

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complement factor D (Homo sapiens (Human)) | BDBM171332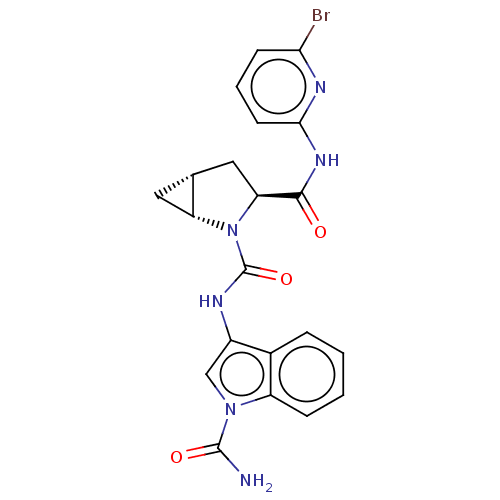 (US9085555, 762) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171332 (US9085555, 762) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171239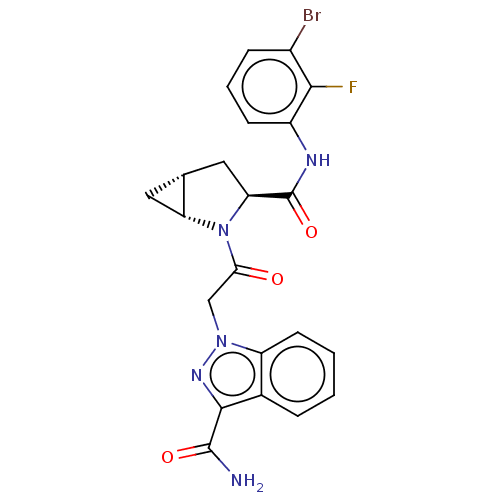 (US9085555, 669) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171350 (1-(2-((1R,3S,5R)-3-((6-Bromopyridin-2-yl)carbamoyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Evaluated for the Non-competitive inhibition constant Ki against TdR varied rat cytoplasmic soluble thymidine kinase | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171350 (1-(2-((1R,3S,5R)-3-((6-Bromopyridin-2-yl)carbamoyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of complement factor D in human whole blood assessed as decrease in zymosan-induced AP activation mediated soluble MAC complex formation p... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238248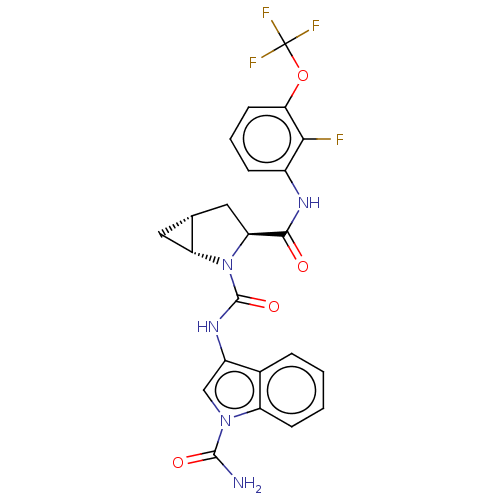 (CHEMBL4094108) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238246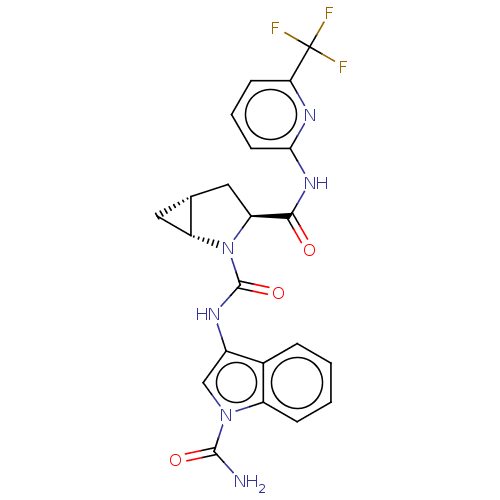 (CHEMBL4098439) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238247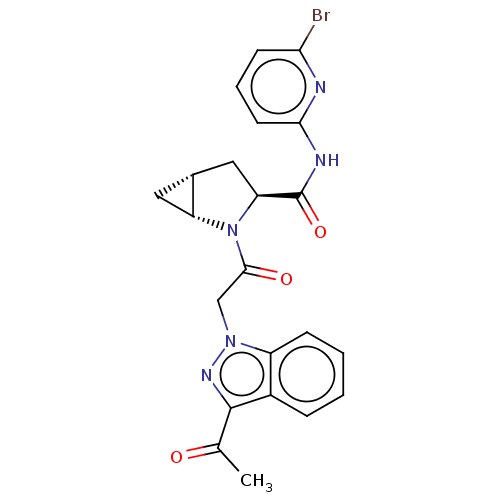 (CHEMBL4081888) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity against alpha-2 adrenergic receptor from calf cerebral cortex, using [3H]clonidine as the radioligand | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238246 (CHEMBL4098439) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of complement factor D in human whole blood assessed as decrease in zymosan-induced AP activation mediated soluble MAC complex formation p... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238245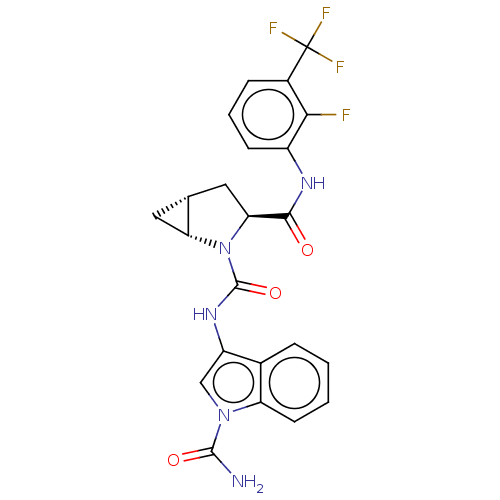 (CHEMBL4070311) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238247 (CHEMBL4081888) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of complement factor D in human whole blood assessed as decrease in zymosan-induced AP activation mediated soluble MAC complex formation p... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238252 (CHEMBL4061643) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238244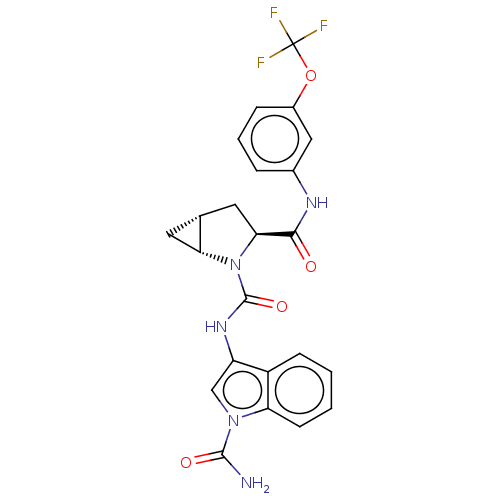 (CHEMBL4065529) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170763 (US9085555, 191) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171323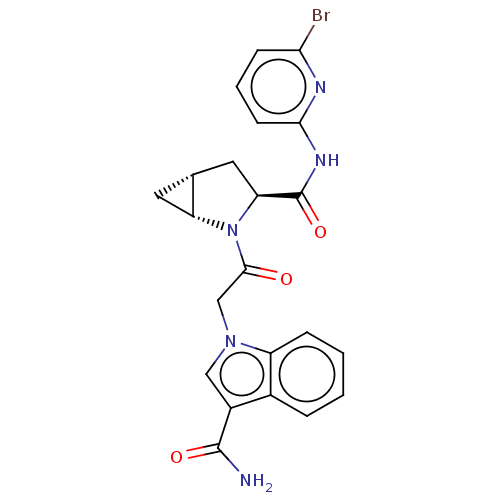 (US9085555, 753) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity against alpha-2 adrenergic receptor from calf cerebral cortex, using [3H]clonidine as the radioligand | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171323 (US9085555, 753) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238251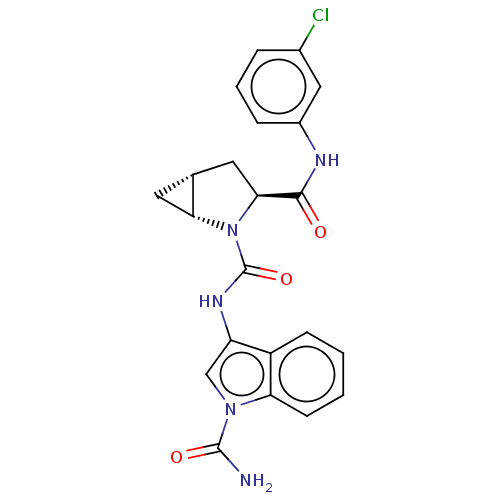 (CHEMBL4095470) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171239 (US9085555, 669) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of complement factor D in human whole blood assessed as decrease in zymosan-induced AP activation mediated soluble MAC complex formation p... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238250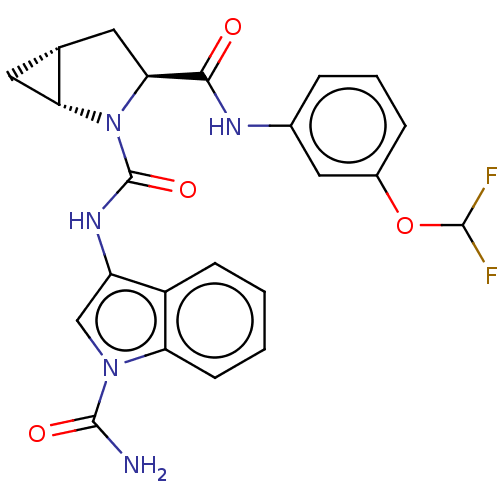 (CHEMBL4075355) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50238245 (CHEMBL4070311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238244 (CHEMBL4065529) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain (G24 to A253 residues) expressed in expressed in Escherichia coli(Rosetta) using... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238248 (CHEMBL4094108) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of complement factor D in human whole blood assessed as decrease in zymosan-induced AP activation mediated soluble MAC complex formation p... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50238248 (CHEMBL4094108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170709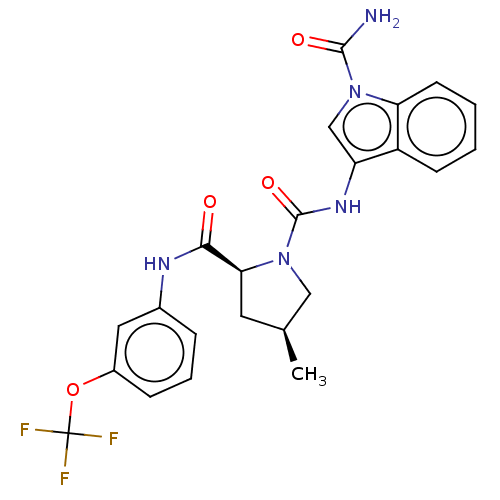 (US9085555, 137) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238276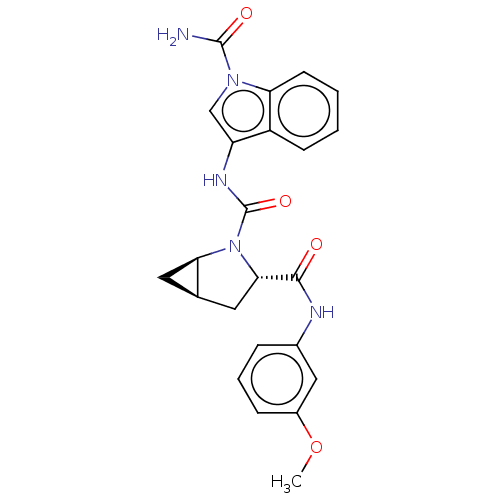 (CHEMBL4075037) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238275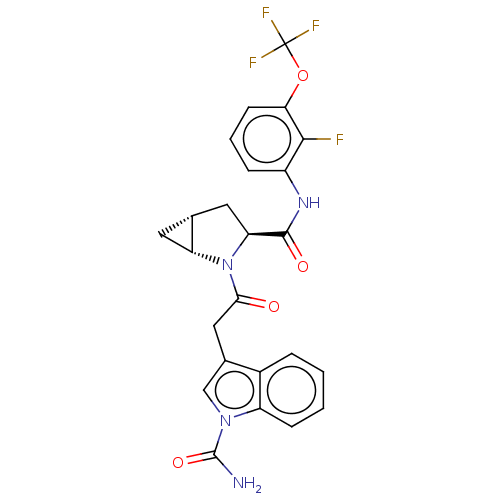 (CHEMBL4076955) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170904 (US9085555, 332) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170966 (US9085555, 396) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM60517 (US9085555, 124) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238245 (CHEMBL4070311) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 587 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of complement factor D in human whole blood assessed as decrease in zymosan-induced AP activation mediated soluble MAC complex formation p... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238249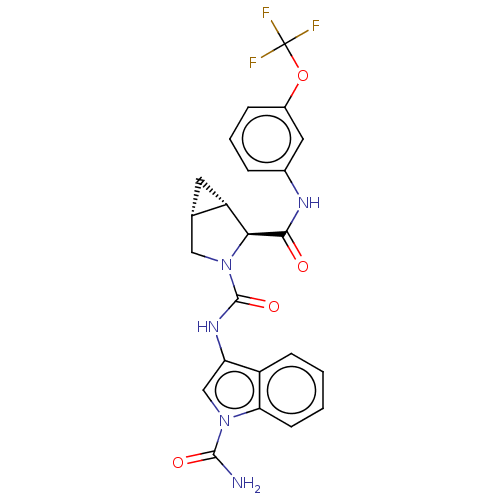 (CHEMBL4068741) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170796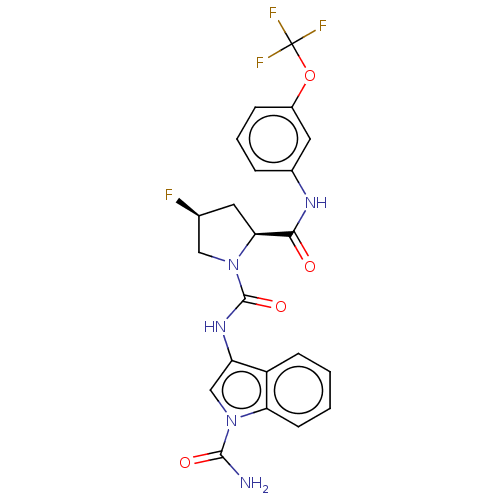 (US9085555, 224) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50238244 (CHEMBL4065529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM60517 (US9085555, 124) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain (G24 to A253 residues) expressed in expressed in Escherichia coli(Rosetta) using... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170729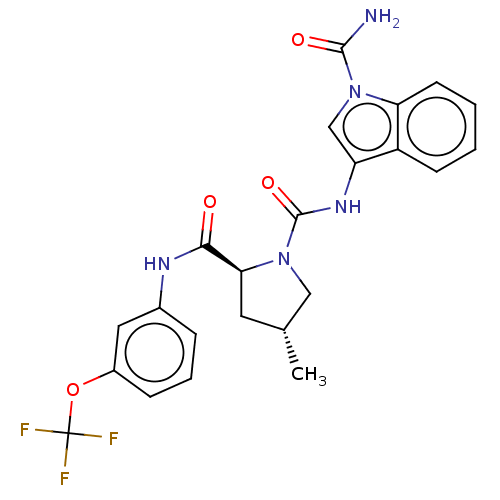 (US9085555, 157) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238244 (CHEMBL4065529) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of complement factor D in human whole blood assessed as decrease in zymosan-induced AP activation mediated soluble MAC complex formation p... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM170821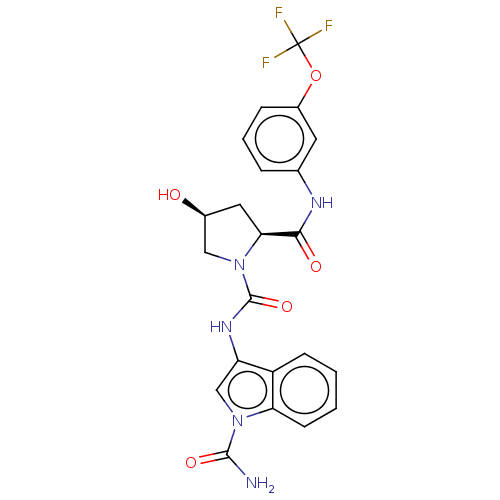 (US9085555, 249) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM170763 (US9085555, 191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Evaluated for the mixed objective Non-competitive inhibition constant Ki against ATP varied cytoplasmic soluble thymidine kinase | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM60517 (US9085555, 124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM171332 (US9085555, 762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50238246 (CHEMBL4098439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM203868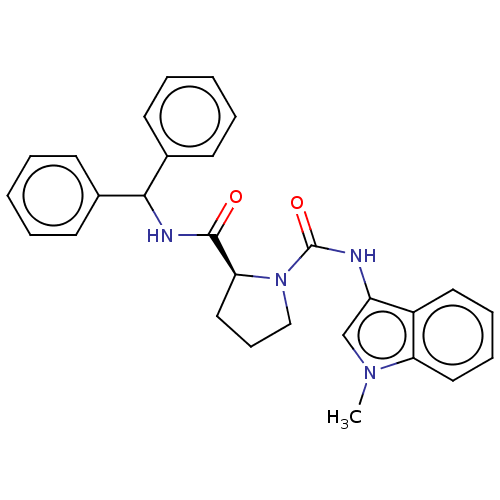 ((S)-N2-benzhydryl-N1-(1-methyl-1H-indol-3-yl)pyrro...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM60517 (US9085555, 124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of COX1 (unknown origin) | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM60517 (US9085555, 124) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine 3 receptor (unknown origin) | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM170966 (US9085555, 396) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM170821 (US9085555, 249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM203865 (Methyl (S)-2-((2-((3-(trifluoromethoxy)phenyl)carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity against alpha-2 adrenergic receptor from calf cerebral cortex, using [3H]prazosin as the radioligand | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM171350 (1-(2-((1R,3S,5R)-3-((6-Bromopyridin-2-yl)carbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM171350 (1-(2-((1R,3S,5R)-3-((6-Bromopyridin-2-yl)carbamoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50238277 (CHEMBL4103449) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain (G24 to A253 residues) expressed in expressed in Escherichia coli(Rosetta) using... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |