 Found 224 hits with Last Name = 'kanno' and Initial = 'h'
Found 224 hits with Last Name = 'kanno' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Oryctolagus cuniculus) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rabbit factor 10a by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Rattus norvegicus (rat)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat factor 10a by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tissue plasminogen activator by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tissue plasminogen activator by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 7a/soluble tissue factor by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358999

(CHEMBL1923892)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(cc(Cl)c1CCC(=O)Nc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C26H26Cl2N4O4S/c1-14(2)32-10-9-20-22(13-32)37-25(31-20)24(34)30-21-12-15(26(35)36)11-19(28)18(21)7-8-23(33)29-17-5-3-16(27)4-6-17/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,29,33)(H,30,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35767
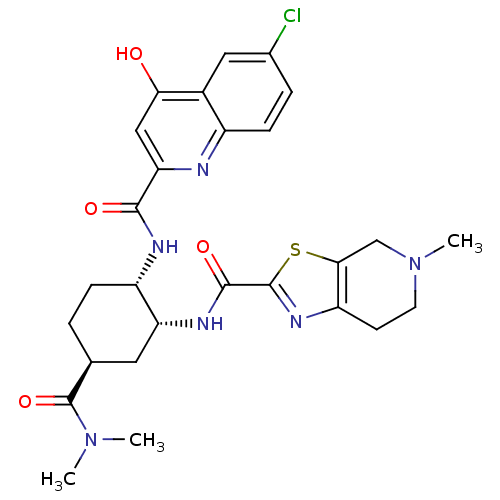
(cis-1,2-diaminocyclohexane derivative, 5h)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc(O)c3cc(Cl)ccc3n2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C27H31ClN6O4S/c1-33(2)27(38)14-4-6-18(30-24(36)21-12-22(35)16-11-15(28)5-7-17(16)29-21)20(10-14)31-25(37)26-32-19-8-9-34(3)13-23(19)39-26/h5,7,11-12,14,18,20H,4,6,8-10,13H2,1-3H3,(H,29,35)(H,30,36)(H,31,37)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Daiichi Sankyo Co, Ltd
| Assay Description
The in vitro anti-fXa activity was measured by using a chromogenic substrate S-2222 and human fXa. Aqueous DMSO or test compounds in aqueous DMSO and... |
Bioorg Med Chem 17: 8221-33 (2009)
Article DOI: 10.1016/j.bmc.2009.10.024
BindingDB Entry DOI: 10.7270/Q2CC0Z1V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50359000
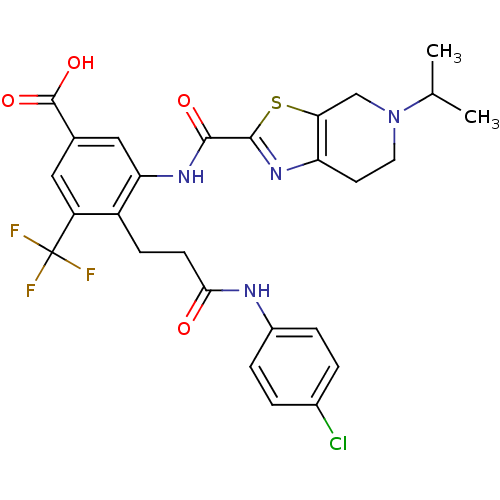
(CHEMBL1923893)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(cc(c1CCC(=O)Nc1ccc(Cl)cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C27H26ClF3N4O4S/c1-14(2)35-10-9-20-22(13-35)40-25(34-20)24(37)33-21-12-15(26(38)39)11-19(27(29,30)31)18(21)7-8-23(36)32-17-5-3-16(28)4-6-17/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,32,36)(H,33,37)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50359001
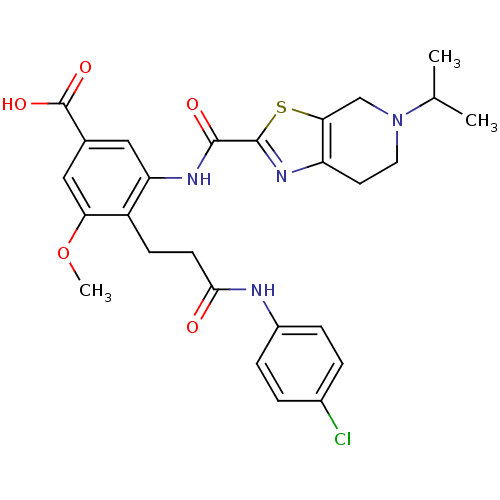
(CHEMBL1923894)Show SMILES COc1cc(cc(NC(=O)c2nc3CCN(Cc3s2)C(C)C)c1CCC(=O)Nc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C27H29ClN4O5S/c1-15(2)32-11-10-20-23(14-32)38-26(31-20)25(34)30-21-12-16(27(35)36)13-22(37-3)19(21)8-9-24(33)29-18-6-4-17(28)5-7-18/h4-7,12-13,15H,8-11,14H2,1-3H3,(H,29,33)(H,30,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50287299
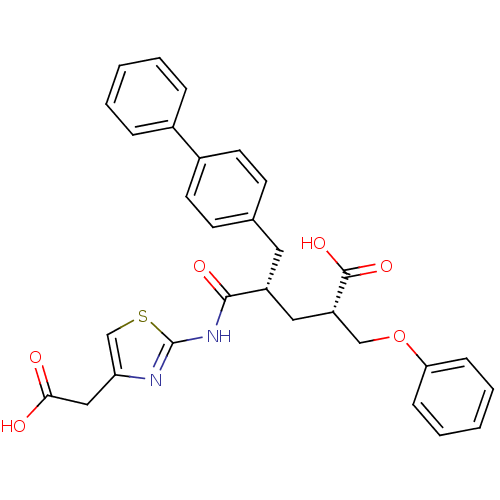
((2S,4S)-5-Biphenyl-4-yl-4-(4-carboxymethyl-thiazol...)Show SMILES OC(=O)Cc1csc(NC(=O)[C@@H](C[C@@H](COc2ccccc2)C(O)=O)Cc2ccc(cc2)-c2ccccc2)n1 Show InChI InChI=1S/C30H28N2O6S/c33-27(34)17-25-19-39-30(31-25)32-28(35)23(16-24(29(36)37)18-38-26-9-5-2-6-10-26)15-20-11-13-22(14-12-20)21-7-3-1-4-8-21/h1-14,19,23-24H,15-18H2,(H,33,34)(H,36,37)(H,31,32,35)/t23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral Endopeptidase |
Bioorg Med Chem Lett 6: 1487-1490 (1996)
Article DOI: 10.1016/S0960-894X(96)00255-7
BindingDB Entry DOI: 10.7270/Q2B27VSQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50359002
(CHEMBL1923895)Show SMILES CCOc1cc(cc(NC(=O)c2nc3CCN(Cc3s2)C(C)C)c1CCC(=O)Nc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C28H31ClN4O5S/c1-4-38-23-14-17(28(36)37)13-22(20(23)9-10-25(34)30-19-7-5-18(29)6-8-19)31-26(35)27-32-21-11-12-33(16(2)3)15-24(21)39-27/h5-8,13-14,16H,4,9-12,15H2,1-3H3,(H,30,34)(H,31,35)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358998

