 Found 229 hits with Last Name = 'kang' and Initial = 'kw'
Found 229 hits with Last Name = 'kang' and Initial = 'kw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50214974
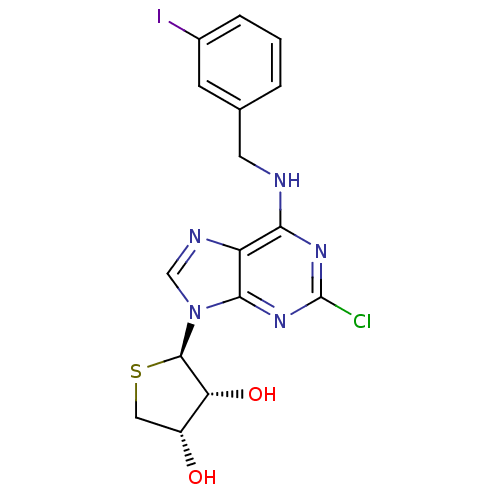
((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15ClIN5O2S/c17-16-21-13(19-5-8-2-1-3-9(18)4-8)11-14(22-16)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]I-AB-MECA from recombinant human A3AR expressed in CHO cell membranes measured after 60 mins by gamma counter analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50383373
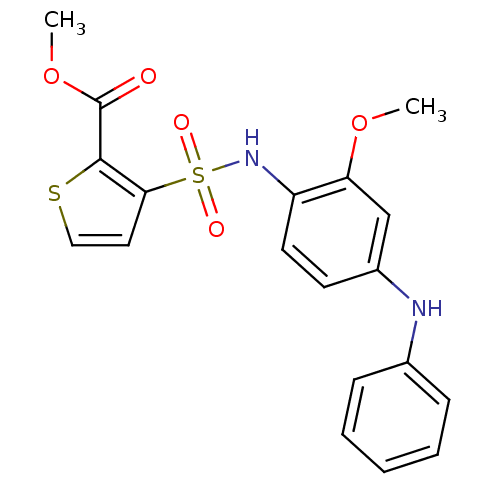
(CHEMBL592652 | GSK0660)Show SMILES COC(=O)c1sccc1S(=O)(=O)Nc1ccc(Nc2ccccc2)cc1OC Show InChI InChI=1S/C19H18N2O5S2/c1-25-16-12-14(20-13-6-4-3-5-7-13)8-9-15(16)21-28(23,24)17-10-11-27-18(17)19(22)26-2/h3-12,20-21H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
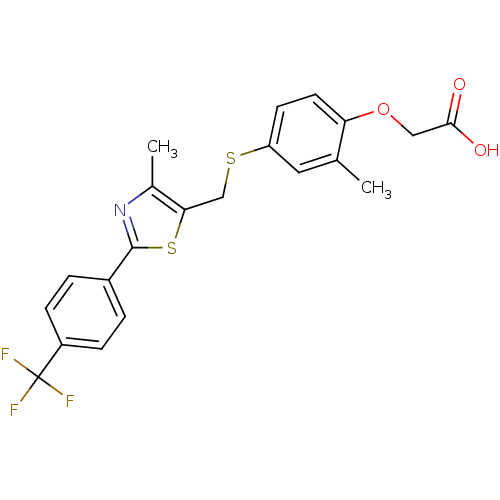
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50214974

((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15ClIN5O2S/c17-16-21-13(19-5-8-2-1-3-9(18)4-8)11-14(22-16)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50103521
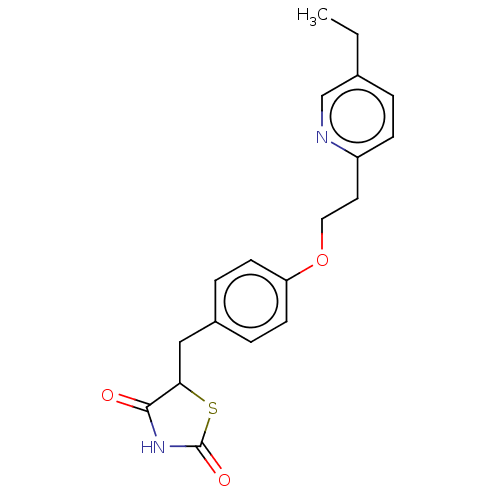
(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50555357

