 Found 119 hits with Last Name = 'kang' and Initial = 'dh'
Found 119 hits with Last Name = 'kang' and Initial = 'dh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM537926

(US11248003, Example 1)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2N(C)S(C)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28G8PWW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,C797S]
(Homo sapiens (Human)) | BDBM537926

(US11248003, Example 1)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2N(C)S(C)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28G8PWW |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50250152
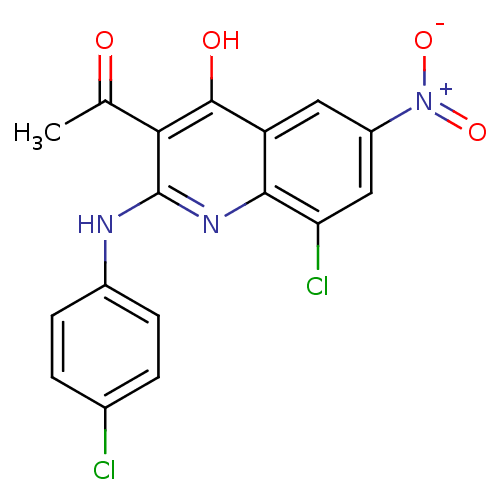
(3-acetyl-8-chloro-2-(4-chlorophenylamino)-6-nitroq...)Show SMILES CC(=O)c1c(Nc2ccc(Cl)cc2)nc2c(Cl)cc(cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C17H11Cl2N3O4/c1-8(23)14-16(24)12-6-11(22(25)26)7-13(19)15(12)21-17(14)20-10-4-2-9(18)3-5-10/h2-7H,1H3,(H2,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM537926

(US11248003, Example 1)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2N(C)S(C)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28G8PWW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125
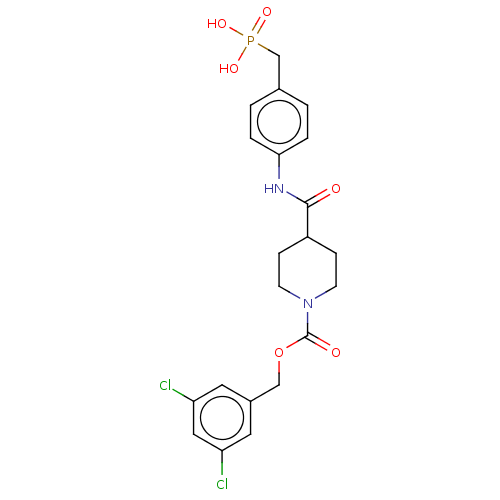
(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human A2058 cells using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458132