(CHEMBL1923891)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(cc(F)c1CCC(=O)Nc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C26H26ClFN4O4S/c1-14(2)32-10-9-20-22(13-32)37-25(31-20)24(34)30-21-12-15(26(35)36)11-19(28)18(21)7-8-23(33)29-17-5-3-16(27)4-6-17/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,29,33)(H,30,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358982

(CHEMBL1923876)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(ccc1CNC(=O)c1ccc(Cl)s1)C(O)=O Show InChI InChI=1S/C23H23ClN4O4S2/c1-12(2)28-8-7-15-18(11-28)34-22(27-15)21(30)26-16-9-13(23(31)32)3-4-14(16)10-25-20(29)17-5-6-19(24)33-17/h3-6,9,12H,7-8,10-11H2,1-2H3,(H,25,29)(H,26,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50340866
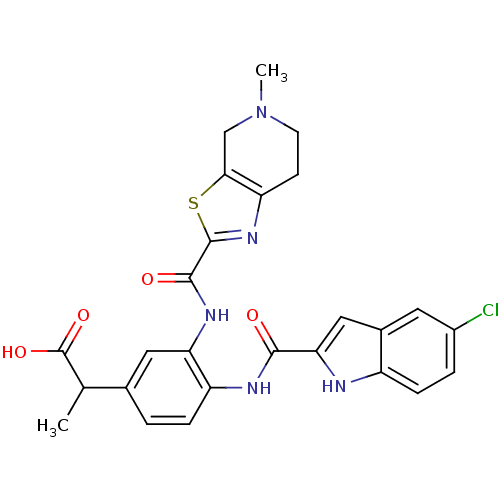
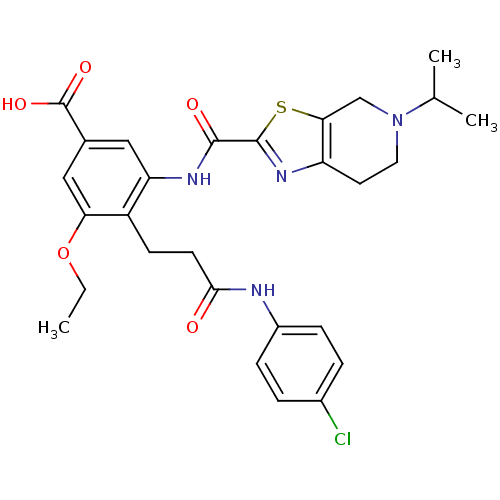
(2-(4-(5-chloro-1H-indole-2-carboxamido)-3-(5-methy...)Show SMILES CC(C(O)=O)c1ccc(NC(=O)c2cc3cc(Cl)ccc3[nH]2)c(NC(=O)c2nc3CCN(C)Cc3s2)c1 Show InChI InChI=1S/C26H24ClN5O4S/c1-13(26(35)36)14-3-5-18(29-23(33)21-11-15-9-16(27)4-6-17(15)28-21)20(10-14)30-24(34)25-31-19-7-8-32(2)12-22(19)37-25/h3-6,9-11,13,28H,7-8,12H2,1-2H3,(H,29,33)(H,30,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 21: 2133-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.132
BindingDB Entry DOI: 10.7270/Q2MK6D60 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358997

