

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343354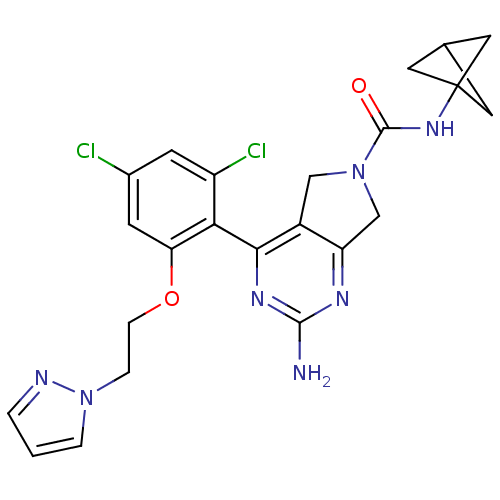 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343355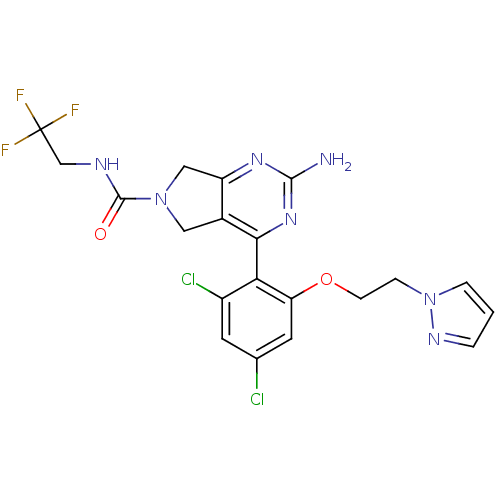 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343372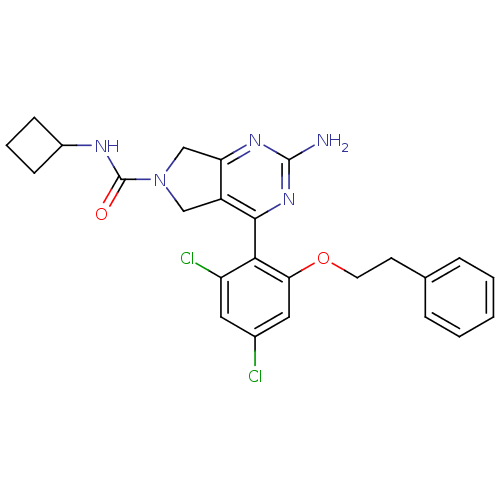 (Amino-4-(2,4-dichloro-6-phenethyloxyphenyl)-5,7-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343380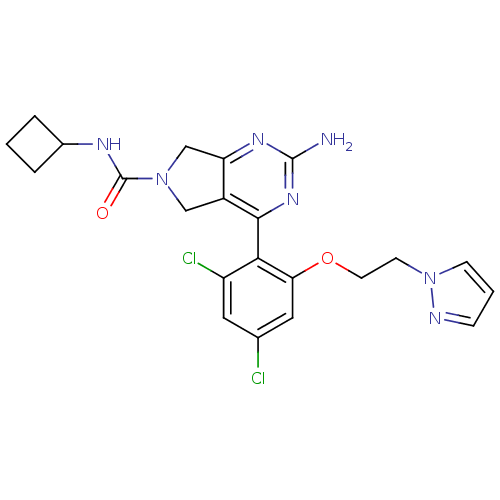 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343370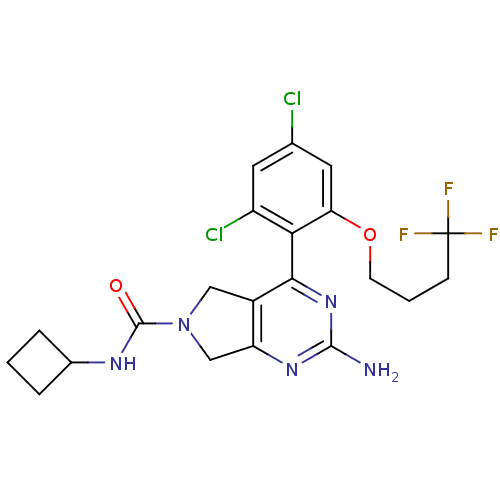 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343386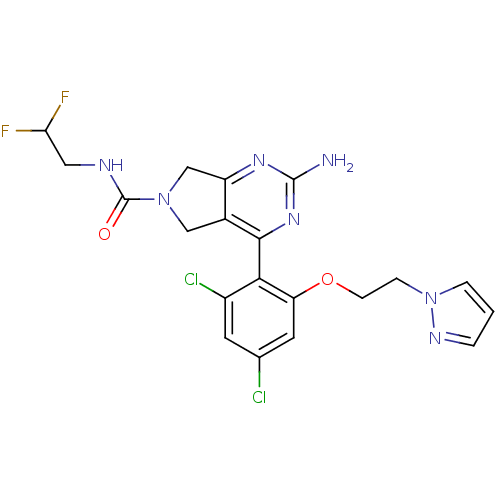 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343385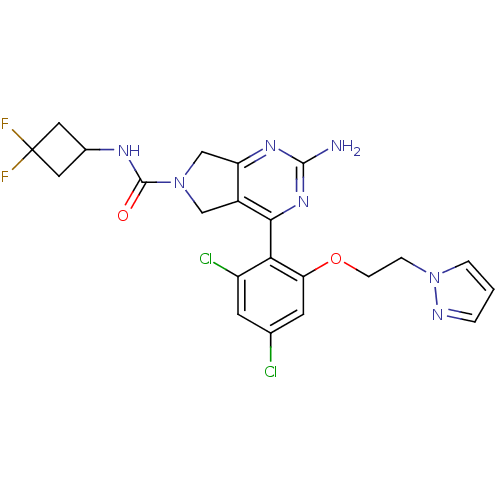 