(CHEMBL4205127)Show SMILES OP(O)(=O)Cc1ccc(cc1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-19(6-8-25)24-20(26)16-3-1-14(2-4-16)13-32(28,29)30/h1-4,9-11,19H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50250190
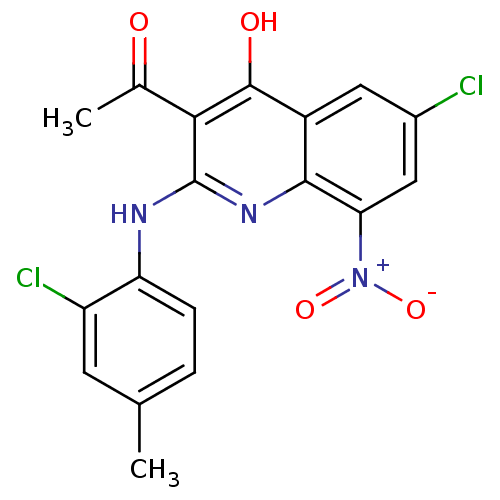
(3-acetyl-6-chloro-2-(2-chloro-4-methylphenylamino)...)Show SMILES CC(=O)c1c(Nc2ccc(C)cc2Cl)nc2c(cc(Cl)cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C18H13Cl2N3O4/c1-8-3-4-13(12(20)5-8)21-18-15(9(2)24)17(25)11-6-10(19)7-14(23(26)27)16(11)22-18/h3-7H,1-2H3,(H2,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458135
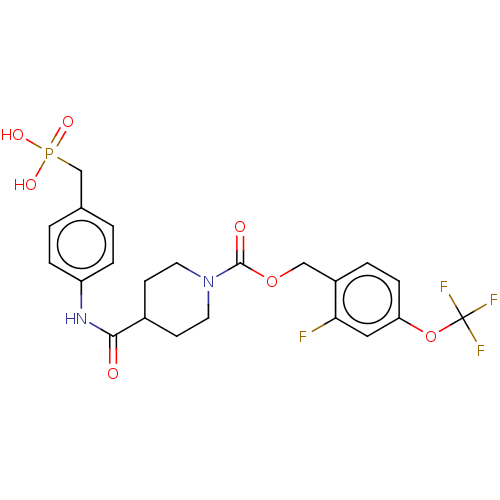
(CHEMBL4214731)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2F)cc1 Show InChI InChI=1S/C22H23F4N2O7P/c23-19-11-18(35-22(24,25)26)6-3-16(19)12-34-21(30)28-9-7-15(8-10-28)20(29)27-17-4-1-14(2-5-17)13-36(31,32)33/h1-6,11,15H,7-10,12-13H2,(H,27,29)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458127
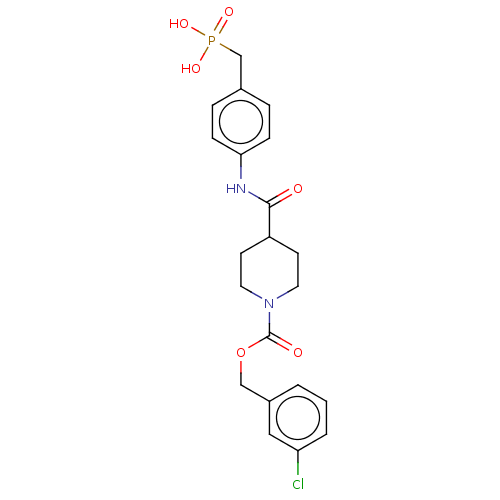
(CHEMBL4211269)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cccc(Cl)c2)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-18-3-1-2-16(12-18)13-30-21(26)24-10-8-17(9-11-24)20(25)23-19-6-4-15(5-7-19)14-31(27,28)29/h1-7,12,17H,8-11,13-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458131

(CHEMBL4212398)Show SMILES OP(O)(=O)Cc1csc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)n1 Show InChI InChI=1S/C18H20Cl2N3O6PS/c19-13-5-11(6-14(20)7-13)8-29-18(25)23-3-1-12(2-4-23)16(24)22-17-21-15(10-31-17)9-30(26,27)28/h5-7,10,12H,1-4,8-9H2,(H,21,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM50185140
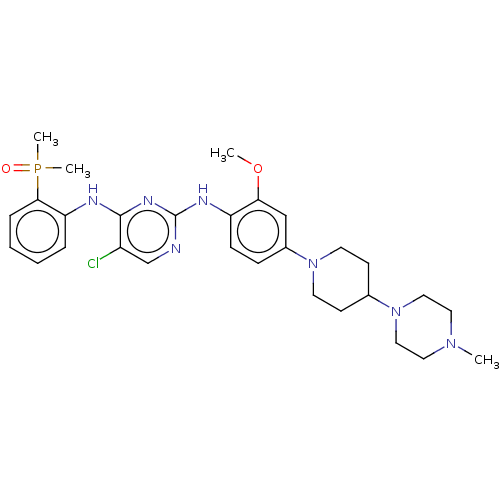
(AP-26113 | Brigatinib | US11248003, Example Brigat...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28G8PWW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458126

(CHEMBL4203076)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C22H24F3N2O7P/c23-22(24,25)34-19-7-3-15(4-8-19)13-33-21(29)27-11-9-17(10-12-27)20(28)26-18-5-1-16(2-6-18)14-35(30,31)32/h1-8,17H,9-14H2,(H,26,28)(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458135

(CHEMBL4214731)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2F)cc1 Show InChI InChI=1S/C22H23F4N2O7P/c23-19-11-18(35-22(24,25)26)6-3-16(19)12-34-21(30)28-9-7-15(8-10-28)20(29)27-17-4-1-14(2-5-17)13-36(31,32)33/h1-6,11,15H,7-10,12-13H2,(H,27,29)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458126