(CHEMBL1921822)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(ccc1CCC(=O)Nc1ccc(Br)cc1)C(O)=O Show InChI InChI=1S/C26H27BrN4O4S/c1-15(2)31-12-11-20-22(14-31)36-25(30-20)24(33)29-21-13-17(26(34)35)4-3-16(21)5-10-23(32)28-19-8-6-18(27)7-9-19/h3-4,6-9,13,15H,5,10-12,14H2,1-2H3,(H,28,32)(H,29,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358994

(CHEMBL1923888)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(ccc1CCC(=O)Nc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C26H27ClN4O4S/c1-15(2)31-12-11-20-22(14-31)36-25(30-20)24(33)29-21-13-17(26(34)35)4-3-16(21)5-10-23(32)28-19-8-6-18(27)7-9-19/h3-4,6-9,13,15H,5,10-12,14H2,1-2H3,(H,28,32)(H,29,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35769
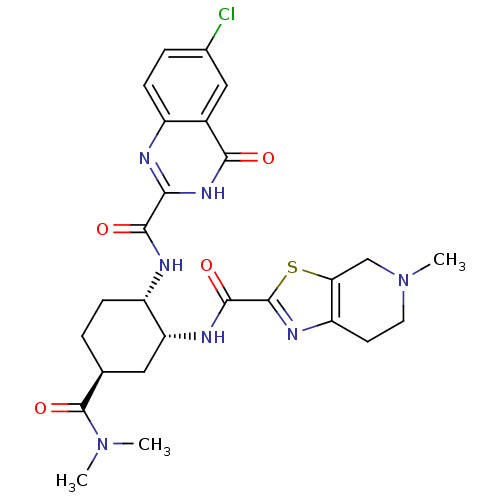
(cis-1,2-diaminocyclohexane derivative, 5j)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2nc3ccc(Cl)cc3c(=O)[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H30ClN7O4S/c1-33(2)26(38)13-4-6-17(29-23(36)21-28-16-7-5-14(27)11-15(16)22(35)32-21)19(10-13)30-24(37)25-31-18-8-9-34(3)12-20(18)39-25/h5,7,11,13,17,19H,4,6,8-10,12H2,1-3H3,(H,29,36)(H,30,37)(H,28,32,35)/t13-,17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Daiichi Sankyo Co, Ltd
| Assay Description
The in vitro anti-fXa activity was measured by using a chromogenic substrate S-2222 and human fXa. Aqueous DMSO or test compounds in aqueous DMSO and... |
Bioorg Med Chem 17: 8221-33 (2009)
Article DOI: 10.1016/j.bmc.2009.10.024
BindingDB Entry DOI: 10.7270/Q2CC0Z1V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35751
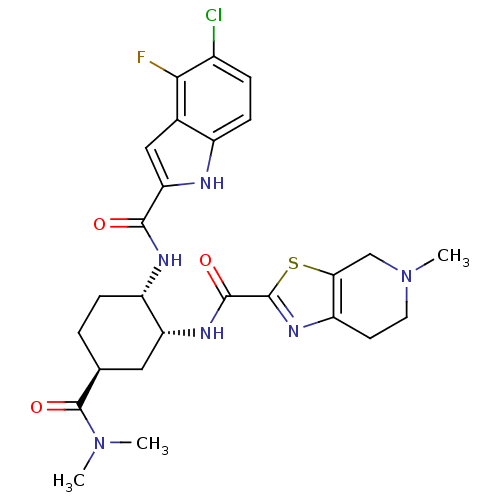
(cis-1,2-diaminocyclohexane derivative, 8h)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3c(F)c(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H30ClFN6O3S/c1-33(2)26(37)13-4-6-17(30-23(35)20-11-14-16(29-20)7-5-15(27)22(14)28)19(10-13)31-24(36)25-32-18-8-9-34(3)12-21(18)38-25/h5,7,11,13,17,19,29H,4,6,8-10,12H2,1-3H3,(H,30,35)(H,31,36)/t13-,17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Daiichi Sankyo Co, Ltd
| Assay Description
The in vitro anti-fXa activity was measured by using a chromogenic substrate S-2222 and human fXa. Aqueous DMSO or test compounds in aqueous DMSO and... |
Bioorg Med Chem 17: 8206-20 (2009)
Article DOI: 10.1016/j.bmc.2009.10.023
BindingDB Entry DOI: 10.7270/Q2H130CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358993
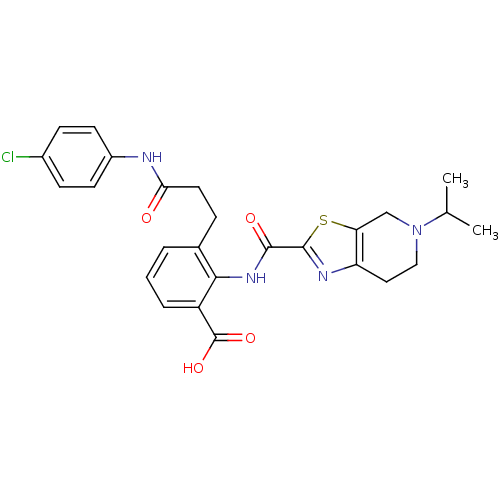
(CHEMBL1923887)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1c(CCC(=O)Nc2ccc(Cl)cc2)cccc1C(O)=O Show InChI InChI=1S/C26H27ClN4O4S/c1-15(2)31-13-12-20-21(14-31)36-25(29-20)24(33)30-23-16(4-3-5-19(23)26(34)35)6-11-22(32)28-18-9-7-17(27)8-10-18/h3-5,7-10,15H,6,11-14H2,1-2H3,(H,28,32)(H,30,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50359003
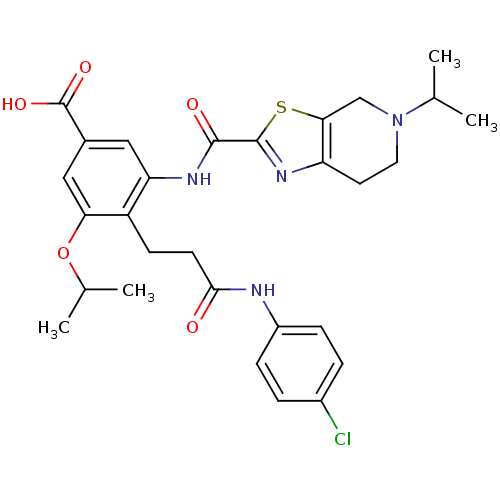
(CHEMBL1923896)Show SMILES CC(C)Oc1cc(cc(NC(=O)c2nc3CCN(Cc3s2)C(C)C)c1CCC(=O)Nc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C29H33ClN4O5S/c1-16(2)34-12-11-22-25(15-34)40-28(33-22)27(36)32-23-13-18(29(37)38)14-24(39-17(3)4)21(23)9-10-26(35)31-20-7-5-19(30)6-8-20/h5-8,13-14,16-17H,9-12,15H2,1-4H3,(H,31,35)(H,32,36)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338329
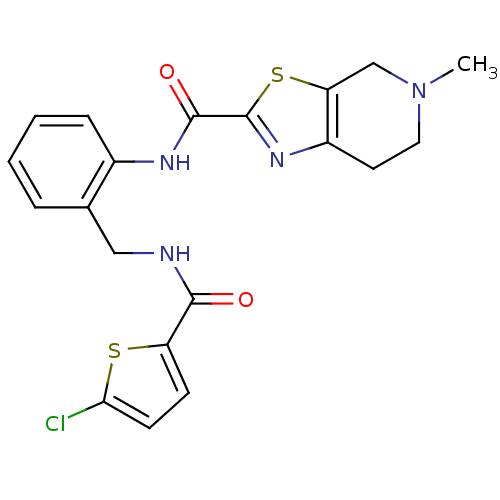
(CHEMBL1681798 | N-(2-{3-[(5-Chlorothiophen-2-yl)ca...)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1ccccc1CNC(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C20H19ClN4O2S2/c1-25-9-8-14-16(11-25)29-20(24-14)19(27)23-13-5-3-2-4-12(13)10-22-18(26)15-6-7-17(21)28-15/h2-7H,8-11H2,1H3,(H,22,26)(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a by S2222 chromogenic substrate assay by chromogenic assay |
Bioorg Med Chem 19: 1623-42 (2011)
Article DOI: 10.1016/j.bmc.2011.01.035
BindingDB Entry DOI: 10.7270/Q22F7NR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338329

