILES COc1ccc(cc1OC)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)CCN(C1)C(=O)c1ccccc1 Show InChI InChI=1S/C41H43FN4O4/c1-49-36-17-14-32(26-37(36)50-2)41(21-25-45(28-41)40(48)31-8-4-3-5-9-31)20-24-44-22-18-30(19-23-44)38(47)39-43-34-10-6-7-11-35(34)46(39)27-29-12-15-33(42)16-13-29/h3-17,26,30H,18-25,27-28H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290705

((3-{2-[4-(1H-Benzoimidazole-2-carbonyl)-piperidin-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3[nH]2)(C1)c1ccccc1 Show InChI InChI=1S/C35H40N4O5/c1-42-29-21-25(22-30(43-2)32(29)44-3)34(41)39-20-16-35(23-39,26-9-5-4-6-10-26)15-19-38-17-13-24(14-18-38)31(40)33-36-27-11-7-8-12-28(27)37-33/h4-12,21-22,24H,13-20,23H2,1-3H3,(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290714

((3-(4-Methoxy-phenyl)-3-{2-[4-(1-pyridin-2-ylmethy...)Show SMILES COc1ccc(cc1)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccccn2)CCN(C1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C42H47N5O6/c1-50-33-14-12-31(13-15-33)42(19-24-46(28-42)41(49)30-25-36(51-2)39(53-4)37(26-30)52-3)18-23-45-21-16-29(17-22-45)38(48)40-44-34-10-5-6-11-35(34)47(40)27-32-9-7-8-20-43-32/h5-15,20,25-26,29H,16-19,21-24,27-28H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290730

((3-(4-Fluoro-phenyl)-3-{2-[4-(1-pyridin-2-ylmethyl...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccccn2)(C1)c1ccc(F)cc1 Show InChI InChI=1S/C41H44FN5O5/c1-50-35-24-29(25-36(51-2)38(35)52-3)40(49)46-23-18-41(27-46,30-11-13-31(42)14-12-30)17-22-45-20-15-28(16-21-45)37(48)39-44-33-9-4-5-10-34(33)47(39)26-32-8-6-7-19-43-32/h4-14,19,24-25,28H,15-18,20-23,26-27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290736
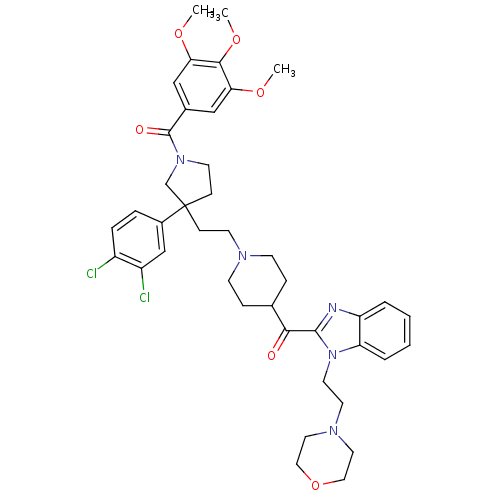
(CHEMBL97240 | [3-(3,4-Dichloro-phenyl)-3-(2-{4-[1-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2CCN2CCOCC2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C41H49Cl2N5O6/c1-51-35-24-29(25-36(52-2)38(35)53-3)40(50)47-17-13-41(27-47,30-8-9-31(42)32(43)26-30)12-16-45-14-10-28(11-15-45)37(49)39-44-33-6-4-5-7-34(33)48(39)19-18-46-20-22-54-23-21-46/h4-9,24-26,28H,10-23,27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290708