(CHEMBL4203076)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C22H24F3N2O7P/c23-22(24,25)34-19-7-3-15(4-8-19)13-33-21(29)27-11-9-17(10-12-27)20(28)26-18-5-1-16(2-6-18)14-35(30,31)32/h1-8,17H,9-14H2,(H,26,28)(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50250153
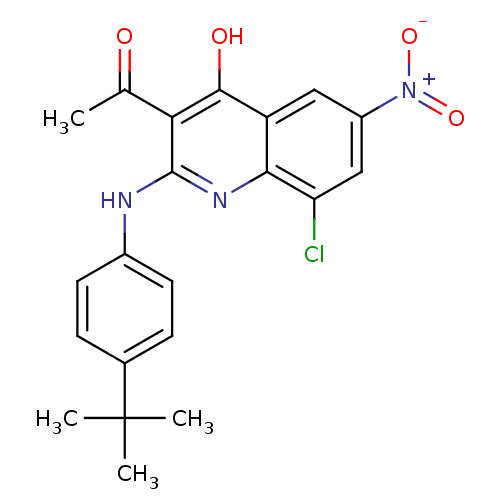
(3-acetyl-2-(4-tert-butylphenylamino)-8-chloro-6-ni...)Show SMILES CC(=O)c1c(Nc2ccc(cc2)C(C)(C)C)nc2c(Cl)cc(cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C21H20ClN3O4/c1-11(26)17-19(27)15-9-14(25(28)29)10-16(22)18(15)24-20(17)23-13-7-5-12(6-8-13)21(2,3)4/h5-10H,1-4H3,(H2,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458136
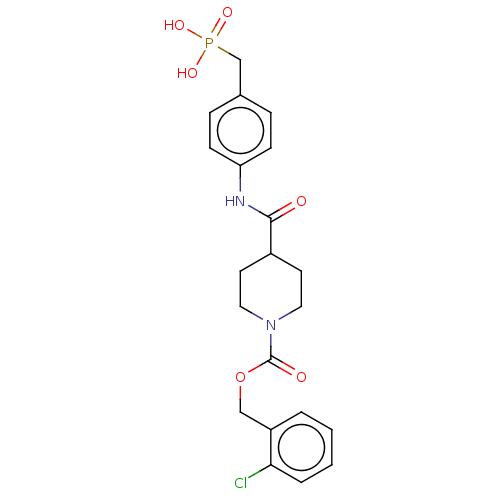
(CHEMBL4216236)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2Cl)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458133

(CHEMBL4216825)Show SMILES OP(O)(=O)Cc1csc(n1)C(=O)NC1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C17H18Cl2N3O6PS/c18-11-3-10(4-12(19)5-11)7-28-17(24)22-2-1-13(6-22)20-15(23)16-21-14(9-30-16)8-29(25,26)27/h3-5,9,13H,1-2,6-8H2,(H,20,23)(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458124
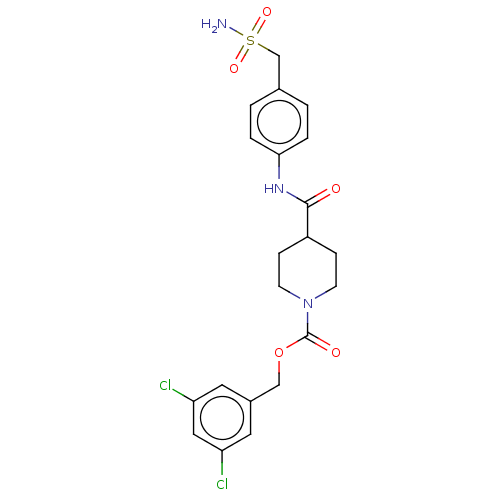
(CHEMBL4208368)Show SMILES NS(=O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N3O5S/c22-17-9-15(10-18(23)11-17)12-31-21(28)26-7-5-16(6-8-26)20(27)25-19-3-1-14(2-4-19)13-32(24,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,25,27)(H2,24,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458132

(CHEMBL4205127)Show SMILES OP(O)(=O)Cc1ccc(cc1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-19(6-8-25)24-20(26)16-3-1-14(2-4-16)13-32(28,29)30/h1-4,9-11,19H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM537979

(US11248003, Example TRE-069)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)C1CCNCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28G8PWW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458128

