 Found 1409 hits with Last Name = 'karcz' and Initial = 't'
Found 1409 hits with Last Name = 'karcz' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14R |
J Med Chem 63: 9563-9589 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00745
BindingDB Entry DOI: 10.7270/Q20R9SZP |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50535225
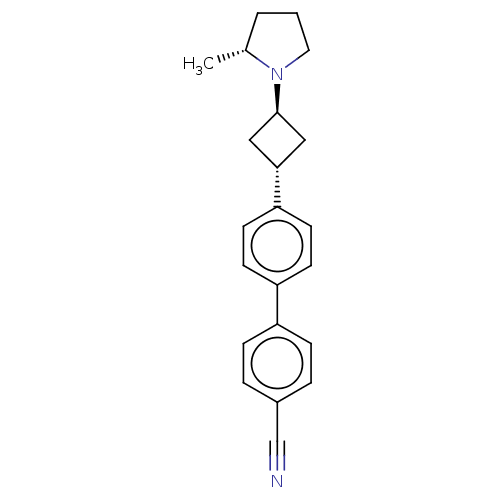
(CHEMBL4516622)Show SMILES C[C@@H]1CCCN1[C@H]1C[C@@H](C1)c1ccc(cc1)-c1ccc(cc1)C#N |r,wU:8.11,1.0,wD:6.6,(34.83,-7.94,;35.15,-9.44,;34.11,-10.59,;34.88,-11.92,;36.39,-11.6,;36.55,-10.07,;37.88,-9.3,;39.37,-9.7,;39.77,-8.21,;38.28,-7.81,;41.1,-7.45,;41.1,-5.9,;42.43,-5.13,;43.77,-5.9,;43.77,-7.45,;42.43,-8.22,;45.09,-5.12,;46.43,-5.89,;47.76,-5.12,;47.75,-3.58,;46.41,-2.81,;45.08,-3.59,;49.08,-2.8,;50.41,-2.03,)| Show InChI InChI=1S/C22H24N2/c1-16-3-2-12-24(16)22-13-21(14-22)20-10-8-19(9-11-20)18-6-4-17(15-23)5-7-18/h4-11,16,21-22H,2-3,12-14H2,1H3/t16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to human H3R |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Rattus norvegicus) | BDBM50455533
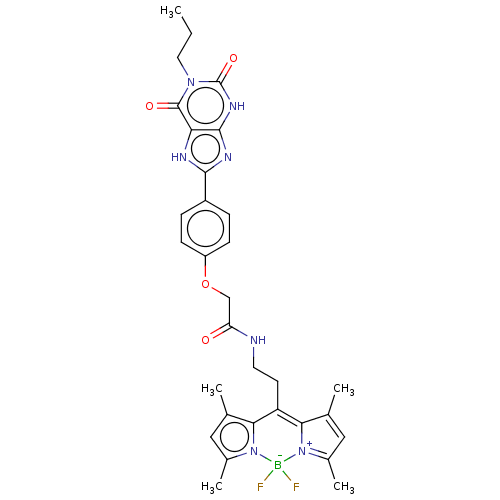
(CHEMBL4205635)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:27,30,33| Show InChI InChI=1S/C31H34BF2N7O4/c1-6-13-39-30(43)25-29(38-31(39)44)37-28(36-25)21-7-9-22(10-8-21)45-16-24(42)35-12-11-23-26-17(2)14-19(4)40(26)32(33,34)41-20(5)15-18(3)27(23)41/h7-10,14-15H,6,11-13,16H2,1-5H3,(H,35,42)(H,36,37)(H,38,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant rat adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation countin... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of human H3 receptor |
Eur J Med Chem 152: 223-234 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.043
BindingDB Entry DOI: 10.7270/Q2697632 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50393167

(CHEMBL2153721)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C1C2CC3OC(=O)C1C3C2 |TLB:22:24:26:19.20,21:20:24.17:26,THB:6:17:26:19.20| Show InChI InChI=1S/C19H24N4O4/c1-3-5-22-16-14(17(24)23(6-4-2)19(22)26)20-15(21-16)12-9-7-10-11(8-9)27-18(25)13(10)12/h9-13H,3-8H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]CPX from adenosine A1 receptor in rat brain cortical membrane |
Eur J Med Chem 46: 3590-607 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.023
BindingDB Entry DOI: 10.7270/Q2ZC840S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem 25: 2701-2712 (2017)
Article DOI: 10.1016/j.bmc.2017.03.031
BindingDB Entry DOI: 10.7270/Q2833VPK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158595