(CHEMBL1254751)Show SMILES O[C@@H]1CS[C@@H](Cn2cnc3c(NCc4cccc(I)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50311581
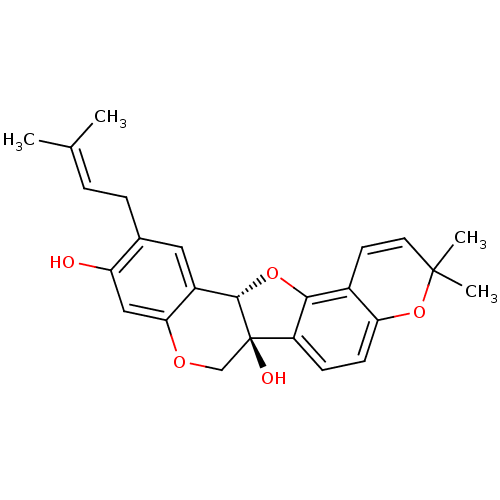
(CHEMBL1086764 | erysubin E)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc2-[#6@@H]3-[#8]-c4c(ccc5-[#8]C([#6])([#6])[#6]=[#6]-c45)[C@]3([#8])[#6]-[#8]-c2cc1-[#8] |r,c:19| Show InChI InChI=1S/C25H26O5/c1-14(2)5-6-15-11-17-21(12-19(15)26)28-13-25(27)18-7-8-20-16(22(18)29-23(17)25)9-10-24(3,4)30-20/h5,7-12,23,26-27H,6,13H2,1-4H3/t23-,25+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317430

(CHEMBL454849 | Erythrabyssin I | cristacarpin | cr...)Show SMILES [#6]-[#8]-c1ccc2c(-[#8]-[#6@H]3-c4ccc(-[#8])cc4-[#8]-[#6][C@@]23[#8])c1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C21H22O5/c1-12(2)4-6-14-17(24-3)9-8-16-19(14)26-20-15-7-5-13(22)10-18(15)25-11-21(16,20)23/h4-5,7-10,20,22-23H,6,11H2,1-3H3/t20-,21+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50555363

(CHEMBL4758410)Show SMILES O[C@@H]1CO[C@@H](Cn2cnc3c(NCc4cccc(I)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50555361

(CHEMBL4799672)Show SMILES O[C@@H]1CO[C@@H](Cn2cnc3c(NCc4cccc(Cl)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 504 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50555358

(CHEMBL1254750)Show SMILES O[C@@H]1CS[C@@H](Cn2cnc3c(NCc4cccc(Br)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317435
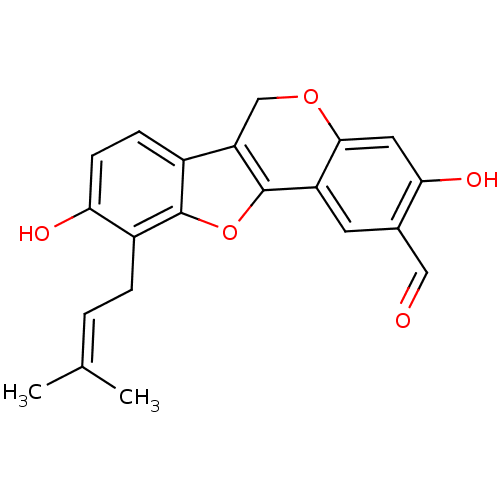
(CHEMBL1096406 | Erythribyssin O)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2c3-[#6]-[#8]-c4cc(-[#8])c(-[#6]=O)cc4-c3oc12 Show InChI InChI=1S/C21H18O5/c1-11(2)3-4-14-17(23)6-5-13-16-10-25-19-8-18(24)12(9-22)7-15(19)21(16)26-20(13)14/h3,5-9,23-24H,4,10H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555357