(CHEMBL1681798 | N-(2-{3-[(5-Chlorothiophen-2-yl)ca...)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1ccccc1CNC(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C20H19ClN4O2S2/c1-25-9-8-14-16(11-25)29-20(24-14)19(27)23-13-5-3-2-4-12(13)10-22-18(26)15-6-7-17(21)28-15/h2-7H,8-11H2,1H3,(H,22,26)(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255631
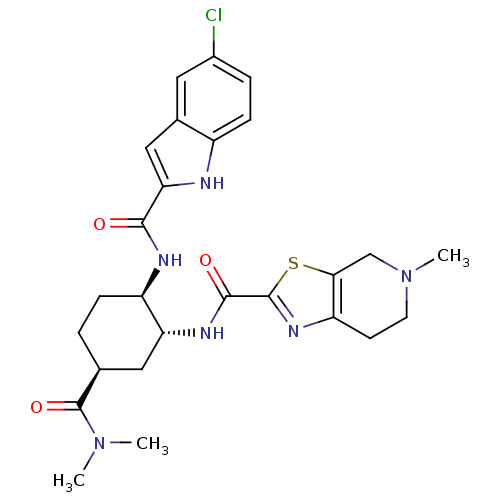
(CHEMBL516922 | N-[(1R,2R,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CN(C)C(=O)[C@H]1CC[C@@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50340864
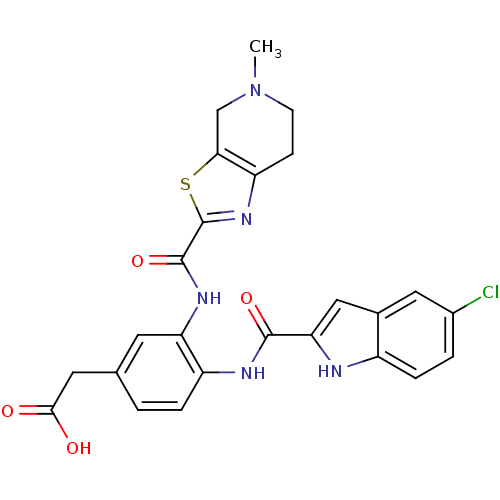
(2-(4-(5-chloro-1H-indole-2-carboxamido)-3-(5-methy...)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1cc(CC(O)=O)ccc1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C25H22ClN5O4S/c1-31-7-6-18-21(12-31)36-25(30-18)24(35)29-19-8-13(9-22(32)33)2-4-17(19)28-23(34)20-11-14-10-15(26)3-5-16(14)27-20/h2-5,8,10-11,27H,6-7,9,12H2,1H3,(H,28,34)(H,29,35)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 21: 2133-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.132
BindingDB Entry DOI: 10.7270/Q2MK6D60 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50340874
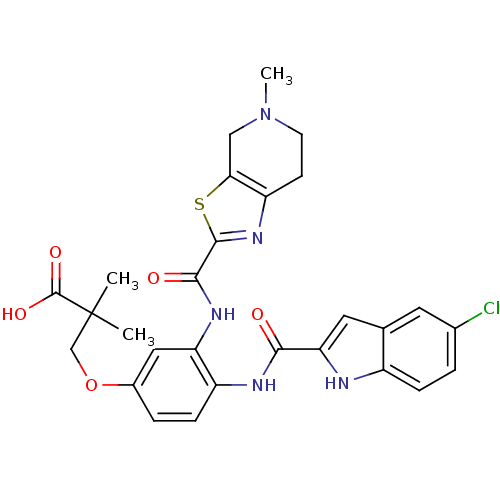
(3-(4-(5-chloro-1H-indole-2-carboxamido)-3-(5-methy...)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1cc(OCC(C)(C)C(O)=O)ccc1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C28H28ClN5O5S/c1-28(2,27(37)38)14-39-17-5-7-19(31-24(35)22-11-15-10-16(29)4-6-18(15)30-22)21(12-17)32-25(36)26-33-20-8-9-34(3)13-23(20)40-26/h4-7,10-12,30H,8-9,13-14H2,1-3H3,(H,31,35)(H,32,36)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 21: 2133-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.132