(CHEMBL4206899)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2F)cc1 Show InChI InChI=1S/C21H24FN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458129
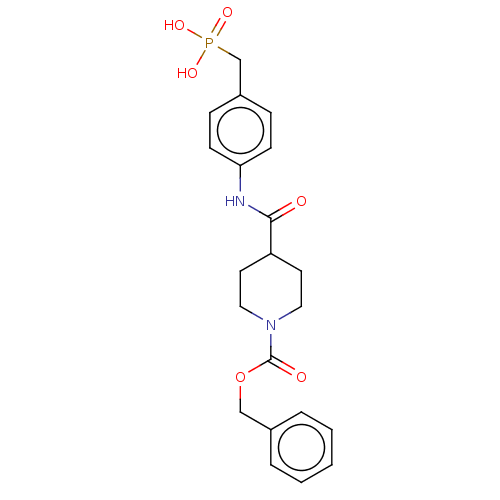
(CHEMBL4209155)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C21H25N2O6P/c24-20(22-19-8-6-17(7-9-19)15-30(26,27)28)18-10-12-23(13-11-18)21(25)29-14-16-4-2-1-3-5-16/h1-9,18H,10-15H2,(H,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human SKHEP1 cells using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-M... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458130
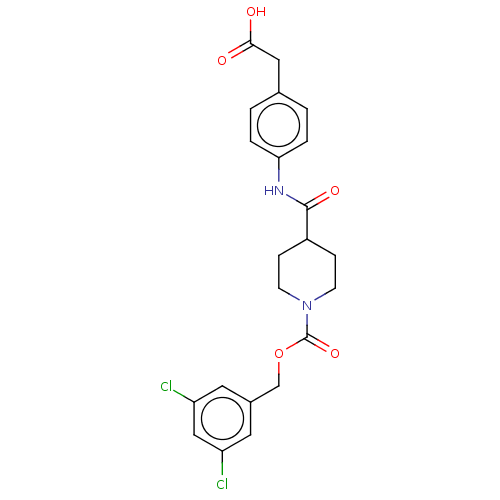
(CHEMBL4204027)Show SMILES OC(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C22H22Cl2N2O5/c23-17-9-15(10-18(24)12-17)13-31-22(30)26-7-5-16(6-8-26)21(29)25-19-3-1-14(2-4-19)11-20(27)28/h1-4,9-10,12,16H,5-8,11,13H2,(H,25,29)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458134

(CHEMBL4203499)Show SMILES NS(=O)(=O)Nc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C20H22Cl2N4O5S/c21-15-9-13(10-16(22)11-15)12-31-20(28)26-7-5-14(6-8-26)19(27)24-17-1-3-18(4-2-17)25-32(23,29)30/h1-4,9-11,14,25H,5-8,12H2,(H,24,27)(H2,23,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50250152

(3-acetyl-8-chloro-2-(4-chlorophenylamino)-6-nitroq...)Show SMILES CC(=O)c1c(Nc2ccc(Cl)cc2)nc2c(Cl)cc(cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C17H11Cl2N3O4/c1-8(23)14-16(24)12-6-11(22(25)26)7-13(19)15(12)21-17(14)20-10-4-2-9(18)3-5-10/h2-7H,1H3,(H2,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50269714

(CHEMBL4092264)Show SMILES OP(O)(=O)Cc1ccc(cc1)-c1csc(n1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C24H24Cl2N3O6PS/c25-18-9-16(10-19(26)11-18)12-35-24(31)29-7-5-20(6-8-29)27-22(30)23-28-21(14-37-23)17-3-1-15(2-4-17)13-36(32,33)34/h1-4,9-11,14,20H,5-8,12-13H2,(H,27,30)(H2,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human plasma using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS/MS a... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458123

(CHEMBL4215710)Show SMILES ONC(=O)c1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H21Cl2N3O5/c22-16-9-13(10-17(23)11-16)12-31-21(29)26-7-5-15(6-8-26)19(27)24-18-3-1-14(2-4-18)20(28)25-30/h1-4,9-11,15,30H,5-8,12H2,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458131

(CHEMBL4212398)Show SMILES OP(O)(=O)Cc1csc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)n1 Show InChI InChI=1S/C18H20Cl2N3O6PS/c19-13-5-11(6-14(20)7-13)8-29-18(25)23-3-1-12(2-4-23)16(24)22-17-21-15(10-31-17)9-30(26,27)28/h5-7,10,12H,1-4,8-9H2,(H,21,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458127