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343356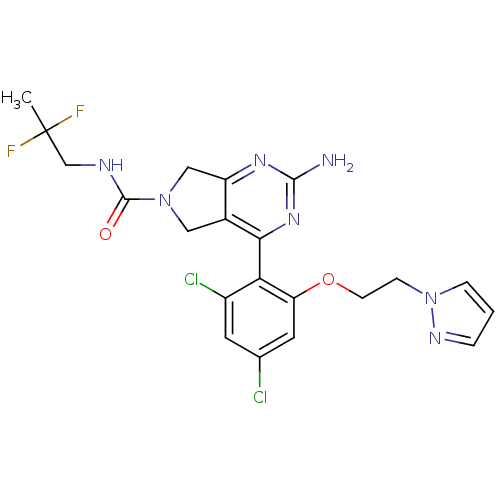 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343384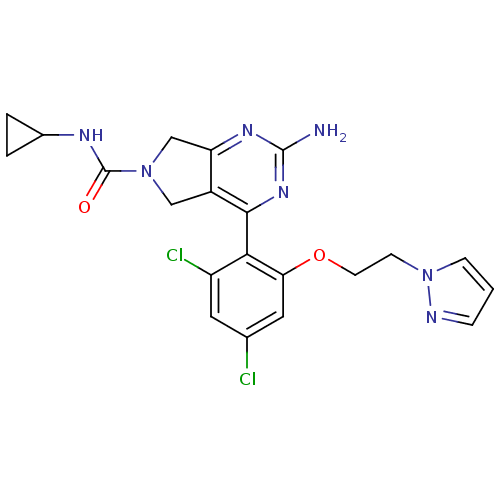 (4-(2-(2-(1H-pyrazol-1-yl)ethoxy)-4,6-dichloropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343383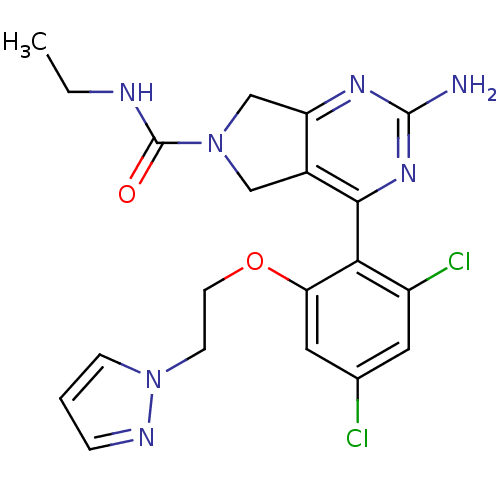 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343378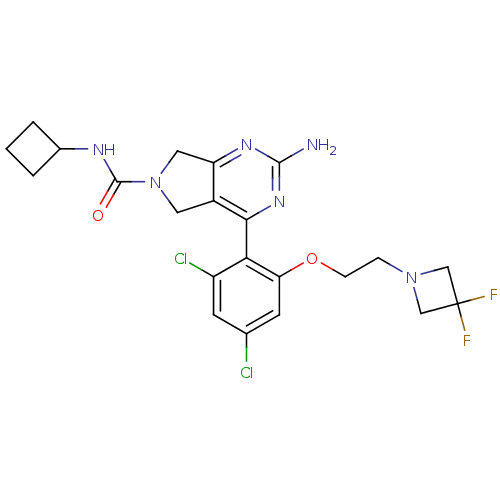 (2-Amino-4-{2,4-dichloro-6-[2-(3,3-difluoro-azetidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343369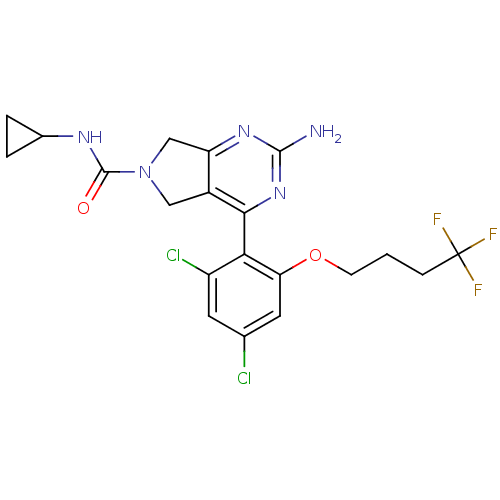 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343382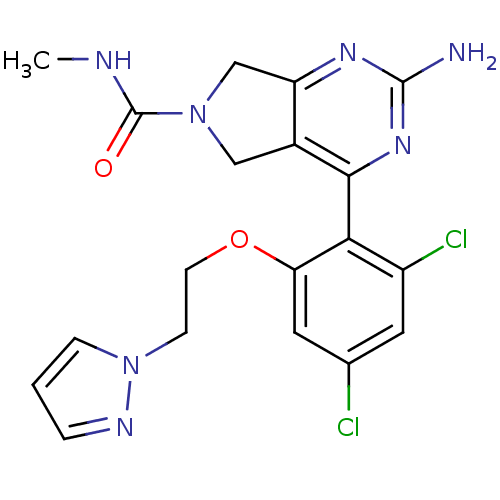 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343368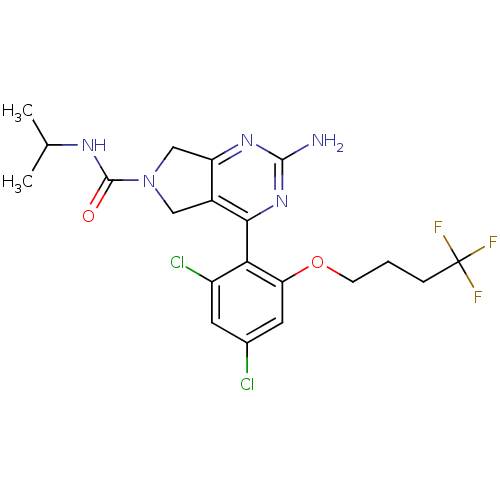 