BindingDB Entry DOI: 10.7270/Q2MK6D60 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
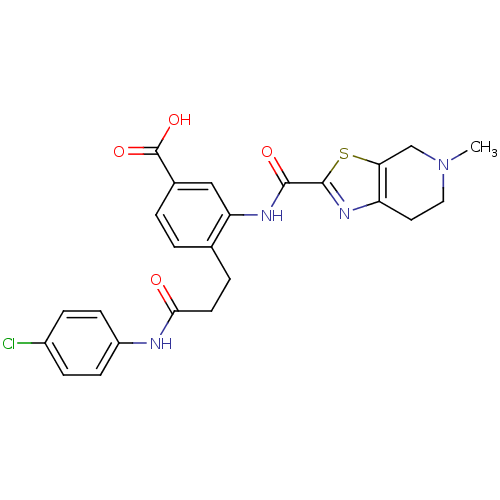
(Homo sapiens (Human)) | BDBM50358991
(CHEMBL1923885)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1cc(ccc1CCC(=O)Nc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C24H23ClN4O4S/c1-29-11-10-18-20(13-29)34-23(28-18)22(31)27-19-12-15(24(32)33)3-2-14(19)4-9-21(30)26-17-7-5-16(25)6-8-17/h2-3,5-8,12H,4,9-11,13H2,1H3,(H,26,30)(H,27,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
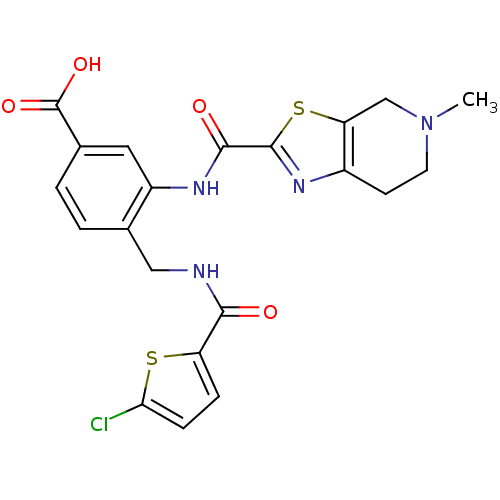
(Homo sapiens (Human)) | BDBM50358970
(CHEMBL1923795)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1cc(ccc1CNC(=O)c1ccc(Cl)s1)C(O)=O Show InChI InChI=1S/C21H19ClN4O4S2/c1-26-7-6-13-16(10-26)32-20(25-13)19(28)24-14-8-11(21(29)30)2-3-12(14)9-23-18(27)15-4-5-17(22)31-15/h2-5,8H,6-7,9-10H2,1H3,(H,23,27)(H,24,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35756
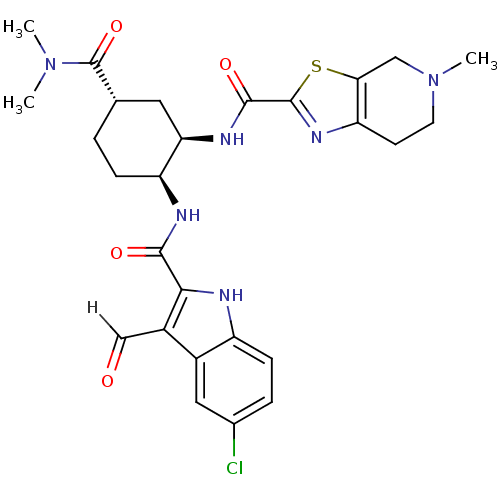
(cis-1,2-diaminocyclohexane derivative, 8m)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2[nH]c3ccc(Cl)cc3c2C=O)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C27H31ClN6O4S/c1-33(2)27(38)14-4-6-19(30-24(36)23-17(13-35)16-11-15(28)5-7-18(16)29-23)21(10-14)31-25(37)26-32-20-8-9-34(3)12-22(20)39-26/h5,7,11,13-14,19,21,29H,4,6,8-10,12H2,1-3H3,(H,30,36)(H,31,37)/t14-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Daiichi Sankyo Co, Ltd
| Assay Description
The in vitro anti-fXa activity was measured by using a chromogenic substrate S-2222 and human fXa. Aqueous DMSO or test compounds in aqueous DMSO and... |
Bioorg Med Chem 17: 8206-20 (2009)
Article DOI: 10.1016/j.bmc.2009.10.023
BindingDB Entry DOI: 10.7270/Q2H130CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358976