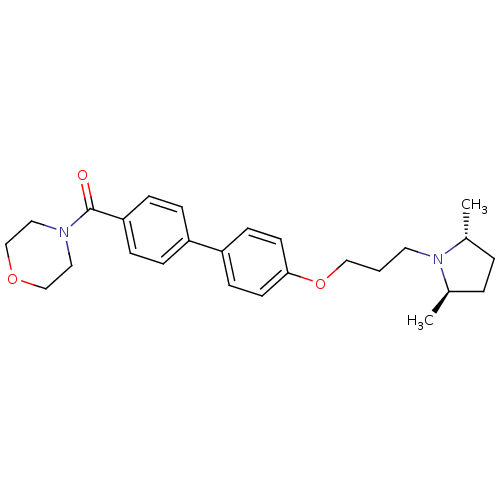
((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to human H3R expressed in rat C6 cells |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2Y14 expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP accumulation incubated for 15 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01964
BindingDB Entry DOI: 10.7270/Q2611470 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146835
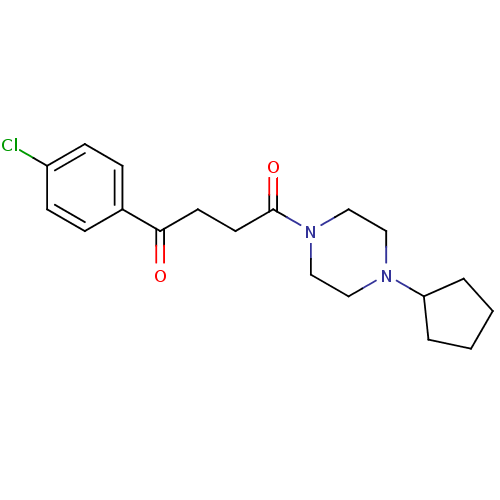
(1-(4-Chloro-phenyl)-4-(4-cyclopentyl-piperazin-1-y...)Show InChI InChI=1S/C19H25ClN2O2/c20-16-7-5-15(6-8-16)18(23)9-10-19(24)22-13-11-21(12-14-22)17-3-1-2-4-17/h5-8,17H,1-4,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human CB1 receptor expressed in CHO cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 7967-77 (2012)
Article DOI: 10.1021/jm3008213
BindingDB Entry DOI: 10.7270/Q2JM2BRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from recombinant human CB1 receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
ACS Med Chem Lett 4: 41-5 (2013)
Article DOI: 10.1021/ml300235q
BindingDB Entry DOI: 10.7270/Q2SF2XHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Mus musculus) | BDBM50455533

(CHEMBL4205635)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:27,30,33| Show InChI InChI=1S/C31H34BF2N7O4/c1-6-13-39-30(43)25-29(38-31(39)44)37-28(36-25)21-7-9-22(10-8-21)45-16-24(42)35-12-11-23-26-17(2)14-19(4)40(26)32(33,34)41-20(5)15-18(3)27(23)41/h7-10,14-15H,6,11-13,16H2,1-5H3,(H,35,42)(H,36,37)(H,38,44) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant mouse adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from recombinant human CB2 receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
ACS Med Chem Lett 4: 41-5 (2013)
Article DOI: 10.1021/ml300235q
BindingDB Entry DOI: 10.7270/Q2SF2XHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human CB2 receptor expressed in CHO cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 7967-77 (2012)
Article DOI: 10.1021/jm3008213
BindingDB Entry DOI: 10.7270/Q2JM2BRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455533

(CHEMBL4205635)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:27,30,33| Show InChI InChI=1S/C31H34BF2N7O4/c1-6-13-39-30(43)25-29(38-31(39)44)37-28(36-25)21-7-9-22(10-8-21)45-16-24(42)35-12-11-23-26-17(2)14-19(4)40(26)32(33,34)41-20(5)15-18(3)27(23)41/h7-10,14-15H,6,11-13,16H2,1-5H3,(H,35,42)(H,36,37)(H,38,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50533199
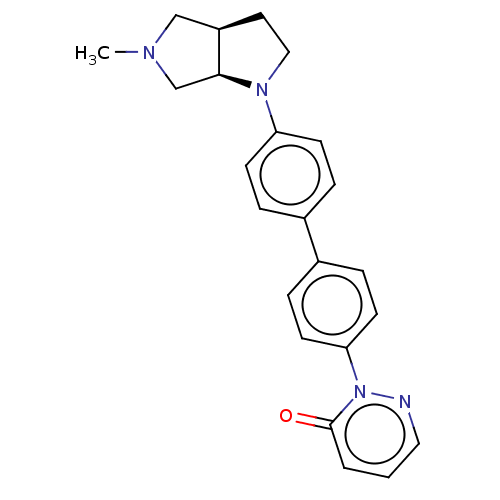