(CHEMBL94834 | [3-(2-{4-[1-(4-Fluoro-benzyl)-1H-ben...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C43H44F4N4O5/c1-54-36-24-30(25-37(55-2)39(36)56-3)41(53)50-23-19-42(27-50,31-10-12-32(13-11-31)43(45,46)47)18-22-49-20-16-29(17-21-49)38(52)40-48-34-6-4-5-7-35(34)51(40)26-28-8-14-33(44)15-9-28/h4-15,24-25,29H,16-23,26-27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290716
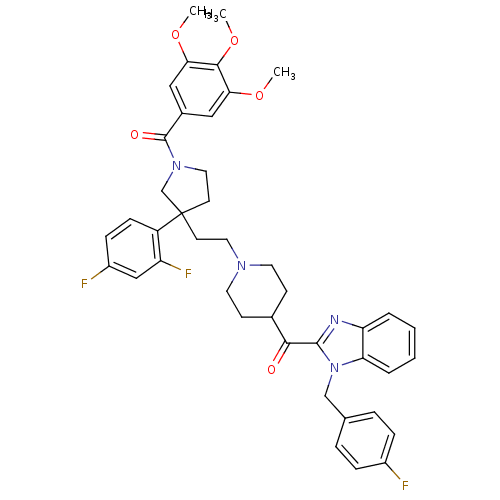
(CHEMBL264007 | [3-(2,4-Difluoro-phenyl)-3-(2-{4-[1...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc(F)cc1F Show InChI InChI=1S/C42H43F3N4O5/c1-52-36-22-29(23-37(53-2)39(36)54-3)41(51)48-21-17-42(26-48,32-13-12-31(44)24-33(32)45)16-20-47-18-14-28(15-19-47)38(50)40-46-34-6-4-5-7-35(34)49(40)25-27-8-10-30(43)11-9-27/h4-13,22-24,28H,14-21,25-26H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290717

((3-Phenyl-3-{2-[4-(1-pyridin-2-ylmethyl-1H-benzoim...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccccn2)(C1)c1ccccc1 Show InChI InChI=1S/C41H45N5O5/c1-49-35-25-30(26-36(50-2)38(35)51-3)40(48)45-24-19-41(28-45,31-11-5-4-6-12-31)18-23-44-21-16-29(17-22-44)37(47)39-43-33-14-7-8-15-34(33)46(39)27-32-13-9-10-20-42-32/h4-15,20,25-26,29H,16-19,21-24,27-28H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290738
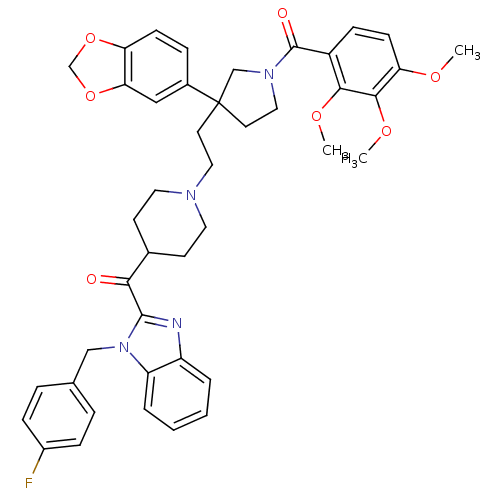
(CHEMBL94726 | [3-Benzo[1,3]dioxol-5-yl-3-(2-{4-[1-...)Show SMILES COc1ccc(C(=O)N2CCC(CCN3CCC(CC3)C(=O)c3nc4ccccc4n3Cc3ccc(F)cc3)(C2)c2ccc3OCOc3c2)c(OC)c1OC Show InChI InChI=1S/C43H45FN4O7/c1-51-36-15-13-32(39(52-2)40(36)53-3)42(50)47-23-19-43(26-47,30-10-14-35-37(24-30)55-27-54-35)18-22-46-20-16-29(17-21-46)38(49)41-45-33-6-4-5-7-34(33)48(41)25-28-8-11-31(44)12-9-28/h4-15,24,29H,16-23,25-27H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290712

((3-Phenyl-3-{2-[4-(1-pyridin-3-ylmethyl-1H-benzoim...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2cccnc2)(C1)c1ccccc1 Show InChI InChI=1S/C41H45N5O5/c1-49-35-24-31(25-36(50-2)38(35)51-3)40(48)45-23-18-41(28-45,32-11-5-4-6-12-32)17-22-44-20-15-30(16-21-44)37(47)39-43-33-13-7-8-14-34(33)46(39)27-29-10-9-19-42-26-29/h4-14,19,24-26,30H,15-18,20-23,27-28H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290702
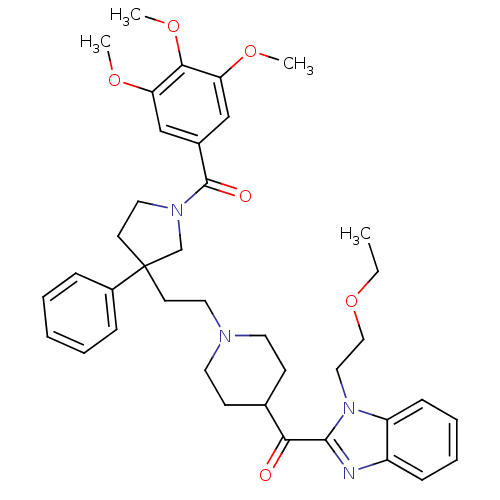
(CHEMBL322194 | [3-(2-{4-[1-(2-Ethoxy-ethyl)-1H-ben...)Show SMILES CCOCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccccc2)CC1 Show InChI InChI=1S/C39H48N4O6/c1-5-49-24-23-43-32-14-10-9-13-31(32)40-37(43)35(44)28-15-19-41(20-16-28)21-17-39(30-11-7-6-8-12-30)18-22-42(27-39)38(45)29-25-33(46-2)36(48-4)34(26-29)47-3/h6-14,25-26,28H,5,15-24,27H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290733