(CHEMBL1254751)Show SMILES O[C@@H]1CS[C@@H](Cn2cnc3c(NCc4cccc(I)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50555362
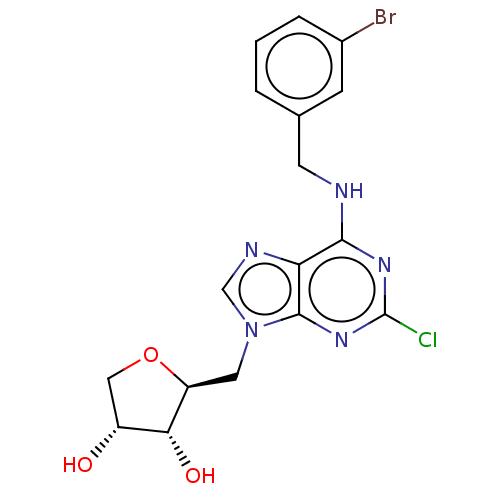
(CHEMBL4748172)Show SMILES O[C@@H]1CO[C@@H](Cn2cnc3c(NCc4cccc(Br)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50311583
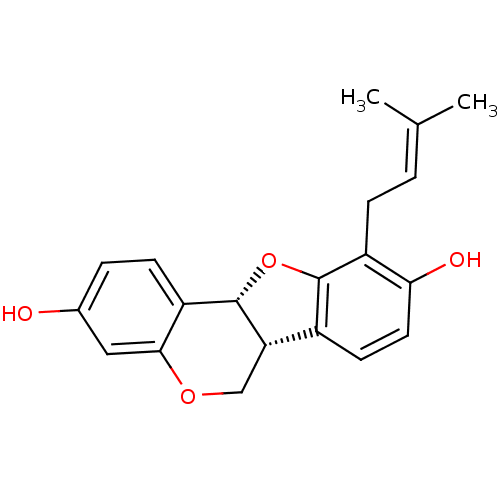
(Abyssinone II | CHEMBL508534 | phaseolidin | phase...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c2-[#8]-[#6@@H]-3-[#6@@H](-[#6]-[#8]-c4cc(-[#8])ccc-34)-c2ccc1-[#8] |r| Show InChI InChI=1S/C20H20O4/c1-11(2)3-5-14-17(22)8-7-13-16-10-23-18-9-12(21)4-6-15(18)20(16)24-19(13)14/h3-4,6-9,16,20-22H,5,10H2,1-2H3/t16-,20-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50311586
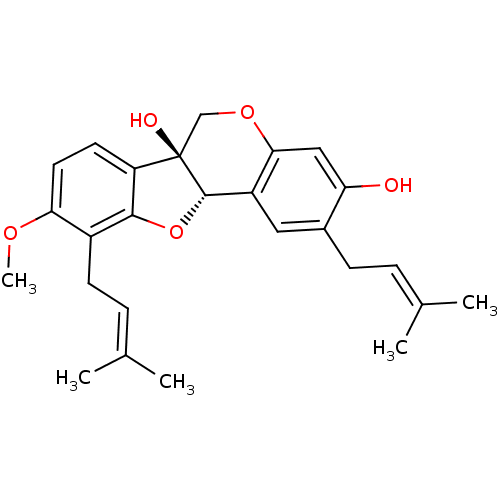
(CHEMBL1088462 | erystagallin A)Show SMILES [#6]-[#8]-c1ccc2c(-[#8]-[#6@H]3-c4cc(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc4-[#8]-[#6][C@@]23[#8])c1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C26H30O5/c1-15(2)6-8-17-12-19-23(13-21(17)27)30-14-26(28)20-10-11-22(29-5)18(9-7-16(3)4)24(20)31-25(19)26/h6-7,10-13,25,27-28H,8-9,14H2,1-5H3/t25-,26+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50555359
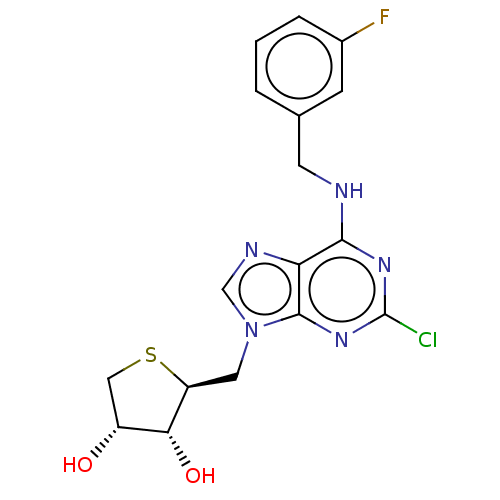
(CHEMBL1254667)Show SMILES O[C@@H]1CS[C@@H](Cn2cnc3c(NCc4cccc(F)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-delta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317436
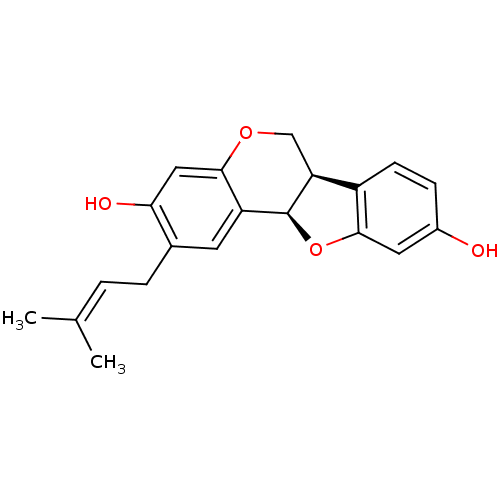
(CHEMBL1096407 | calopocarpin)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2-[#6@@H]-3-[#8]-c4cc(-[#8])ccc4-[#6@@H]-3-[#6]-[#8]-c2cc1-[#8] |r| Show InChI InChI=1S/C20H20O4/c1-11(2)3-4-12-7-15-18(9-17(12)22)23-10-16-14-6-5-13(21)8-19(14)24-20(15)16/h3,5-9,16,20-22H,4,10H2,1-2H3/t16-,20-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317434

(CHEMBL1097045 | eryvarin D)Show SMILES [#6]-[#8]-c1ccc2c3-[#6]-[#8]-c4cc(-[#8])ccc4-c3oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C21H20O4/c1-12(2)4-6-15-18(23-3)9-8-14-17-11-24-19-10-13(22)5-7-16(19)21(17)25-20(14)15/h4-5,7-10,22H,6,11H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50214974

((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15ClIN5O2S/c17-16-21-13(19-5-8-2-1-3-9(18)4-8)11-14(22-16)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555363