(CHEMBL4211269)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cccc(Cl)c2)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-18-3-1-2-16(12-18)13-30-21(26)24-10-8-17(9-11-24)20(25)23-19-6-4-15(5-7-19)14-31(27,28)29/h1-7,12,17H,8-11,13-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50250191
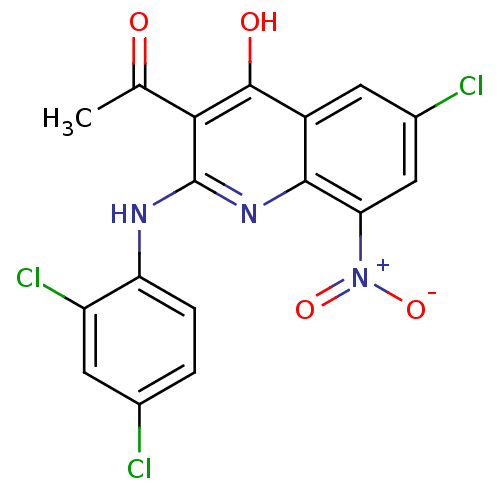
(3-acetyl-6-chloro-2-(2,4-dichlorophenylamino)-8-ni...)Show SMILES CC(=O)c1c(Nc2ccc(Cl)cc2Cl)nc2c(cc(Cl)cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C17H10Cl3N3O4/c1-7(24)14-16(25)10-4-9(19)6-13(23(26)27)15(10)22-17(14)21-12-3-2-8(18)5-11(12)20/h2-6H,1H3,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin D after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458133

(CHEMBL4216825)Show SMILES OP(O)(=O)Cc1csc(n1)C(=O)NC1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C17H18Cl2N3O6PS/c18-11-3-10(4-12(19)5-11)7-28-17(24)22-2-1-13(6-22)20-15(23)16-21-14(9-30-16)8-29(25,26)27/h3-5,9,13H,1-2,6-8H2,(H,20,23)(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50185140

(AP-26113 | Brigatinib | US11248003, Example Brigat...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28G8PWW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458124

(CHEMBL4208368)Show SMILES NS(=O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N3O5S/c22-17-9-15(10-18(23)11-17)12-31-21(28)26-7-5-16(6-8-26)20(27)25-19-3-1-14(2-4-19)13-32(24,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,25,27)(H2,24,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM537979

(US11248003, Example TRE-069)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)C1CCNCC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28G8PWW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human plasma using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS/MS a... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Pro-cathepsin H
(Homo sapiens (Human)) | BDBM50250152

(3-acetyl-8-chloro-2-(4-chlorophenylamino)-6-nitroq...)Show SMILES CC(=O)c1c(Nc2ccc(Cl)cc2)nc2c(Cl)cc(cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C17H11Cl2N3O4/c1-8(23)14-16(24)12-6-11(22(25)26)7-13(19)15(12)21-17(14)20-10-4-2-9(18)3-5-10/h2-7H,1H3,(H2,20,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin H after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50073850
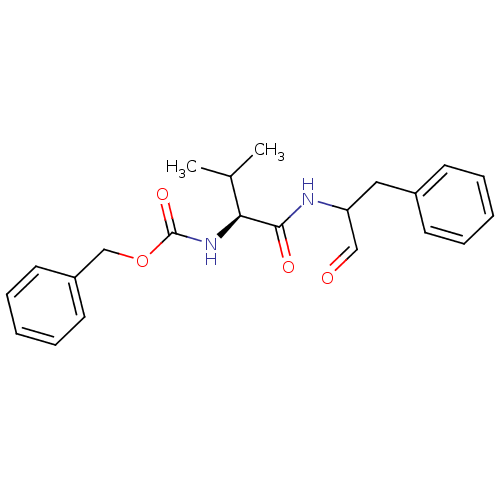
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes mu-calpain by fluorometric assay using pep2 as substrate |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50073850

((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes mu-calpain after 30 mins by fluorometric assay using pep1 as substrate |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458136

(CHEMBL4216236)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2Cl)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458134

(CHEMBL4203499)Show SMILES NS(=O)(=O)Nc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C20H22Cl2N4O5S/c21-15-9-13(10-16(22)11-15)12-31-20(28)26-7-5-14(6-8-26)19(27)24-17-1-3-18(4-2-17)25-32(23,29)30/h1-4,9-11,14,25H,5-8,12H2,(H,24,27)(H2,23,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458129