(CHEMBL320003 | [3-{2-[4-(1H-Benzoimidazole-2-carbo...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3[nH]2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C35H38Cl2N4O5/c1-44-29-18-23(19-30(45-2)32(29)46-3)34(43)41-17-13-35(21-41,24-8-9-25(36)26(37)20-24)12-16-40-14-10-22(11-15-40)31(42)33-38-27-6-4-5-7-28(27)39-33/h4-9,18-20,22H,10-17,21H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290732

(CHEMBL95076 | {3-[2-(4-{1-[2-(Furan-2-ylmethoxy)-e...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2CCOCc2ccco2)(C1)c1ccccc1 Show InChI InChI=1S/C42H48N4O7/c1-49-36-26-31(27-37(50-2)39(36)51-3)41(48)45-22-18-42(29-45,32-10-5-4-6-11-32)17-21-44-19-15-30(16-20-44)38(47)40-43-34-13-7-8-14-35(34)46(40)23-25-52-28-33-12-9-24-53-33/h4-14,24,26-27,30H,15-23,25,28-29H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290713
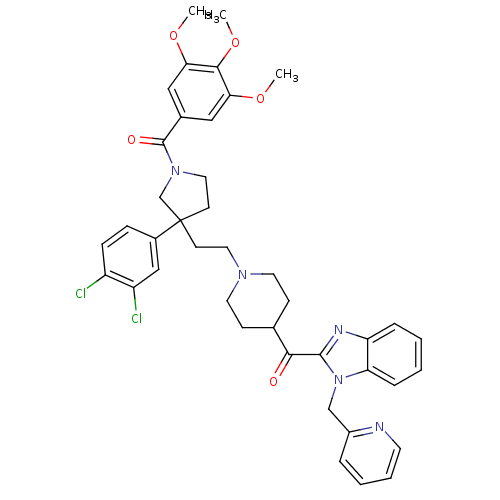
((3-(3,4-Dichloro-phenyl)-3-{2-[4-(1-pyridin-2-ylme...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccccn2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C41H43Cl2N5O5/c1-51-35-22-28(23-36(52-2)38(35)53-3)40(50)47-21-16-41(26-47,29-11-12-31(42)32(43)24-29)15-20-46-18-13-27(14-19-46)37(49)39-45-33-9-4-5-10-34(33)48(39)25-30-8-6-7-17-44-30/h4-12,17,22-24,27H,13-16,18-21,25-26H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50175497

(CHEMBL93556 | [3-(3,4-Dimethoxy-phenyl)-3-(2-{4-[1...)Show SMILES COc1ccc(cc1OC)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)CCN(C1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C44H49FN4O7/c1-52-36-15-12-32(26-37(36)53-2)44(19-23-48(28-44)43(51)31-24-38(54-3)41(56-5)39(25-31)55-4)18-22-47-20-16-30(17-21-47)40(50)42-46-34-8-6-7-9-35(34)49(42)27-29-10-13-33(45)14-11-29/h6-15,24-26,30H,16-23,27-28H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290711

((3-{2-[4-(1-Furan-2-ylmethyl-1H-benzoimidazole-2-c...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccco2)(C1)c1ccccc1 Show InChI InChI=1S/C40H44N4O6/c1-47-34-24-29(25-35(48-2)37(34)49-3)39(46)43-22-18-40(27-43,30-10-5-4-6-11-30)17-21-42-19-15-28(16-20-42)36(45)38-41-32-13-7-8-14-33(32)44(38)26-31-12-9-23-50-31/h4-14,23-25,28H,15-22,26-27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290716