(CHEMBL4527011)Show SMILES [H][C@]12CCN(c3ccc(cc3)-c3ccc(cc3)-n3ncccc3=O)[C@@]1([H])CN(C)C2 |r| Show InChI InChI=1S/C23H24N4O/c1-25-15-19-12-14-26(22(19)16-25)20-8-4-17(5-9-20)18-6-10-21(11-7-18)27-23(28)3-2-13-24-27/h2-11,13,19,22H,12,14-16H2,1H3/t19-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Antagonist activity at human H3R expressed in rat C6 cells incubated for 20 mins by [35S]GTPgammaS binding assay |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50268815
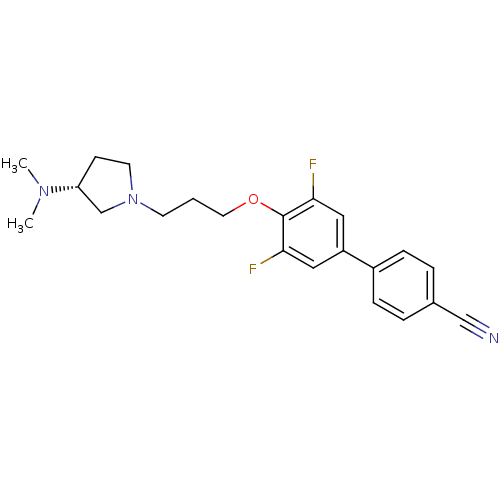
((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2c(F)cc(cc2F)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H25F2N3O/c1-26(2)19-8-10-27(15-19)9-3-11-28-22-20(23)12-18(13-21(22)24)17-6-4-16(14-25)5-7-17/h4-7,12-13,19H,3,8-11,15H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50240709
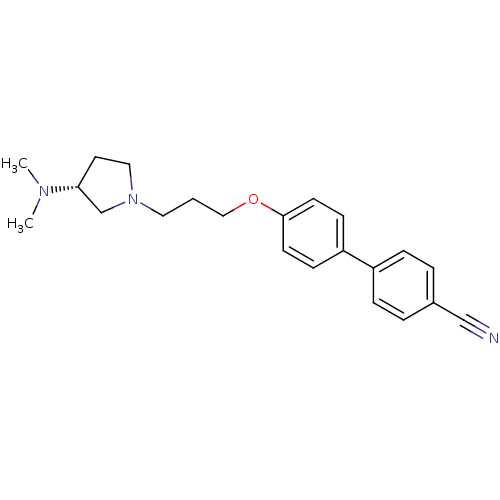
((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-MeHA from rat brain H3 receptor |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodoproxyfan from human striatal full length H3 receptor after 60 mins by gamma counting method |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50274767
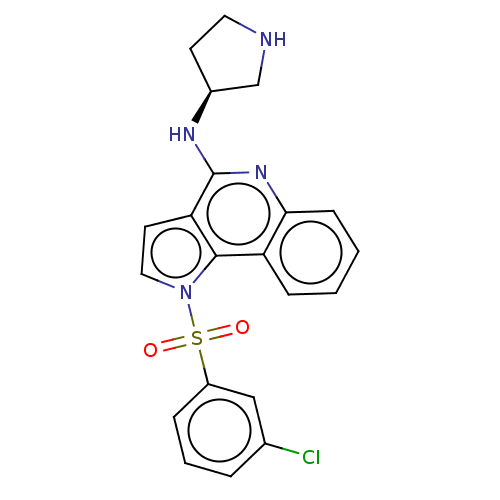
(CHEMBL4125735)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N[C@H]3CCNC3)nc3ccccc3c12 |r| Show InChI InChI=1S/C21H19ClN4O2S/c22-14-4-3-5-16(12-14)29(27,28)26-11-9-18-20(26)17-6-1-2-7-19(17)25-21(18)24-15-8-10-23-13-15/h1-7,9,11-12,15,23H,8,10,13H2,(H,24,25)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114329
BindingDB Entry DOI: 10.7270/Q2RF6040 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562553
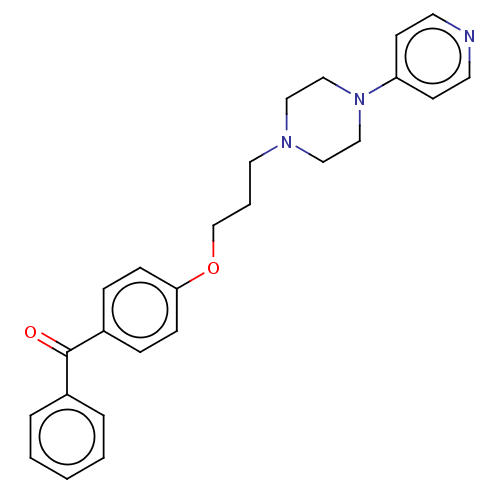
(CHEMBL4759150)Show SMILES O=C(c1ccccc1)c1ccc(OCCCN2CCN(CC2)c2ccncc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562541

(CHEMBL4783744) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]UR-PI294 from human SP-FLAG-tagged H3R expressed in HEK293T cells measured after 60 to 120 mins by liquid scintillation counting ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455527
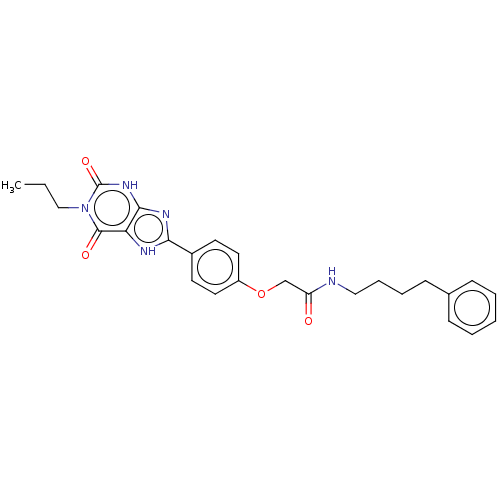
(CHEMBL4218460)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCCCc2ccccc2)cc1 Show InChI InChI=1S/C26H29N5O4/c1-2-16-31-25(33)22-24(30-26(31)34)29-23(28-22)19-11-13-20(14-12-19)35-17-21(32)27-15-7-6-10-18-8-4-3-5-9-18/h3-5,8-9,11-14H,2,6-7,10,15-17H2,1H3,(H,27,32)(H,28,29)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50004587