(CHEMBL1923801)Show SMILES CN(C)C(=O)c1cccc(CNC(=O)c2ccc(Cl)s2)c1NC(=O)c1nc2CCN(C)Cc2s1 Show InChI InChI=1S/C23H24ClN5O3S2/c1-28(2)23(32)14-6-4-5-13(11-25-20(30)16-7-8-18(24)33-16)19(14)27-21(31)22-26-15-9-10-29(3)12-17(15)34-22/h4-8H,9-12H2,1-3H3,(H,25,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255577

(CHEMBL473243 | N-{(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES COCCN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C28H35ClN6O4S/c1-34-9-8-21-24(15-34)40-27(33-21)26(37)32-22-13-16(28(38)35(2)10-11-39-3)4-6-20(22)31-25(36)23-14-17-12-18(29)5-7-19(17)30-23/h5,7,12,14,16,20,22,30H,4,6,8-11,13,15H2,1-3H3,(H,31,36)(H,32,37)/t16-,20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50340872

(2-(4-(5-chloro-1H-indole-2-carboxamido)-3-(5-methy...)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1cc(OC(C)(C)C(O)=O)ccc1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C27H26ClN5O5S/c1-27(2,26(36)37)38-16-5-7-18(30-23(34)21-11-14-10-15(28)4-6-17(14)29-21)20(12-16)31-24(35)25-32-19-8-9-33(3)13-22(19)39-25/h4-7,10-12,29H,8-9,13H2,1-3H3,(H,30,34)(H,31,35)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 21: 2133-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.132
BindingDB Entry DOI: 10.7270/Q2MK6D60 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358995