(CHEMBL264007 | [3-(2,4-Difluoro-phenyl)-3-(2-{4-[1...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc(F)cc1F Show InChI InChI=1S/C42H43F3N4O5/c1-52-36-22-29(23-37(53-2)39(36)54-3)41(51)48-21-17-42(26-48,32-13-12-31(44)24-33(32)45)16-20-47-18-14-28(15-19-47)38(50)40-46-34-6-4-5-7-35(34)49(40)25-27-8-10-30(43)11-9-27/h4-13,22-24,28H,14-21,25-26H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290734

(1-[3-Benzo[1,3]dioxol-5-yl-3-(2-{4-[1-(4-fluoro-be...)Show SMILES CC(C)Oc1cccc(CC(=O)N2CCC(CCN3CCC(CC3)C(=O)c3nc4ccccc4n3Cc3ccc(F)cc3)(C2)c2ccc3OCOc3c2)c1 Show InChI InChI=1S/C44H47FN4O5/c1-30(2)54-36-7-5-6-32(24-36)25-41(50)48-23-19-44(28-48,34-12-15-39-40(26-34)53-29-52-39)18-22-47-20-16-33(17-21-47)42(51)43-46-37-8-3-4-9-38(37)49(43)27-31-10-13-35(45)14-11-31/h3-15,24,26,30,33H,16-23,25,27-29H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290737

(CHEMBL96141 | [3-(3,4-Dichloro-phenyl)-3-(2-{4-[1-...)Show SMILES CCOCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C39H46Cl2N4O6/c1-5-51-21-20-45-32-9-7-6-8-31(32)42-37(45)35(46)26-12-16-43(17-13-26)18-14-39(28-10-11-29(40)30(41)24-28)15-19-44(25-39)38(47)27-22-33(48-2)36(50-4)34(23-27)49-3/h6-11,22-24,26H,5,12-21,25H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290745
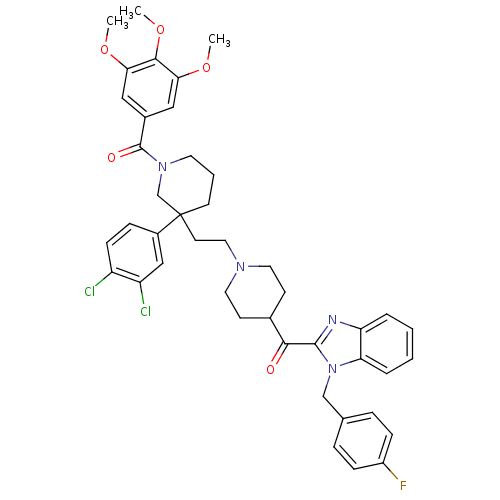
(CHEMBL329055 | [3-(3,4-Dichloro-phenyl)-3-(2-{4-[1...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C43H45Cl2FN4O5/c1-53-37-23-30(24-38(54-2)40(37)55-3)42(52)49-19-6-17-43(27-49,31-11-14-33(44)34(45)25-31)18-22-48-20-15-29(16-21-48)39(51)41-47-35-7-4-5-8-36(35)50(41)26-28-9-12-32(46)13-10-28/h4-5,7-14,23-25,29H,6,15-22,26-27H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290734

(1-[3-Benzo[1,3]dioxol-5-yl-3-(2-{4-[1-(4-fluoro-be...)Show SMILES CC(C)Oc1cccc(CC(=O)N2CCC(CCN3CCC(CC3)C(=O)c3nc4ccccc4n3Cc3ccc(F)cc3)(C2)c2ccc3OCOc3c2)c1 Show InChI InChI=1S/C44H47FN4O5/c1-30(2)54-36-7-5-6-32(24-36)25-41(50)48-23-19-44(28-48,34-12-15-39-40(26-34)53-29-52-39)18-22-47-20-16-33(17-21-47)42(51)43-46-37-8-3-4-9-38(37)49(43)27-31-10-13-35(45)14-11-31/h3-15,24,26,30,33H,16-23,25,27-29H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290738