(CHEMBL4209155)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C21H25N2O6P/c24-20(22-19-8-6-17(7-9-19)15-30(26,27)28)18-10-12-23(13-11-18)21(25)29-14-16-4-2-1-3-5-16/h1-9,18H,10-15H2,(H,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50249659
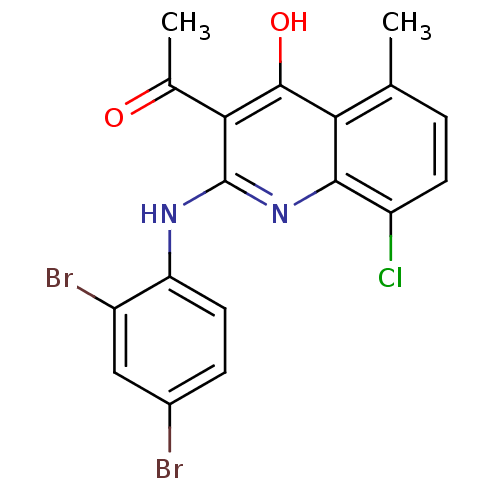
(3-acetyl-8-chloro-2-(2,4-dibromophenylamino)-5-met...)Show SMILES CC(=O)c1c(O)c2c(C)ccc(Cl)c2nc1Nc1ccc(Br)cc1Br Show InChI InChI=1S/C18H13Br2ClN2O2/c1-8-3-5-12(21)16-14(8)17(25)15(9(2)24)18(23-16)22-13-6-4-10(19)7-11(13)20/h3-7H,1-2H3,(H2,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes mu-calpain after 30 mins by fluorometric assay using pep1 as substrate |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24778

(2-methyl-1,4-dihydronaphthalene-1,4-dione | 2-meth...)Show InChI InChI=1S/C11H8O2/c1-7-6-10(12)8-4-2-3-5-9(8)11(7)13/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of ascorbic acid/methylene blue activated recombinant human IDO expressed in Escherichia coli using L-Tryptophan as substrate after 60 min... |
J Nat Prod 80: 1378-1386 (2017)
Article DOI: 10.1021/acs.jnatprod.6b01059
BindingDB Entry DOI: 10.7270/Q2902687 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50250190

(3-acetyl-6-chloro-2-(2-chloro-4-methylphenylamino)...)Show SMILES CC(=O)c1c(Nc2ccc(C)cc2Cl)nc2c(cc(Cl)cc2c1O)[N+]([O-])=O Show InChI InChI=1S/C18H13Cl2N3O4/c1-8-3-4-13(12(20)5-8)21-18-15(9(2)24)17(25)11-6-10(19)7-14(23(26)27)16(11)22-18/h3-7H,1-2H3,(H2,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 397 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B after 30 mins by fluorometric end-point assay |
J Med Chem 52: 3093-7 (2009)
Article DOI: 10.1021/jm8014734
BindingDB Entry DOI: 10.7270/Q2KK9CQ4 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458130

(CHEMBL4204027)Show SMILES OC(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C22H22Cl2N2O5/c23-17-9-15(10-18(24)12-17)13-31-22(30)26-7-5-16(6-8-26)21(29)25-19-3-1-14(2-4-19)11-20(27)28/h1-4,9-10,12,16H,5-8,11,13H2,(H,25,29)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269626

(Naphthomycin A)Show SMILES C[C@H]1\C=C\C=C/C=C(C)\C(=O)NC2=C(Cl)C(=O)c3c(cc(C)c(O)c3C(=O)\C(C)=C\[C@H](C)[C@@H](O)[C@@H](C)\C=C\[C@@H](O)C\C=C(C)\C(=O)C[C@@H]1O)C2=O |r,c:4,12,42,t:2,6,29,37| Show InChI InChI=1S/C40H46ClNO9/c1-20-11-9-8-10-12-23(4)40(51)42-34-33(41)39(50)31-28(38(34)49)18-26(7)37(48)32(31)36(47)25(6)17-24(5)35(46)22(3)14-16-27(43)15-13-21(2)30(45)19-29(20)44/h8-14,16-18,20,22,24,27,29,35,43-44,46,48H,15,19H2,1-7H3,(H,42,51)/b10-8-,11-9+,16-14+,21-13+,23-12-,25-17+/t20-,22-,24-,27-,29-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of ascorbic acid/methylene blue activated recombinant human IDO expressed in Escherichia coli using L-Tryptophan as substrate after 60 min... |
J Nat Prod 80: 1378-1386 (2017)
Article DOI: 10.1021/acs.jnatprod.6b01059
BindingDB Entry DOI: 10.7270/Q2902687 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458128

(CHEMBL4206899)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2F)cc1 Show InChI InChI=1S/C21H24FN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 453 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data