(8-Adamantan-1-yl-1,3-dipropyl-3,7-dihydro-purine-2...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CC3CC(CC(C3)C1)C2 |TLB:24:19:26:25.23.22,24:23:26:18.19.20,20:21:25:18.19.24,THB:20:19:25:26.21.22| Show InChI InChI=1S/C21H30N4O2/c1-3-5-24-17-16(18(26)25(6-4-2)20(24)27)22-19(23-17)21-10-13-7-14(11-21)9-15(8-13)12-21/h13-15H,3-12H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]CPX from adenosine A1 receptor in rat brain cortical membrane |
Eur J Med Chem 46: 3590-607 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.023
BindingDB Entry DOI: 10.7270/Q2ZC840S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562552
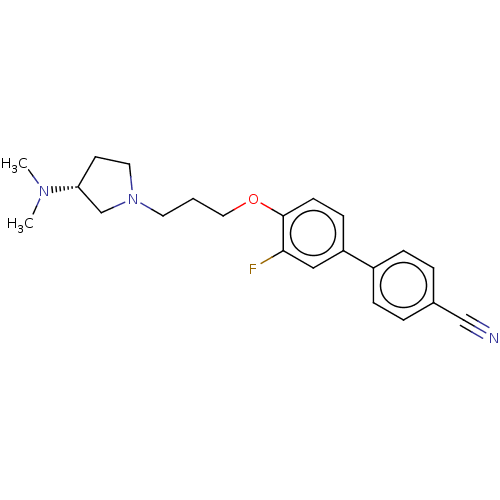
(CHEMBL4796748)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2F)-c2ccc(cc2)C#N)C1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50438370
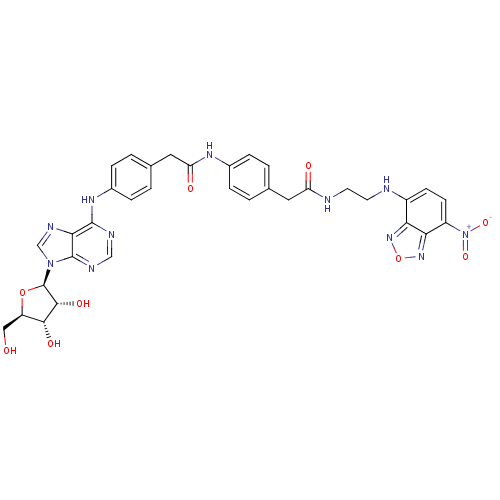
(CHEMBL2413101)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(Nc3ccc(CC(=O)Nc4ccc(CC(=O)NCCNc5ccc([N+]([O-])=O)c6nonc56)cc4)cc3)ncnc12 |r| Show InChI InChI=1S/C34H33N11O9/c46-15-24-30(49)31(50)34(53-24)44-17-39-29-32(37-16-38-33(29)44)41-21-7-3-19(4-8-21)14-26(48)40-20-5-1-18(2-6-20)13-25(47)36-12-11-35-22-9-10-23(45(51)52)28-27(22)42-54-43-28/h1-10,16-17,24,30-31,34-35,46,49-50H,11-15H2,(H,36,47)(H,40,48)(H,37,38,41)/t24-,30-,31-,34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ADAC from adenosine A1 receptor in rat cerebral cortex membranes after 120 mins by filter binding method |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50176050
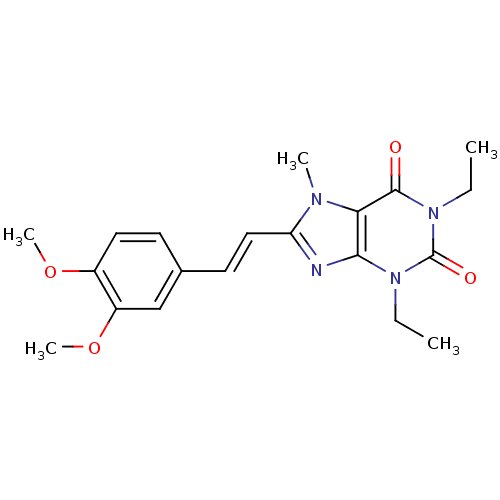
(8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-1H-pu...)Show SMILES CCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C20H24N4O4/c1-6-23-18-17(19(25)24(7-2)20(23)26)22(3)16(21-18)11-9-13-8-10-14(27-4)15(12-13)28-5/h8-12H,6-7H2,1-5H3/b11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCPA from rat adenosine A2a receptor |
Bioorg Med Chem 27: 1195-1210 (2019)
Article DOI: 10.1016/j.bmc.2019.02.004
BindingDB Entry DOI: 10.7270/Q2ZS312P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50176050

(8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-1H-pu...)Show SMILES CCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C20H24N4O4/c1-6-23-18-17(19(25)24(7-2)20(23)26)22(3)16(21-18)11-9-13-8-10-14(27-4)15(12-13)28-5/h8-12H,6-7H2,1-5H3/b11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]MSX-2 from adenosine A2A receptor in rat brain striatal membrane |
Eur J Med Chem 46: 3590-607 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.023
BindingDB Entry DOI: 10.7270/Q2ZC840S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50520939
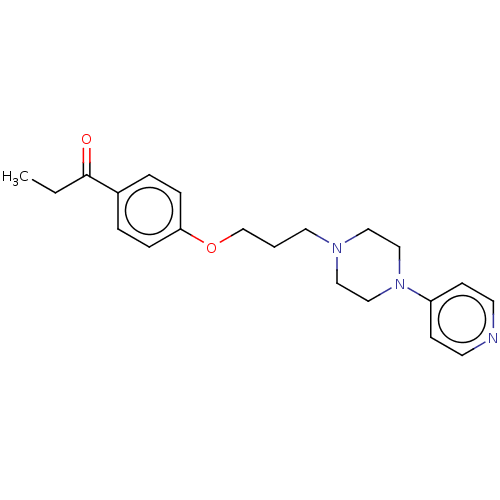