(CHEMBL4758410)Show SMILES O[C@@H]1CO[C@@H](Cn2cnc3c(NCc4cccc(I)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317431
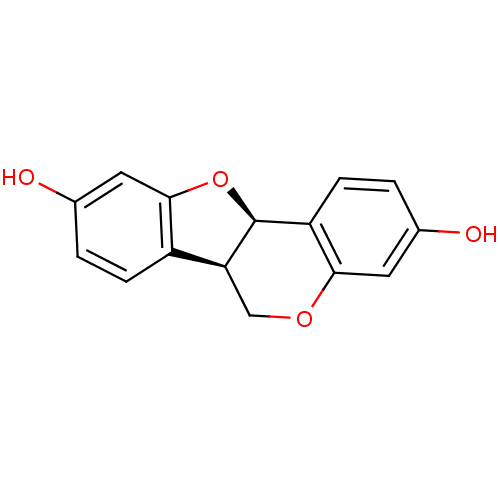
(CHEMBL1098413 | demethylmedicarpin)Show SMILES Oc1ccc2[C@@H]3COc4cc(O)ccc4[C@@H]3Oc2c1 |r| Show InChI InChI=1S/C15H12O4/c16-8-2-4-11-13(5-8)18-7-12-10-3-1-9(17)6-14(10)19-15(11)12/h1-6,12,15-17H,7H2/t12-,15-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555359

(CHEMBL1254667)Show SMILES O[C@@H]1CS[C@@H](Cn2cnc3c(NCc4cccc(F)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555358

(CHEMBL1254750)Show SMILES O[C@@H]1CS[C@@H](Cn2cnc3c(NCc4cccc(Br)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317437
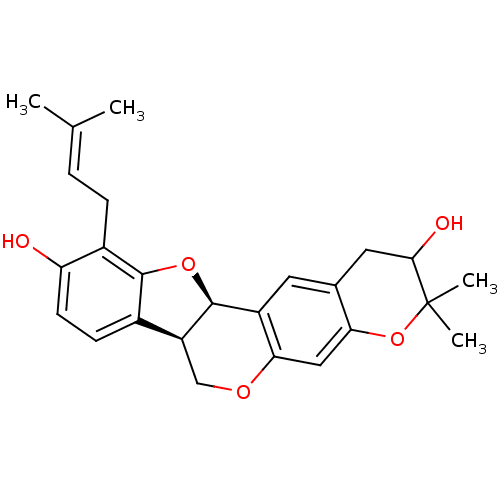
(CHEMBL1096408 | Erythribyssin L)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c2-[#8]-[#6@@H]-3-[#6@@H](-[#6]-[#8]-c4cc5-[#8]C([#6])([#6])[#6](-[#8])-[#6]-c5cc-34)-c2ccc1-[#8] |r| Show InChI InChI=1S/C25H28O5/c1-13(2)5-6-16-19(26)8-7-15-18-12-28-21-11-20-14(10-22(27)25(3,4)30-20)9-17(21)24(18)29-23(15)16/h5,7-9,11,18,22,24,26-27H,6,10,12H2,1-4H3/t18-,22?,24-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317438
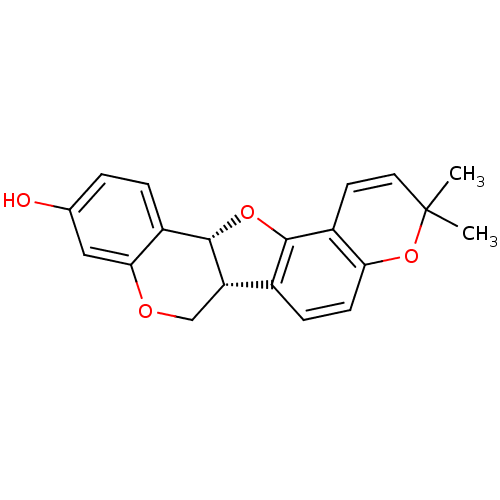
(Abyssinone I | CHEMBL448350 | phaseollin)Show SMILES CC1(C)Oc2ccc3[C@@H]4COc5cc(O)ccc5[C@@H]4Oc3c2C=C1 |r,c:26| Show InChI InChI=1S/C20H18O4/c1-20(2)8-7-14-16(24-20)6-5-12-15-10-22-17-9-11(21)3-4-13(17)19(15)23-18(12)14/h3-9,15,19,21H,10H2,1-2H3/t15-,19-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555355

(CHEMBL4760772)Show SMILES CC#Cc1nc(NCc2cccc(Br)c2)c2ncn(C[C@@H]3SC[C@@H](O)[C@H]3O)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555360

(CHEMBL4740559)Show SMILES CC#Cc1nc(NCc2cccc(I)c2)c2ncn(C[C@@H]3SC[C@@H](O)[C@H]3O)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555361

(CHEMBL4799672)Show SMILES O[C@@H]1CO[C@@H](Cn2cnc3c(NCc4cccc(Cl)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555356
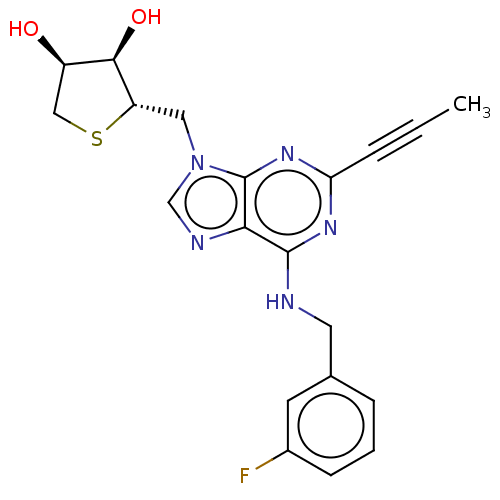
(CHEMBL4756797)Show SMILES CC#Cc1nc(NCc2cccc(F)c2)c2ncn(C[C@@H]3SC[C@@H](O)[C@H]3O)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50555362

(CHEMBL4748172)Show SMILES O[C@@H]1CO[C@@H](Cn2cnc3c(NCc4cccc(Br)c4)nc(Cl)nc23)[C@@H]1O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPAR-gamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01874
BindingDB Entry DOI: 10.7270/Q2VX0M6T |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317433
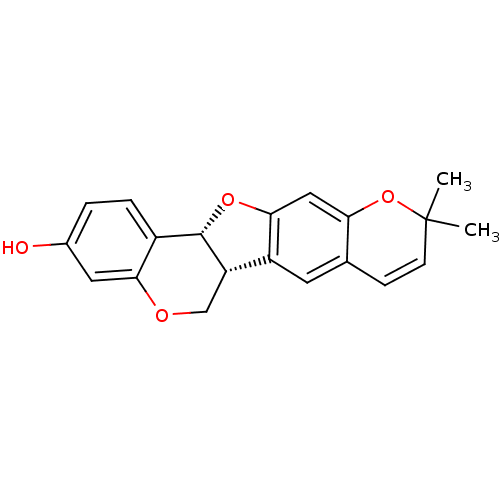
(CHEMBL1098729 | isoneorautenol)Show SMILES CC1(C)Oc2cc3O[C@@H]4[C@@H](COc5cc(O)ccc45)c3cc2C=C1 |r,c:26| Show InChI InChI=1S/C20H18O4/c1-20(2)6-5-11-7-14-15-10-22-17-8-12(21)3-4-13(17)19(15)23-18(14)9-16(11)24-20/h3-9,15,19,21H,10H2,1-2H3/t15-,19-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317439
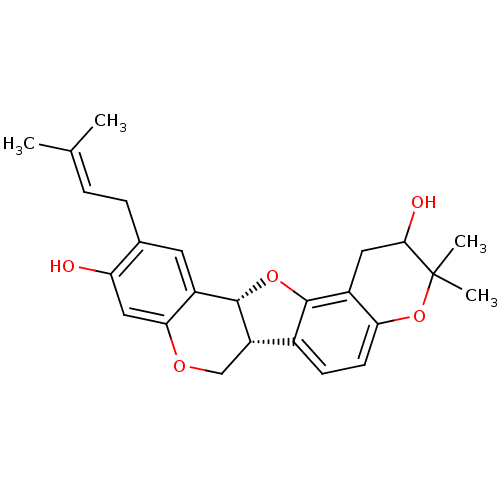
(CHEMBL1095422 | erysubin D)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2-[#6@@H]-3-[#8]-c4c(ccc5-[#8]C([#6])([#6])[#6](-[#8])-[#6]-c45)-[#6@@H]-3-[#6]-[#8]-c2cc1-[#8] |r| Show InChI InChI=1S/C25H28O5/c1-13(2)5-6-14-9-16-21(11-19(14)26)28-12-18-15-7-8-20-17(23(15)29-24(16)18)10-22(27)25(3,4)30-20/h5,7-9,11,18,22,24,26-27H,6,10,12H2,1-4H3/t18-,22?,24-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50317432
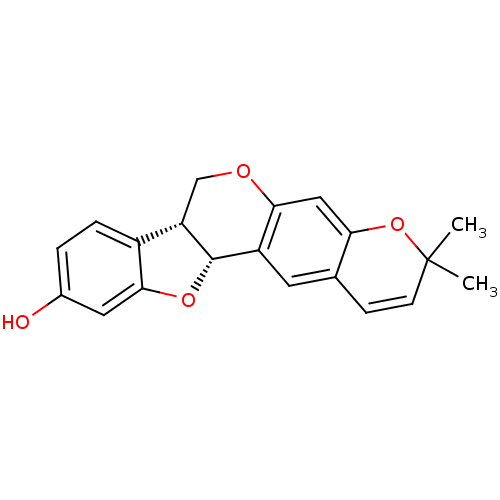
(CHEMBL1098728 | NEORAUTENOL)Show SMILES CC1(C)Oc2cc3OC[C@@H]4[C@@H](Oc5cc(O)ccc45)c3cc2C=C1 |r,c:26| Show InChI InChI=1S/C20H18O4/c1-20(2)6-5-11-7-14-17(9-16(11)24-20)22-10-15-13-4-3-12(21)8-18(13)23-19(14)15/h3-9,15,19,21H,10H2,1-2H3/t15-,19-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Clostridium perfringens neuraminidase |
Bioorg Med Chem 18: 3335-44 (2010)
Article DOI: 10.1016/j.bmc.2010.03.005
BindingDB Entry DOI: 10.7270/Q2MW2HBM |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50386567