(CHEMBL94726 | [3-Benzo[1,3]dioxol-5-yl-3-(2-{4-[1-...)Show SMILES COc1ccc(C(=O)N2CCC(CCN3CCC(CC3)C(=O)c3nc4ccccc4n3Cc3ccc(F)cc3)(C2)c2ccc3OCOc3c2)c(OC)c1OC Show InChI InChI=1S/C43H45FN4O7/c1-51-36-15-13-32(39(52-2)40(36)53-3)42(50)47-23-19-43(26-47,30-10-14-35-37(24-30)55-27-54-35)18-22-46-20-16-29(17-21-46)38(49)41-45-33-6-4-5-7-34(33)48(41)25-28-8-11-31(44)12-9-28/h4-15,24,29H,16-23,25-27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290740

(3-(2-{4-[1-(2-Ethoxy-ethyl)-1H-benzoimidazole-2-ca...)Show SMILES CCOCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(Cc3cc(OC)c(OC)c(OC)c3)C2=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C39H47FN4O6/c1-5-50-23-22-44-32-9-7-6-8-31(32)41-37(44)35(45)28-14-18-42(19-15-28)20-16-39(29-10-12-30(40)13-11-29)17-21-43(38(39)46)26-27-24-33(47-2)36(49-4)34(25-27)48-3/h6-13,24-25,28H,5,14-23,26H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290704

(CHEMBL330430 | CHEMBL97357 | [3-(3,4-Dichloro-phen...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C42H43Cl2FN4O5/c1-52-36-22-29(23-37(53-2)39(36)54-3)41(51)48-21-17-42(26-48,30-10-13-32(43)33(44)24-30)16-20-47-18-14-28(15-19-47)38(50)40-46-34-6-4-5-7-35(34)49(40)25-27-8-11-31(45)12-9-27/h4-13,22-24,28H,14-21,25-26H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290701

((3-Phenyl-3-{2-[4-(1-pyridin-4-ylmethyl-1H-benzoim...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccncc2)(C1)c1ccccc1 Show InChI InChI=1S/C41H45N5O5/c1-49-35-25-31(26-36(50-2)38(35)51-3)40(48)45-24-18-41(28-45,32-9-5-4-6-10-32)17-23-44-21-15-30(16-22-44)37(47)39-43-33-11-7-8-12-34(33)46(39)27-29-13-19-42-20-14-29/h4-14,19-20,25-26,30H,15-18,21-24,27-28H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290706
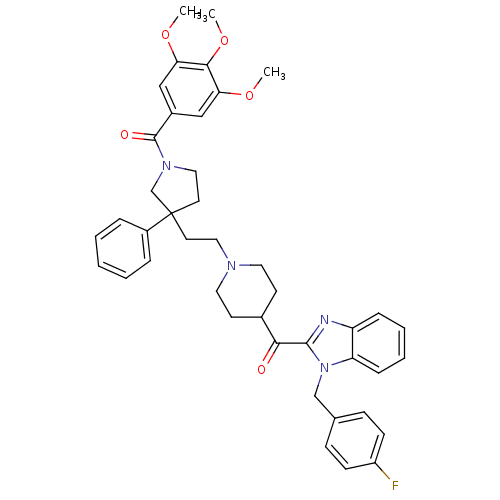
(CHEMBL431458 | CHEMBL98079 | [3-(2-{4-[1-(4-Fluoro...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccccc1 Show InChI InChI=1S/C42H45FN4O5/c1-50-36-25-31(26-37(51-2)39(36)52-3)41(49)46-24-20-42(28-46,32-9-5-4-6-10-32)19-23-45-21-17-30(18-22-45)38(48)40-44-34-11-7-8-12-35(34)47(40)27-29-13-15-33(43)16-14-29/h4-16,25-26,30H,17-24,27-28H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290715

((1-{2-[1-Benzoyl-3-(3,4-dimethoxy-phenyl)-pyrrolid...)Show SMILES COc1ccc(cc1OC)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)CCN(C1)C(=O)c1ccccc1 Show InChI InChI=1S/C41H43FN4O4/c1-49-36-17-14-32(26-37(36)50-2)41(21-25-45(28-41)40(48)31-8-4-3-5-9-31)20-24-44-22-18-30(19-23-44)38(47)39-43-34-10-6-7-11-35(34)46(39)27-29-12-15-33(42)16-13-29/h3-17,26,30H,18-25,27-28H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290745

(CHEMBL329055 | [3-(3,4-Dichloro-phenyl)-3-(2-{4-[1...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C43H45Cl2FN4O5/c1-53-37-23-30(24-38(54-2)40(37)55-3)42(52)49-19-6-17-43(27-49,31-11-14-33(44)34(45)25-31)18-22-48-20-15-29(16-21-48)39(51)41-47-35-7-4-5-8-36(35)50(41)26-28-9-12-32(46)13-10-28/h4-5,7-14,23-25,29H,6,15-22,26-27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290709
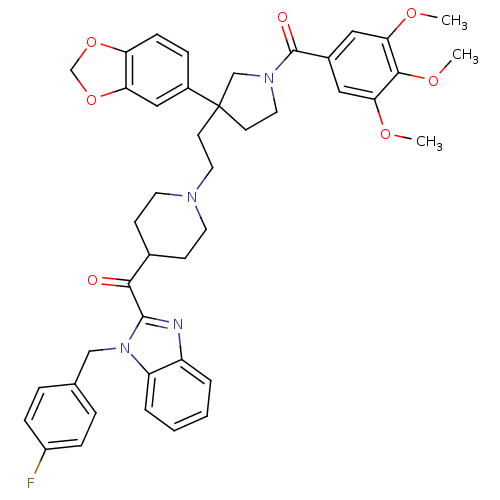
(CHEMBL317524 | CHEMBL320002 | [3-Benzo[1,3]dioxol-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc2OCOc2c1 Show InChI InChI=1S/C43H45FN4O7/c1-51-37-22-30(23-38(52-2)40(37)53-3)42(50)47-21-17-43(26-47,31-10-13-35-36(24-31)55-27-54-35)16-20-46-18-14-29(15-19-46)39(49)41-45-33-6-4-5-7-34(33)48(41)25-28-8-11-32(44)12-9-28/h4-13,22-24,29H,14-21,25-27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290709

(CHEMBL317524 | CHEMBL320002 | [3-Benzo[1,3]dioxol-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc2OCOc2c1 Show InChI InChI=1S/C43H45FN4O7/c1-51-37-22-30(23-38(52-2)40(37)53-3)42(50)47-21-17-43(26-47,31-10-13-35-36(24-31)55-27-54-35)16-20-46-18-14-29(15-19-46)39(49)41-45-33-6-4-5-7-34(33)48(41)25-28-8-11-32(44)12-9-28/h4-13,22-24,29H,14-21,25-27H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290707

(CHEMBL330367 | CHEMBL330462 | [3-(3,4-Dimethoxy-ph...)Show SMILES CCOc1cc(cc(OCC)c1OCC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(F)cc2)(C1)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C47H55FN4O7/c1-6-57-41-27-34(28-42(58-7-2)44(41)59-8-3)46(54)51-26-22-47(31-51,35-15-18-39(55-4)40(29-35)56-5)21-25-50-23-19-33(20-24-50)43(53)45-49-37-11-9-10-12-38(37)52(45)30-32-13-16-36(48)17-14-32/h9-18,27-29,33H,6-8,19-26,30-31H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290731

(CHEMBL96466 | [3-Benzo[1,3]dioxol-5-yl-3-(2-{4-[1-...)Show SMILES Fc1ccc(Cn2c(nc3ccccc23)C(=O)C2CCN(CCC3(CCN(C3)C(=O)c3ccc(Cl)cc3Cl)c3ccc4OCOc4c3)CC2)cc1 Show InChI InChI=1S/C40H37Cl2FN4O4/c41-29-8-11-31(32(42)22-29)39(49)46-20-16-40(24-46,28-7-12-35-36(21-28)51-25-50-35)15-19-45-17-13-27(14-18-45)37(48)38-44-33-3-1-2-4-34(33)47(38)23-26-5-9-30(43)10-6-26/h1-12,21-22,27H,13-20,23-25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290740

(3-(2-{4-[1-(2-Ethoxy-ethyl)-1H-benzoimidazole-2-ca...)Show SMILES CCOCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(Cc3cc(OC)c(OC)c(OC)c3)C2=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C39H47FN4O6/c1-5-50-23-22-44-32-9-7-6-8-31(32)41-37(44)35(45)28-14-18-42(19-15-28)20-16-39(29-10-12-30(40)13-11-29)17-21-43(38(39)46)26-27-24-33(47-2)36(49-4)34(25-27)48-3/h6-13,24-25,28H,5,14-23,26H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against Tachykinin receptor 1 |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data