(CHEMBL4449790)Show InChI InChI=1S/C21H27N3O2/c1-2-21(25)18-4-6-20(7-5-18)26-17-3-12-23-13-15-24(16-14-23)19-8-10-22-11-9-19/h4-11H,2-3,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from full length recombinant human H3R expressed in HEK293 cell membranes after 90 mins by beta scintilla... |
Bioorg Med Chem 26: 6056-6066 (2018)
Article DOI: 10.1016/j.bmc.2018.11.010
BindingDB Entry DOI: 10.7270/Q2NV9NNX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50272040
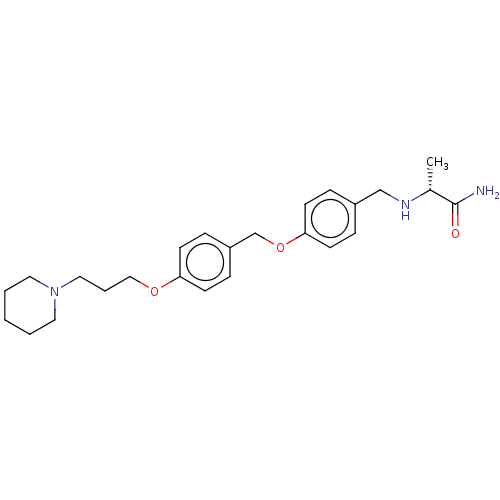
(CHEMBL4129950)Show SMILES C[C@@H](NCc1ccc(OCc2ccc(OCCCN3CCCCC3)cc2)cc1)C(N)=O |r| Show InChI InChI=1S/C25H35N3O3/c1-20(25(26)29)27-18-21-6-10-24(11-7-21)31-19-22-8-12-23(13-9-22)30-17-5-16-28-14-3-2-4-15-28/h6-13,20,27H,2-5,14-19H2,1H3,(H2,26,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Nalpha-MeHA from human H3 receptor expressed in HEK293 cell membranes by scintillation counting method |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562543
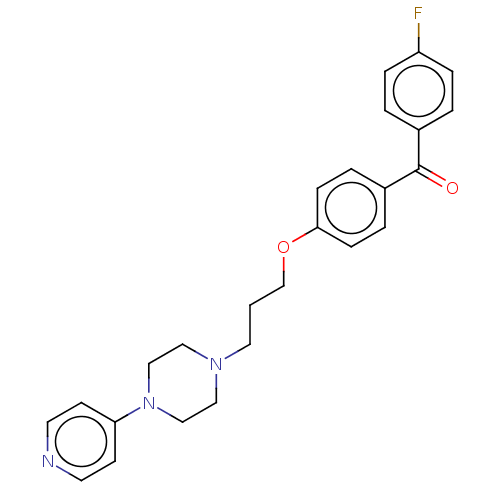
(CHEMBL4741145)Show SMILES Fc1ccc(cc1)C(=O)c1ccc(OCCCN2CCN(CC2)c2ccncc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]UR-PI294 from human SP-FLAG-tagged H3R expressed in HEK293T cells measured after 60 to 120 mins by liquid scintillation counting ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455526

(CHEMBL4218673)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCCCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:30,33,36| Show InChI InChI=1S/C34H40BF2N7O4/c1-6-16-42-33(46)28-32(41-34(42)47)40-31(39-28)24-11-13-25(14-12-24)48-19-27(45)38-15-9-7-8-10-26-29-20(2)17-22(4)43(29)35(36,37)44-23(5)18-21(3)30(26)44/h11-14,17-18H,6-10,15-16,19H2,1-5H3,(H,38,45)(H,39,40)(H,41,47) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50455530
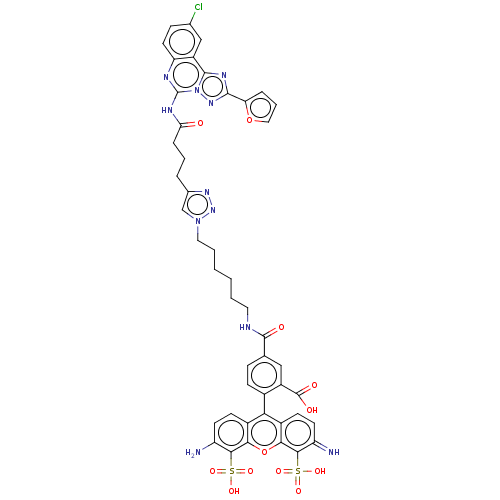
(CHEMBL2413248)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCC(=O)Nc4nc5ccc(Cl)cc5c5nc(nn45)-c4ccco4)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(2.4,-1.39,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-2.67,3.08,;-4,3.85,;-4,5.39,;-2.67,6.16,;-1.34,5.39,;-1.33,3.85,;0,3.09,;.01,1.86,;1.07,3.71,;-2.67,7.7,;-3.74,8.32,;-1.34,8.47,;-1.34,10.01,;-0,10.78,;-0,12.32,;1.33,13.1,;1.33,14.64,;2.67,15.41,;2.67,16.95,;3.91,17.83,;3.43,19.3,;4.33,20.54,;3.7,21.95,;4.6,23.2,;3.97,24.61,;2.75,24.73,;4.87,25.86,;4.24,27.26,;2.71,27.41,;2.08,28.82,;.55,28.97,;-.09,30.37,;.81,31.63,;.3,32.75,;2.34,31.47,;2.98,30.07,;4.51,29.92,;5.65,30.95,;6.98,30.19,;6.67,28.68,;5.14,28.51,;8.39,30.82,;9.64,29.94,;10.86,30.89,;10.33,32.34,;8.8,32.29,;1.89,19.29,;1.42,17.83,;-4,.77,;-5.33,1.54,;-6.67,.77,;-6.67,-.77,;-7.74,-1.39,;-5.33,-1.54,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;,-1.54,;,-3.08,;0,-4.31,;-1.07,-3.7,;1.07,-3.7,;-5.33,-3.08,;-5.33,-4.31,;-6.4,-3.7,;-4.27,-3.7,)| Show