(CHEMBL2048437)Show SMILES Clc1cncc(Cl)c1NNC(=O)C1C2CC3CC(C2)C(Br)C1C3 |TLB:22:21:16.15.14:18,20:19:16:14.13.18,20:19:16.15.14:18,THB:10:12:16.15.14:18,22:15:18:19.21.12,20:19:16.15.22:13.18.12,12:21:16:14.13.18,12:13:16:19.22.21| Show InChI InChI=1S/C16H18BrCl2N3O/c17-14-9-2-7-1-8(4-9)13(10(14)3-7)16(23)22-21-15-11(18)5-20-6-12(15)19/h5-10,13-14H,1-4H2,(H,20,21)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... |
J Med Chem 55: 3687-98 (2012)
Article DOI: 10.1021/jm2012326
BindingDB Entry DOI: 10.7270/Q21G0NB3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) measured after 1 hr in presence of ATP by TR-FRET assay |
Bioorg Med Chem 24: 5036-5046 (2016)
Article DOI: 10.1016/j.bmc.2016.08.008
BindingDB Entry DOI: 10.7270/Q2C82DRC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using GEEPLYWSFPAKKK as substrate measured after 40 mins in presence of ATP by scintillation counting method |
Bioorg Med Chem 24: 5036-5046 (2016)
Article DOI: 10.1016/j.bmc.2016.08.008
BindingDB Entry DOI: 10.7270/Q2C82DRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using GEEPLYWSFPAKKK as substrate measured after 40 mins in presence of ATP by scintillation counting method |
Bioorg Med Chem 24: 5036-5046 (2016)
Article DOI: 10.1016/j.bmc.2016.08.008
BindingDB Entry DOI: 10.7270/Q2C82DRC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal 6His-tagged JAK2 (808 to end amino acids) expressed in Sf21 cells measured after 1 hr in presence of ATP b... |
Bioorg Med Chem 24: 5036-5046 (2016)
Article DOI: 10.1016/j.bmc.2016.08.008
BindingDB Entry DOI: 10.7270/Q2C82DRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal 6His-tagged JAK2 (808 to end amino acids) expressed in Sf21 cells measured after 1 hr in presence of ATP b... |
Bioorg Med Chem 24: 5036-5046 (2016)
Article DOI: 10.1016/j.bmc.2016.08.008
BindingDB Entry DOI: 10.7270/Q2C82DRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50386567

(CHEMBL2048437)Show SMILES Clc1cncc(Cl)c1NNC(=O)C1C2CC3CC(C2)C(Br)C1C3 |TLB:22:21:16.15.14:18,20:19:16:14.13.18,20:19:16.15.14:18,THB:10:12:16.15.14:18,22:15:18:19.21.12,20:19:16.15.22:13.18.12,12:21:16:14.13.18,12:13:16:19.22.21| Show InChI InChI=1S/C16H18BrCl2N3O/c17-14-9-2-7-1-8(4-9)13(10(14)3-7)16(23)22-21-15-11(18)5-20-6-12(15)19/h5-10,13-14H,1-4H2,(H,20,21)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... |
J Med Chem 55: 3687-98 (2012)
Article DOI: 10.1021/jm2012326
BindingDB Entry DOI: 10.7270/Q21G0NB3 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50386566
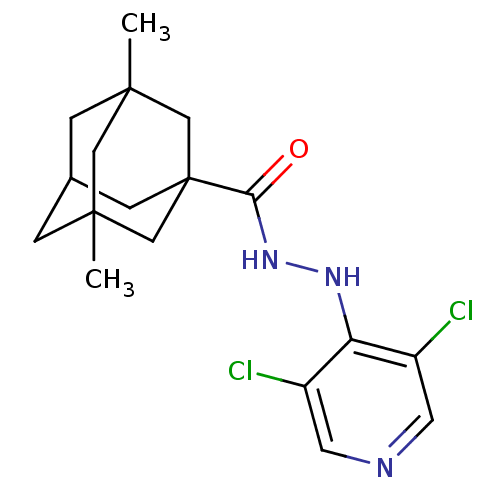
(CHEMBL2048438)Show SMILES CC12CC3CC(C)(C1)CC(C3)(C2)C(=O)NNc1c(Cl)cncc1Cl |TLB:12:9:4:2.7.1,7:1:4.5.8:10,0:1:4:8.9.10,THB:7:5:10:2.1.11,6:5:10:2.1.11,11:1:4:8.9.10,11:9:4:2.7.1| Show InChI InChI=1S/C18H23Cl2N3O/c1-16-3-11-4-17(2,8-16)10-18(5-11,9-16)15(24)23-22-14-12(19)6-21-7-13(14)20/h6-7,11H,3-5,8-10H2,1-2H3,(H,21,22)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... |
J Med Chem 55: 3687-98 (2012)
Article DOI: 10.1021/jm2012326
BindingDB Entry DOI: 10.7270/Q21G0NB3 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50386566