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343366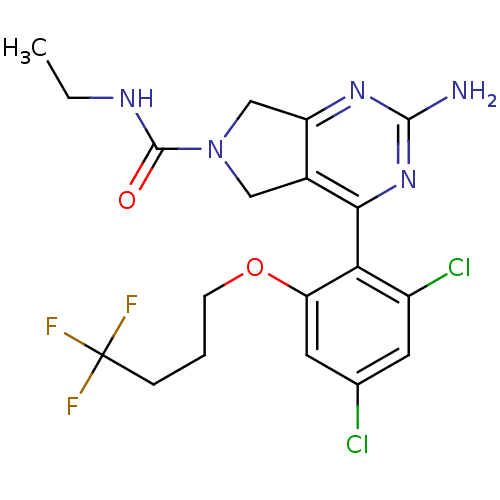 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343373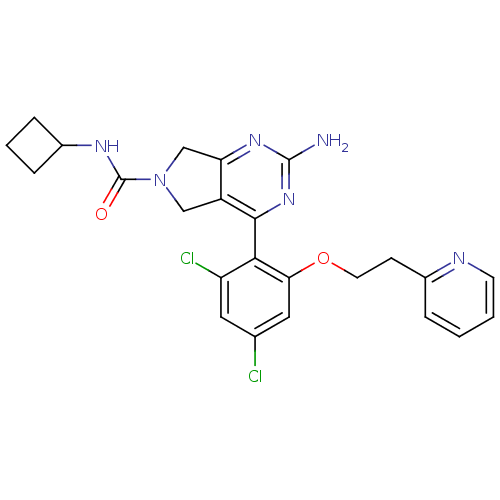 (2-Amino-4-[2,4-dichloro-6-(2-pyridin-2-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343381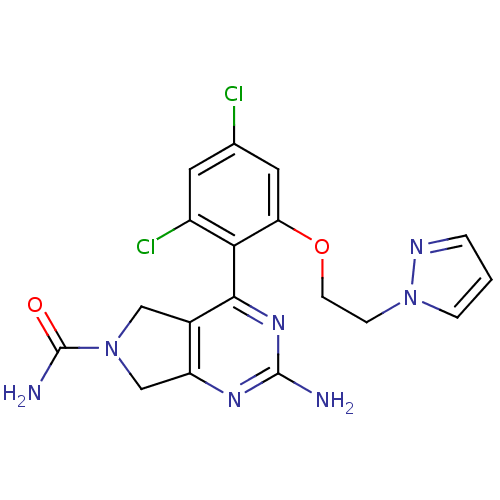 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343367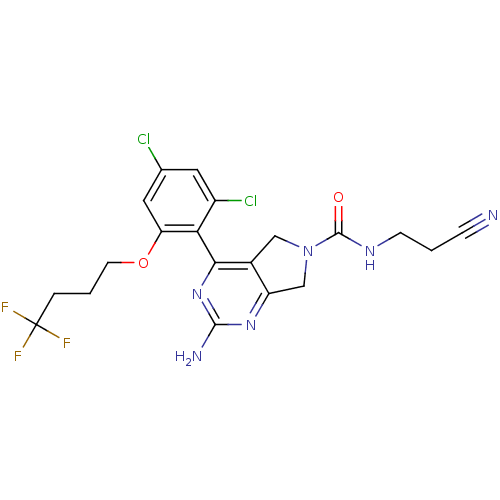 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343374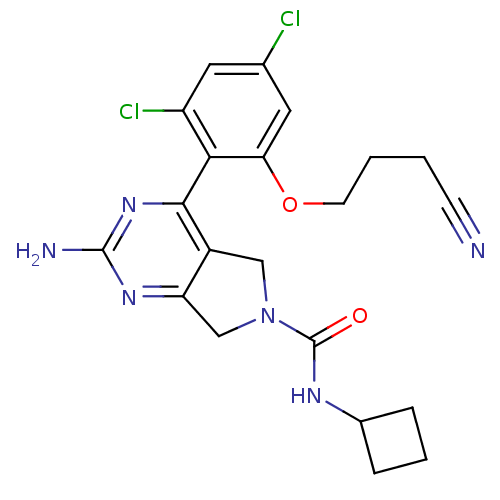 (2-amino-N-cyclobutyl-4-(2,4-dichloro-6-(3-cyanopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343365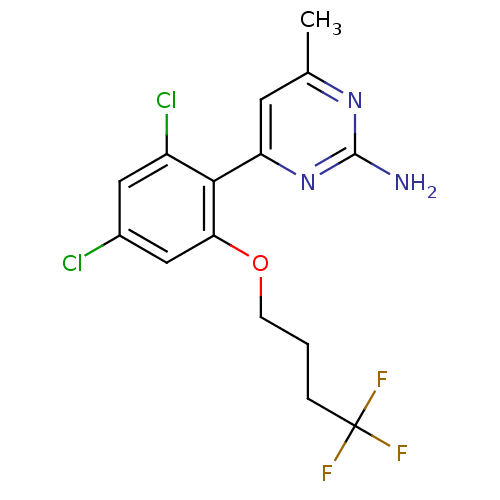 (4-[2,4-Dichloro-6-(4,4,4-trifluoro-butoxy)-phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343371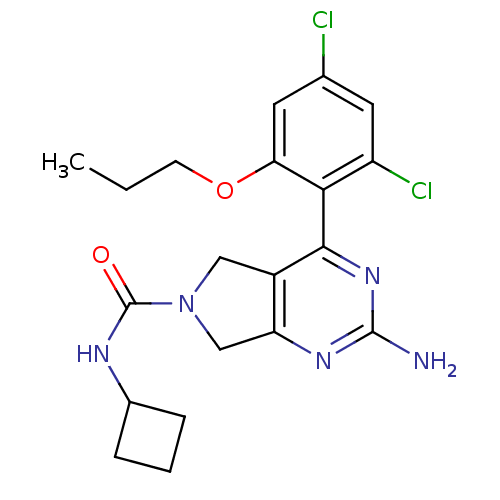 (2-amino-N-cyclobutyl-4-(2,4-dichloro-6-propoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343377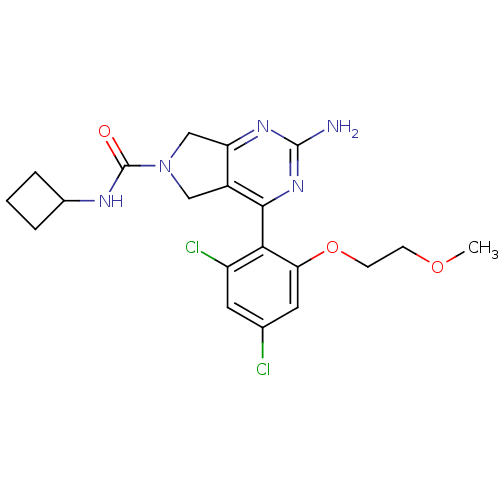 (2-Amino-4-[2,4-dichloro-6-(2-methoxyethoxy)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088503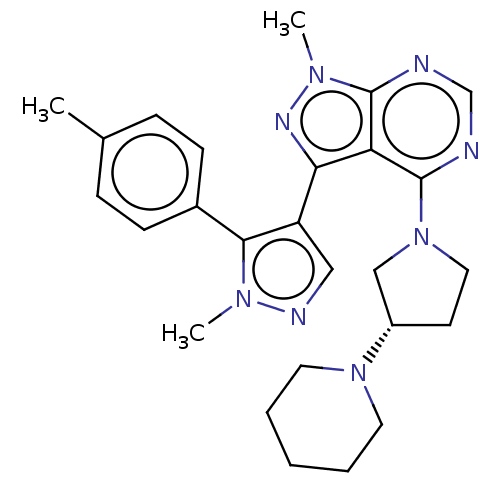 (CHEMBL3527048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A4 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343364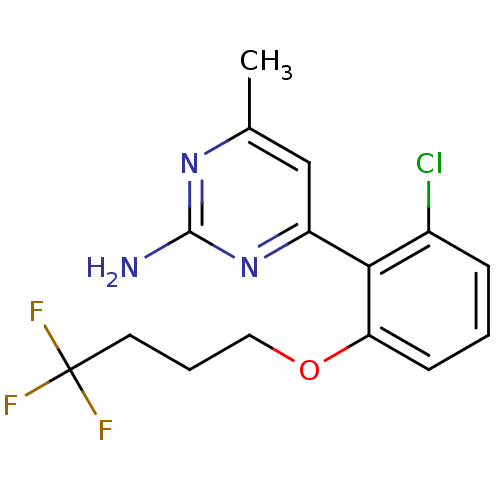 (4-[2-Chloro-6-(4,4,4-trifluoro-butoxy)-phenyl]-6-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A4 in presence of human P450 oxidoreductase and b5 assessed as decreas... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343376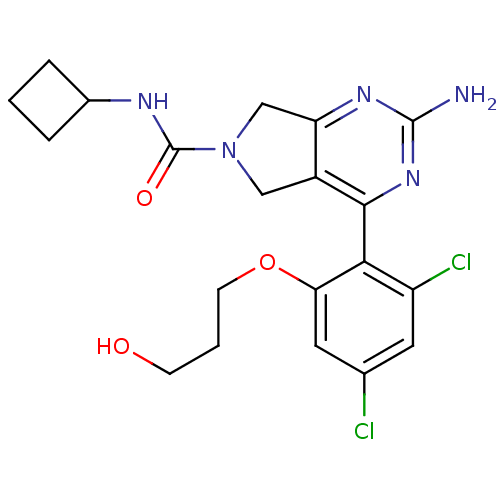 (2-Amino-4-[2,4-dichloro-6-(3-hydroxypropoxy)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343379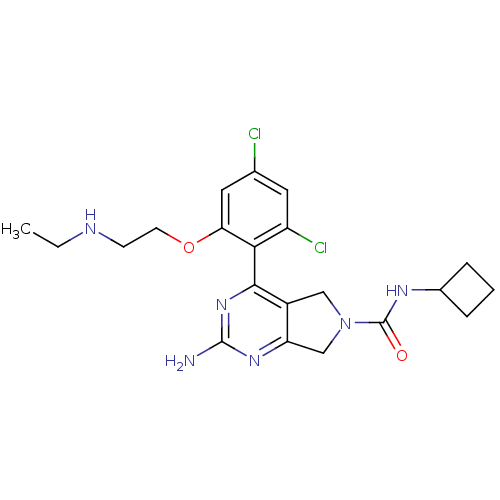 (2-Amino-4-[2,4-dichloro-6-(2-ethylamino-ethoxy)-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343375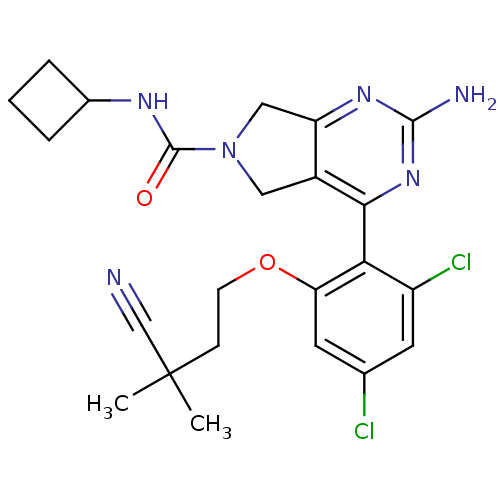 (2-Amino-4-[2,4-dichloro-6-(3-cyano-3,3-dimethylpro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343362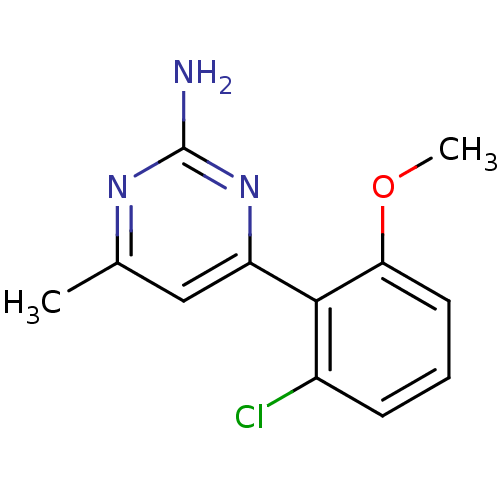 (4-(2-chloro-6-methoxyphenyl)-6-methylpyrimidin-2-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343360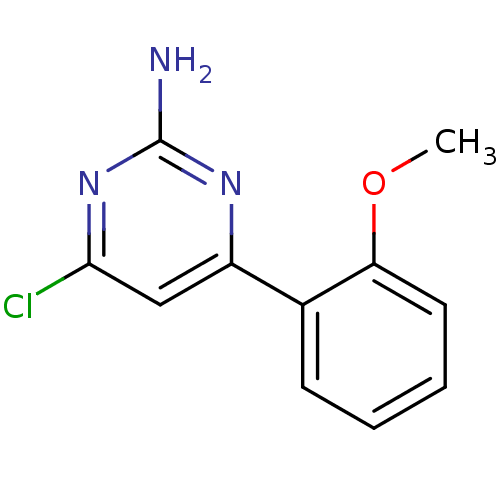 (4-Chloro-6-(2-methoxyphenyl)-pyrimidin-2-ylamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A5 in presence of human P450 oxidoreductase and b5 assessed as decreas... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343363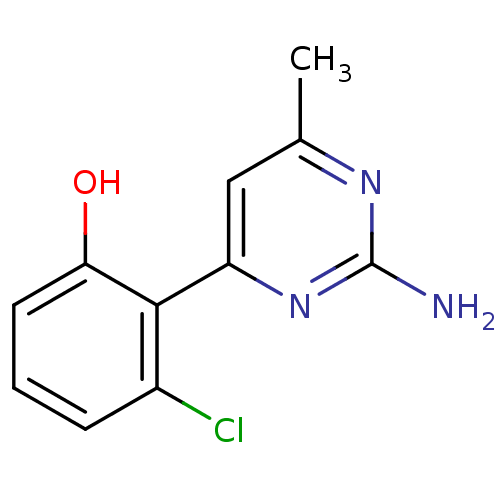 (2-(2-amino-6-methylpyrimidin-4-yl)-3-chlorophenol ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A5 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343361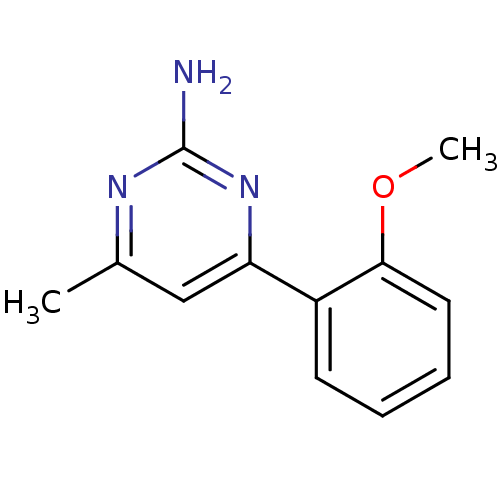 (4-(2-methoxyphenyl)-6-methylpyrimidin-2-amine | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50343365 (4-[2,4-Dichloro-6-(4,4,4-trifluoro-butoxy)-phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG channel expressed in HEK293 cells | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456821 (CHEMBL4208961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456822 (CHEMBL4205764) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456825 (CHEMBL4210401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456826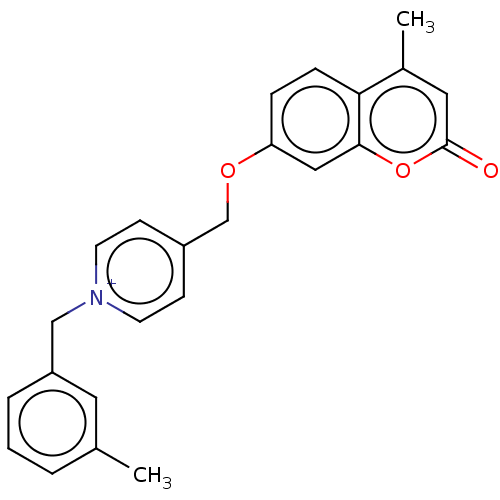 (CHEMBL4205520) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456827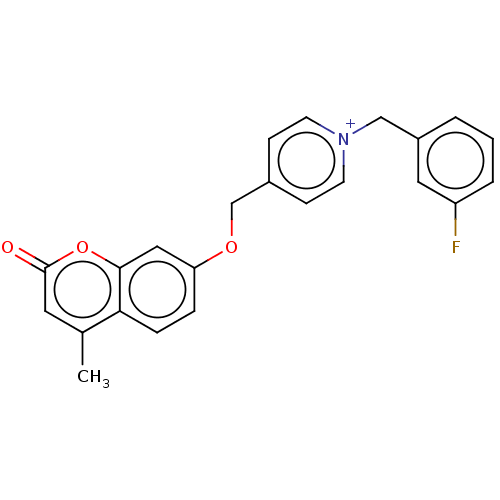 (CHEMBL4206041) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456823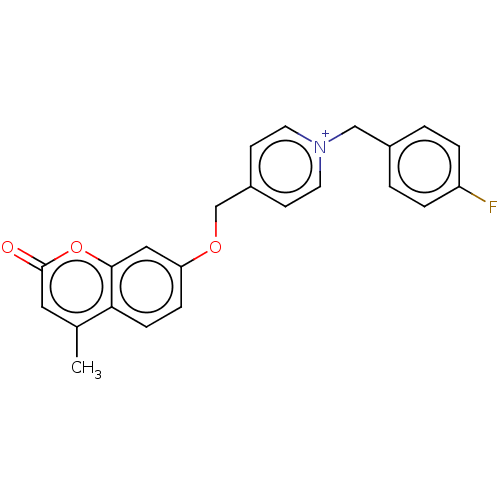 (CHEMBL4217755) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456824 (CHEMBL4217466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456817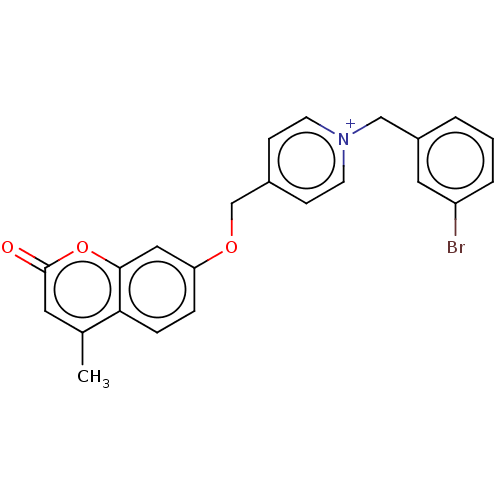 (CHEMBL4218281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456820 (CHEMBL4205110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456818 (CHEMBL4213345) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456819 (CHEMBL4213874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50356991 (CHEMBL1916247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay | J Med Chem 54: 7693-704 (2011) Article DOI: 10.1021/jm201059s BindingDB Entry DOI: 10.7270/Q2348KS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50356988 (CHEMBL1916245) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human AR overexpressed in human LNCAP cells by luciferase reporter gene assay | J Med Chem 54: 7693-704 (2011) Article DOI: 10.1021/jm201059s BindingDB Entry DOI: 10.7270/Q2348KS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456825 (CHEMBL4210401) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition measured up to 180... | Eur J Med Chem 139: 48-59 (2017) Article DOI: 10.1016/j.ejmech.2017.07.055 BindingDB Entry DOI: 10.7270/Q2GM89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 464 total ) | Next | Last >> |