InChI InChI=1S/C46H40ClN11O12S2/c47-25-11-17-34-31(22-25)43-53-42(35-8-6-20-69-35)55-58(43)46(51-34)52-36(59)9-5-7-26-23-57(56-54-26)19-4-2-1-3-18-50-44(60)24-10-12-27(30(21-24)45(61)62)37-28-13-15-32(48)40(71(63,64)65)38(28)70-39-29(37)14-16-33(49)41(39)72(66,67)68/h6,8,10-17,20-23,48H,1-5,7,9,18-19,49H2,(H,50,60)(H,61,62)(H,51,52,59)(H,63,64,65)(H,66,67,68) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cell membranes after 1 hr by gamma counting method |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodoproxyfan from human striatal full length H3 receptor expressed in CHOK1 cells after 60 mins |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455534
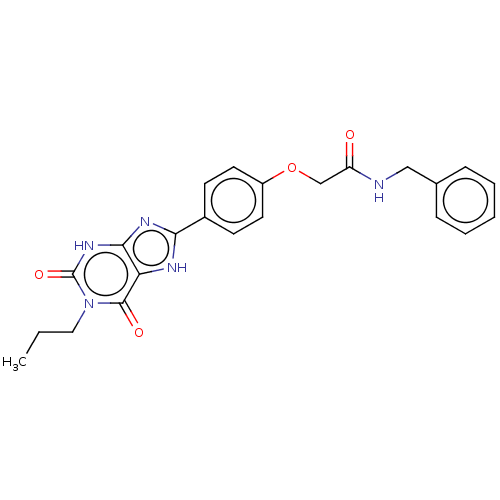
(CHEMBL4213768)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C23H23N5O4/c1-2-12-28-22(30)19-21(27-23(28)31)26-20(25-19)16-8-10-17(11-9-16)32-14-18(29)24-13-15-6-4-3-5-7-15/h3-11H,2,12-14H2,1H3,(H,24,29)(H,25,26)(H,27,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50455531
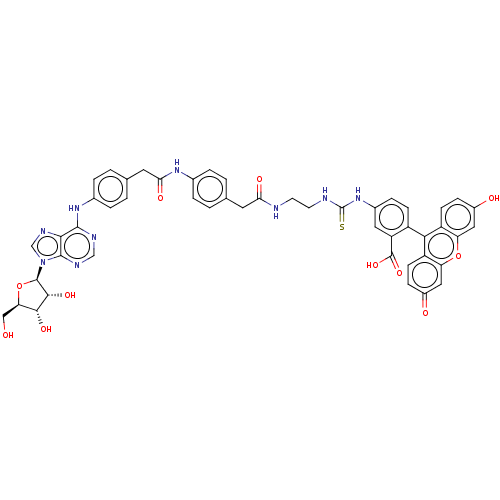
(CHEMBL4213551)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(Nc3ccc(CC(=O)Nc4ccc(CC(=O)NCCNC(=S)Nc5ccc(c(c5)C(O)=O)-c5c6ccc(O)cc6oc6cc(=O)ccc56)cc4)cc3)ncnc12 |r,wU:4.9,2.1,wD:5.5,7.8,(9.7,-25.89,;10.78,-24.8,;12.25,-25.26,;13.49,-24.35,;14.74,-25.24,;14.28,-26.71,;15.2,-27.97,;12.74,-26.73,;11.83,-27.99,;16.22,-24.77,;16.67,-23.3,;18.21,-23.3,;18.7,-24.76,;20.1,-25.38,;21.34,-24.47,;22.68,-25.22,;22.69,-26.76,;24.03,-27.52,;25.36,-26.73,;26.71,-27.49,;28.03,-26.7,;28.01,-25.16,;29.38,-27.45,;30.7,-26.66,;32.04,-27.42,;33.37,-26.63,;33.35,-25.09,;34.67,-24.3,;36.02,-25.05,;36.04,-26.59,;37.34,-24.26,;38.69,-25.01,;40.01,-24.22,;41.36,-24.98,;42.68,-24.19,;42.66,-22.64,;44.03,-24.94,;44.05,-26.48,;42.72,-27.26,;42.74,-28.8,;44.09,-29.56,;45.42,-28.76,;45.39,-27.22,;46.95,-28.76,;47.72,-27.42,;47.72,-30.09,;44.11,-31.09,;42.79,-31.88,;41.46,-31.13,;40.14,-31.92,;40.16,-33.46,;38.84,-34.25,;41.5,-34.2,;42.81,-33.42,;44.16,-34.17,;45.48,-33.37,;46.82,-34.11,;48.14,-33.32,;49.49,-34.06,;48.11,-31.78,;46.76,-31.04,;45.46,-31.83,;31.99,-24.34,;30.68,-25.13,;25.34,-25.18,;24,-24.44,;20.26,-26.9,;19.03,-27.81,;17.63,-27.18,;17.46,-25.66,)| Show InChI InChI=1S/C49H43N9O11S/c59-22-38-43(64)44(65)47(69-38)58-24-54-42-45(52-23-53-46(42)58)56-28-7-3-26(4-8-28)18-40(63)55-27-5-1-25(2-6-27)17-39(62)50-15-16-51-49(70)57-29-9-12-32(35(19-29)48(66)67)41-33-13-10-30(60)20-36(33)68-37-21-31(61)11-14-34(37)41/h1-14,19-21,23-24,38,43-44,47,59-60,64-65H,15-18,22H2,(H,50,62)(H,55,63)(H,66,67)(H2,51,57,70)(H,52,53,56)/t38-,43-,44-,47-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ADAC from adenosine A1 receptor in rat cerebral cortex membranes after 120 mins by filter binding method |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455536