(CHEMBL2048438)Show SMILES CC12CC3CC(C)(C1)CC(C3)(C2)C(=O)NNc1c(Cl)cncc1Cl |TLB:12:9:4:2.7.1,7:1:4.5.8:10,0:1:4:8.9.10,THB:7:5:10:2.1.11,6:5:10:2.1.11,11:1:4:8.9.10,11:9:4:2.7.1| Show InChI InChI=1S/C18H23Cl2N3O/c1-16-3-11-4-17(2,8-16)10-18(5-11,9-16)15(24)23-22-14-12(19)6-21-7-13(14)20/h6-7,11H,3-5,8-10H2,1-2H3,(H,21,22)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... |
J Med Chem 55: 3687-98 (2012)
Article DOI: 10.1021/jm2012326
BindingDB Entry DOI: 10.7270/Q21G0NB3 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50075452
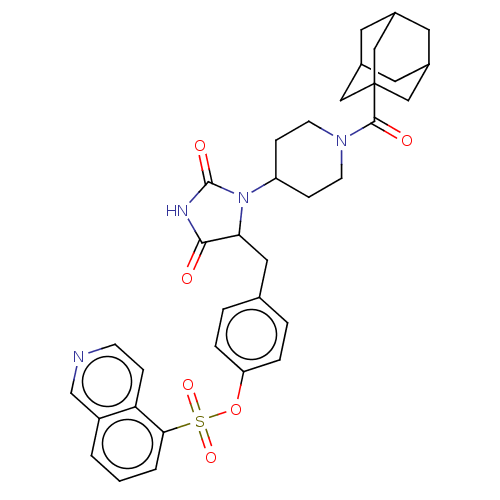
(CHEMBL3415315)Show SMILES O=C(N1CCC(CC1)N1C(Cc2ccc(OS(=O)(=O)c3cccc4cnccc34)cc2)C(=O)NC1=O)C12CC3CC(CC(C3)C1)C2 |TLB:45:36:43:39.40.41,THB:41:40:37:43.42.44,41:42:39.40.45:37,45:40:43:36.37.44,1:36:43:39.40.41| Show InChI InChI=1S/C35H38N4O6S/c40-32-30(17-22-4-6-28(7-5-22)45-46(43,44)31-3-1-2-26-21-36-11-8-29(26)31)39(34(42)37-32)27-9-12-38(13-10-27)33(41)35-18-23-14-24(19-35)16-25(15-23)20-35/h1-8,11,21,23-25,27,30H,9-10,12-20H2,(H,37,40,42) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor in LPS/IFN-gamma-differentiated human THP1 cells assessed as inhibition of BzATP-induced IL-1beta release preinc... |
J Med Chem 58: 2114-34 (2015)
Article DOI: 10.1021/jm500324g
BindingDB Entry DOI: 10.7270/Q2H133QD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50075454

(CHEMBL3415330)Show SMILES O=C(CC12CC3CC(CC(C3)C1)C2)N1CCC(CC1)N1C(Cc2ccc(OS(=O)(=O)c3cccc4cnccc34)cc2)C(=O)NC1=O |TLB:12:3:10:6.7.8,THB:8:7:4:10.9.11,8:9:6.7.12:4,12:7:10:3.4.11,2:3:10:6.7.8| Show InChI InChI=1S/C36H40N4O6S/c41-33(21-36-18-24-14-25(19-36)16-26(15-24)20-36)39-12-9-28(10-13-39)40-31(34(42)38-35(40)43)17-23-4-6-29(7-5-23)46-47(44,45)32-3-1-2-27-22-37-11-8-30(27)32/h1-8,11,22,24-26,28,31H,9-10,12-21H2,(H,38,42,43) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor in LPS/IFN-gamma-differentiated human THP1 cells assessed as inhibition of BzATP-induced IL-1beta release preinc... |
J Med Chem 58: 2114-34 (2015)
Article DOI: 10.1021/jm500324g
BindingDB Entry DOI: 10.7270/Q2H133QD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50075452

(CHEMBL3415315)Show SMILES O=C(N1CCC(CC1)N1C(Cc2ccc(OS(=O)(=O)c3cccc4cnccc34)cc2)C(=O)NC1=O)C12CC3CC(CC(C3)C1)C2 |TLB:45:36:43:39.40.41,THB:41:40:37:43.42.44,41:42:39.40.45:37,45:40:43:36.37.44,1:36:43:39.40.41| Show InChI InChI=1S/C35H38N4O6S/c40-32-30(17-22-4-6-28(7-5-22)45-46(43,44)31-3-1-2-26-21-36-11-8-29(26)31)39(34(42)37-32)27-9-12-38(13-10-27)33(41)35-18-23-14-24(19-35)16-25(15-23)20-35/h1-8,11,21,23-25,27,30H,9-10,12-20H2,(H,37,40,42) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced intracellular ethidium bromi... |
J Med Chem 58: 2114-34 (2015)
Article DOI: 10.1021/jm500324g
BindingDB Entry DOI: 10.7270/Q2H133QD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50075455

(CHEMBL3415331)Show SMILES BrC12CC3CC(C1)CC(C3)(C2)C(=O)N1CCC(CC1)N1C(Cc2ccc(OS(=O)(=O)c3cccc4cnccc34)cc2)C(=O)NC1=O |TLB:4:5:2.3.9:10,9:8:6:2.3.4,THB:9:3:6:8.10.7| Show InChI InChI=1S/C35H37BrN4O6S/c36-35-18-23-14-24(19-35)17-34(16-23,21-35)32(42)39-12-9-26(10-13-39)40-29(31(41)38-33(40)43)15-22-4-6-27(7-5-22)46-47(44,45)30-3-1-2-25-20-37-11-8-28(25)30/h1-8,11,20,23-24,26,29H,9-10,12-19,21H2,(H,38,41,43) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor in LPS/IFN-gamma-differentiated human THP1 cells assessed as inhibition of BzATP-induced IL-1beta release preinc... |
J Med Chem 58: 2114-34 (2015)
Article DOI: 10.1021/jm500324g
BindingDB Entry DOI: 10.7270/Q2H133QD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50075458
(CHEMBL3415334)Show SMILES O=C(CC1CC2CCC1C2)N1CCC(CC1)N1C(Cc2ccc(OS(=O)(=O)c3cccc4cnccc34)cc2)C(=O)NC1=O Show InChI InChI=1S/C33H36N4O6S/c38-31(19-25-17-22-4-7-23(25)16-22)36-14-11-26(12-15-36)37-29(32(39)35-33(37)40)18-21-5-8-27(9-6-21)43-44(41,42)30-3-1-2-24-20-34-13-10-28(24)30/h1-3,5-6,8-10,13,20,22-23,25-26,29H,4,7,11-12,14-19H2,(H,35,39,40) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor in LPS/IFN-gamma-differentiated human THP1 cells assessed as inhibition of BzATP-induced IL-1beta release preinc... |
J Med Chem 58: 2114-34 (2015)
Article DOI: 10.1021/jm500324g
BindingDB Entry DOI: 10.7270/Q2H133QD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50075455

(CHEMBL3415331)Show SMILES BrC12CC3CC(C1)CC(C3)(C2)C(=O)N1CCC(CC1)N1C(Cc2ccc(OS(=O)(=O)c3cccc4cnccc34)cc2)C(=O)NC1=O |TLB:4:5:2.3.9:10,9:8:6:2.3.4,THB:9:3:6:8.10.7| Show InChI InChI=1S/C35H37BrN4O6S/c36-35-18-23-14-24(19-35)17-34(16-23,21-35)32(42)39-12-9-26(10-13-39)40-29(31(41)38-33(40)43)15-22-4-6-27(7-5-22)46-47(44,45)30-3-1-2-25-20-37-11-8-28(25)30/h1-8,11,20,23-24,26,29H,9-10,12-19,21H2,(H,38,41,43) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced intracellular ethidium bromi... |
J Med Chem 58: 2114-34 (2015)
Article DOI: 10.1021/jm500324g
BindingDB Entry DOI: 10.7270/Q2H133QD |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50230061
((S)-4-(3-(4-benzoylpiperazin-1-yl)-2-(benzyloxycar...)Show SMILES [O-][N+](=O)c1ccc(cc1)S(=O)(=O)Oc1ccc(C[C@H](NC(=O)OCc2ccccc2)C(=O)N2CCN(CC2)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C34H32N4O9S/c39-32(27-9-5-2-6-10-27)36-19-21-37(22-20-36)33(40)31(35-34(41)46-24-26-7-3-1-4-8-26)23-25-11-15-29(16-12-25)47-48(44,45)30-17-13-28(14-18-30)38(42)43/h1-18,31H,19-24H2,(H,35,41)/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST)
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor |
J Med Chem 58: 2114-34 (2015)
Article DOI: 10.1021/jm500324g
BindingDB Entry DOI: 10.7270/Q2H133QD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data