(CHEMBL1923889)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(ccc1CCC(=O)Nc1ccc(Cl)cn1)C(O)=O Show InChI InChI=1S/C25H26ClN5O4S/c1-14(2)31-10-9-18-20(13-31)36-24(29-18)23(33)28-19-11-16(25(34)35)4-3-15(19)5-8-22(32)30-21-7-6-17(26)12-27-21/h3-4,6-7,11-12,14H,5,8-10,13H2,1-2H3,(H,28,33)(H,34,35)(H,27,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Daiichi Sankyo Co, Ltd
| Assay Description
The in vitro anti-fXa activity was measured by using a chromogenic substrate S-2222 and human fXa. Aqueous DMSO or test compounds in aqueous DMSO and... |
Bioorg Med Chem 17: 8221-33 (2009)
Article DOI: 10.1016/j.bmc.2009.10.024
BindingDB Entry DOI: 10.7270/Q2CC0Z1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286907
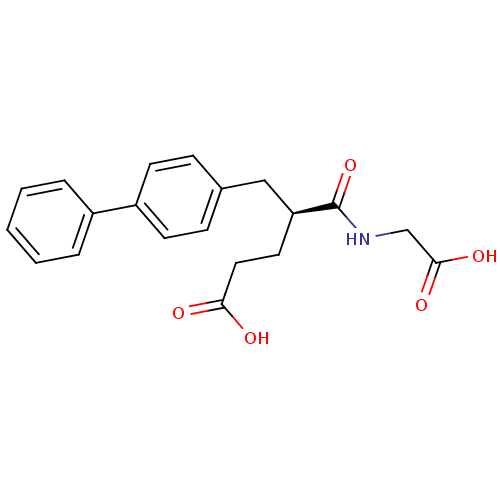
((S)-5-Biphenyl-4-yl-4-(carboxymethyl-carbamoyl)-pe...)Show SMILES OC(=O)CC[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)NCC(O)=O Show InChI InChI=1S/C20H21NO5/c22-18(23)11-10-17(20(26)21-13-19(24)25)12-14-6-8-16(9-7-14)15-4-2-1-3-5-15/h1-9,17H,10-13H2,(H,21,26)(H,22,23)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255205
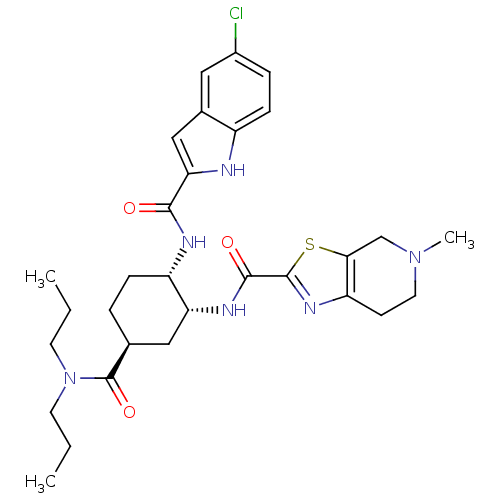
(CHEMBL508906 | N-[(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CCCN(CCC)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C30H39ClN6O3S/c1-4-11-37(12-5-2)30(40)18-6-8-22(33-27(38)25-16-19-14-20(31)7-9-21(19)32-25)24(15-18)34-28(39)29-35-23-10-13-36(3)17-26(23)41-29/h7,9,14,16,18,22,24,32H,4-6,8,10-13,15,17H2,1-3H3,(H,33,38)(H,34,39)/t18-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Daiichi Sankyo Co, Ltd
| Assay Description
The in vitro anti-fXa activity was measured by using a chromogenic substrate S-2222 and human fXa. Aqueous DMSO or test compounds in aqueous DMSO and... |
Bioorg Med Chem 17: 8206-20 (2009)
Article DOI: 10.1016/j.bmc.2009.10.023
BindingDB Entry DOI: 10.7270/Q2H130CP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358992

(CHEMBL1923886)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1cc(ccc1CCC(=O)Nc1ccc(Cl)cn1)C(O)=O Show InChI InChI=1S/C23H22ClN5O4S/c1-29-9-8-16-18(12-29)34-22(27-16)21(31)26-17-10-14(23(32)33)3-2-13(17)4-7-20(30)28-19-6-5-15(24)11-25-19/h2-3,5-6,10-11H,4,7-9,12H2,1H3,(H,26,31)(H,32,33)(H,25,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50340868