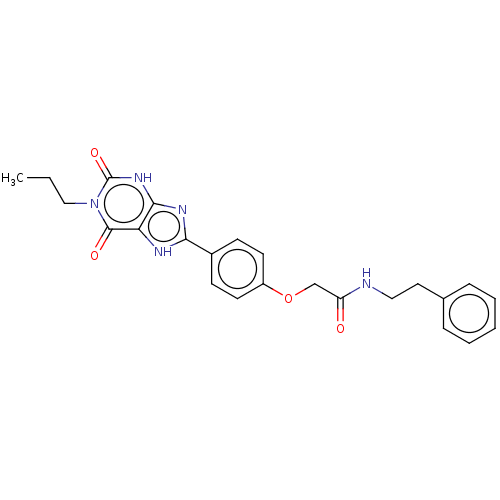
(CHEMBL4204683)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C24H25N5O4/c1-2-14-29-23(31)20-22(28-24(29)32)27-21(26-20)17-8-10-18(11-9-17)33-15-19(30)25-13-12-16-6-4-3-5-7-16/h3-11H,2,12-15H2,1H3,(H,25,30)(H,26,27)(H,28,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562540
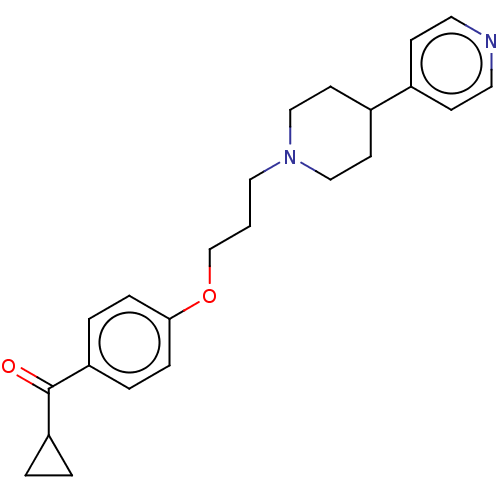
(CHEMBL4776141) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]UR-PI294 from human SP-FLAG-tagged H3R expressed in HEK293T cells measured after 60 to 120 mins by liquid scintillation counting ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455535
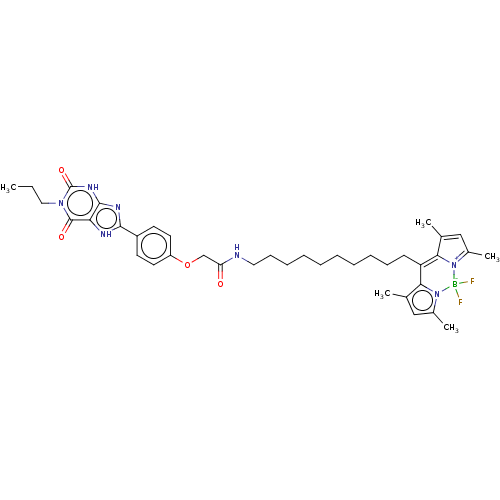
(CHEMBL4217459)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCCCCCCCCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:35,38,41| Show InChI InChI=1S/C39H50BF2N7O4/c1-6-21-47-38(51)33-37(46-39(47)52)45-36(44-33)29-16-18-30(19-17-29)53-24-32(50)43-20-14-12-10-8-7-9-11-13-15-31-34-25(2)22-27(4)48(34)40(41,42)49-28(5)23-26(3)35(31)49/h16-19,22-23H,6-15,20-21,24H2,1-5H3,(H,43,50)(H,44,45)(H,46,52) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562547
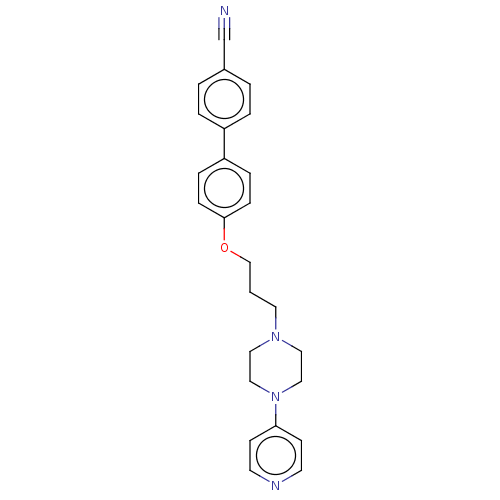
(CHEMBL4743583)Show SMILES N#Cc1ccc(cc1)-c1ccc(OCCCN2CCN(CC2)c2ccncc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]UR-PI294 from human SP-FLAG-tagged H3R expressed in HEK293T cells measured after 60 to 120 mins by liquid scintillation counting ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50455536

(CHEMBL4204683)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C24H25N5O4/c1-2-14-29-23(31)20-22(28-24(29)32)27-21(26-20)17-8-10-18(11-9-17)33-15-19(30)25-13-12-16-6-4-3-5-7-16/h3-11H,2,12-15H2,1H3,(H,25,30)(H,26,27)(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCPA from adenosine A1 receptor in rat brain cortical membranes after 90 mins by liquid scintillation counting method |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50084860
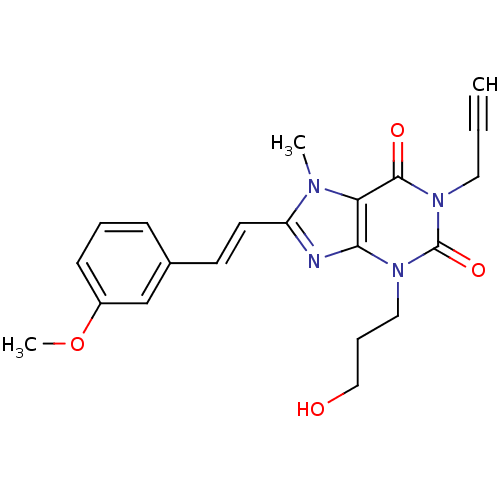
(3-(3-Hydroxy-propyl)-8-[2-(3-methoxy-phenyl)-vinyl...)Show SMILES COc1cccc(\C=C\c2nc3n(CCCO)c(=O)n(CC#C)c(=O)c3n2C)c1 Show InChI InChI=1S/C21H22N4O4/c1-4-11-25-20(27)18-19(24(21(25)28)12-6-13-26)22-17(23(18)2)10-9-15-7-5-8-16(14-15)29-3/h1,5,7-10,14,26H,6,11-13H2,2-3H3/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from adenosine A2A receptor in rat brain striatal membrane |
Eur J Med Chem 46: 3590-607 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.023
BindingDB Entry DOI: 10.7270/Q2ZC840S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50405715

(CHEMBL4173079)Show InChI InChI=1S/C19H21NO3S2/c1-12(11-24)18(21)20-10-16(9-17(20)19(22)23)25-15-7-6-13-4-2-3-5-14(13)8-15/h2-8,12,16-17,24H,9-11H2,1H3,(H,22,23)/t12-,16+,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from recombinant human H3 receptor expressed in HEK293 cells after 90 mins by liquid scintillation countin... |
Eur J Med Chem 152: 223-234 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.043
BindingDB Entry DOI: 10.7270/Q2697632 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562548

(CHEMBL4758080)Show SMILES C(COc1ccc(Nc2ccccc2)cc1)CN1CCN(CC1)c1ccncc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]UR-PI294 from human SP-FLAG-tagged H3R expressed in HEK293T cells measured after 60 to 120 mins by liquid scintillation counting ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50459836

(CHEMBL4227989)Show SMILES OC(=O)C(O)=O.CCC(C)(C)c1ccc(OCCCCCN2CCCCC2)cc1 Show InChI InChI=1S/C21H35NO/c1-4-21(2,3)19-11-13-20(14-12-19)23-18-10-6-9-17-22-15-7-5-8-16-22/h11-14H,4-10,15-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodoproxyfan from recombinant human histamine H3 receptor expressed in CHOK1 cell membranes after 90 mins |
Bioorg Med Chem 25: 2701-2712 (2017)
Article DOI: 10.1016/j.bmc.2017.03.031
BindingDB Entry DOI: 10.7270/Q2833VPK |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50021553

(CHEMBL3290570)Show InChI InChI=1S/C14H16ClN5/c1-19-6-8-20(9-7-19)14-17-10-16-13(18-14)11-2-4-12(15)5-3-11/h2-5,10H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from human histamine H4 receptor after 45 mins by scintillation counting analysis |
Eur J Med Chem 83: 534-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.032
BindingDB Entry DOI: 10.7270/Q2W097HQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50520922

(CHEMBL4547780)Show InChI InChI=1S/C20H25N3O2/c1-17(24)18-3-5-20(6-4-18)25-16-2-11-22-12-14-23(15-13-22)19-7-9-21-10-8-19/h3-10H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from full length recombinant human H3R expressed in HEK293 cell membranes after 90 mins by beta scintilla... |
Bioorg Med Chem 26: 6056-6066 (2018)
Article DOI: 10.1016/j.bmc.2018.11.010
BindingDB Entry DOI: 10.7270/Q2NV9NNX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data