(2-(4-(5-chloro-1H-indole-2-carboxamido)-3-(5-methy...)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1cc(OCC(O)=O)ccc1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C25H22ClN5O5S/c1-31-7-6-18-21(11-31)37-25(30-18)24(35)29-19-10-15(36-12-22(32)33)3-5-17(19)28-23(34)20-9-13-8-14(26)2-4-16(13)27-20/h2-5,8-10,27H,6-7,11-12H2,1H3,(H,28,34)(H,29,35)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 21: 2133-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.132
BindingDB Entry DOI: 10.7270/Q2MK6D60 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255207
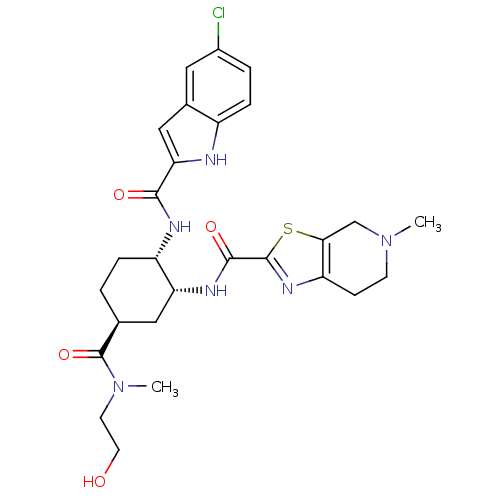
(CHEMBL474459 | N-{(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CN(CCO)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C27H33ClN6O4S/c1-33-8-7-20-23(14-33)39-26(32-20)25(37)31-21-12-15(27(38)34(2)9-10-35)3-5-19(21)30-24(36)22-13-16-11-17(28)4-6-18(16)29-22/h4,6,11,13,15,19,21,29,35H,3,5,7-10,12,14H2,1-2H3,(H,30,36)(H,31,37)/t15-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255159

(CHEMBL515569 | N-{(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CCN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C27H33ClN6O3S/c1-4-34(3)27(37)15-5-7-19(30-24(35)22-13-16-11-17(28)6-8-18(16)29-22)21(12-15)31-25(36)26-32-20-9-10-33(2)14-23(20)38-26/h6,8,11,13,15,19,21,29H,4-5,7,9-10,12,14H2,1-3H3,(H,30,35)(H,31,36)/t15-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50340876

(1-((4-(5-chloro-1H-indole-2-carboxamido)-3-(5-meth...)Show SMILES CN1CCc2nc(sc2C1)C(=O)Nc1cc(OCC2(CC2)C(O)=O)ccc1NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C28H26ClN5O5S/c1-34-9-6-20-23(13-34)40-26(33-20)25(36)32-21-12-17(39-14-28(7-8-28)27(37)38)3-5-19(21)31-24(35)22-11-15-10-16(29)2-4-18(15)30-22/h2-5,10-12,30H,6-9,13-14H2,1H3,(H,31,35)(H,32,36)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S2222 chromogenic substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 21: 2133-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.132
BindingDB Entry DOI: 10.7270/Q2MK6D60 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286905
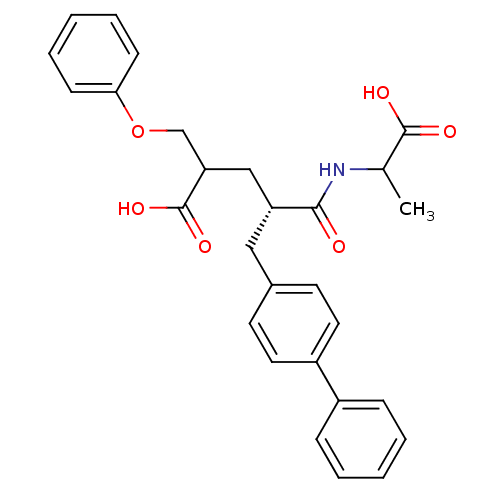
((S)-5-Biphenyl-4-yl-4-(1-carboxy-ethylcarbamoyl)-2...)Show SMILES CC(NC(=O)[C@@H](CC(COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C28H29NO6/c1-19(27(31)32)29-26(30)23(17-24(28(33)34)18-35-25-10-6-3-7-11-25)16-20-12-14-22(15-13-20)21-8-4-2-5-9-21/h2-15,19,23-24H,16-18H2,1H3,(H,29,30)(H,31,32)(H,33,34)/t19?,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50286905

((S)-5-Biphenyl-4-yl-4-(1-carboxy-ethylcarbamoyl)-2...)Show SMILES CC(NC(=O)[C@@H](CC(COc1ccccc1)C(O)=O)Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C28H29NO6/c1-19(27(31)32)29-26(30)23(17-24(28(33)34)18-35-25-10-6-3-7-11-25)16-20-12-14-22(15-13-20)21-8-4-2-5-9-21/h2-15,19,23-24H,16-18H2,1H3,(H,29,30)(H,31,32)(H,33,34)/t19?,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase from rat kidney. |
Bioorg Med Chem Lett 6: 65-70 (1996)
Article DOI: 10.1016/0960-894X(95)00551-4
BindingDB Entry DOI: 10.7270/Q2BZ66J9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data