 Found 982 hits with Last Name = 'karnachi' and Initial = 'p'
Found 982 hits with Last Name = 'karnachi' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50160855
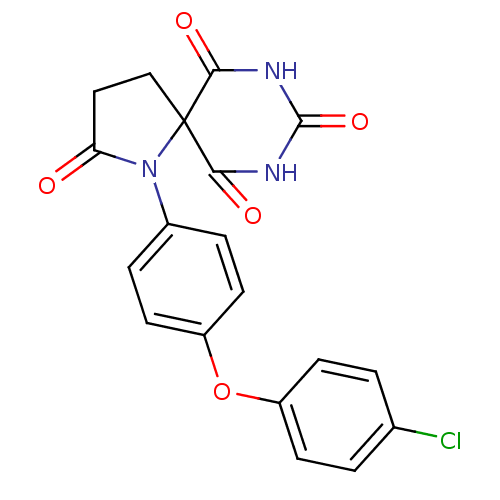
(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11551
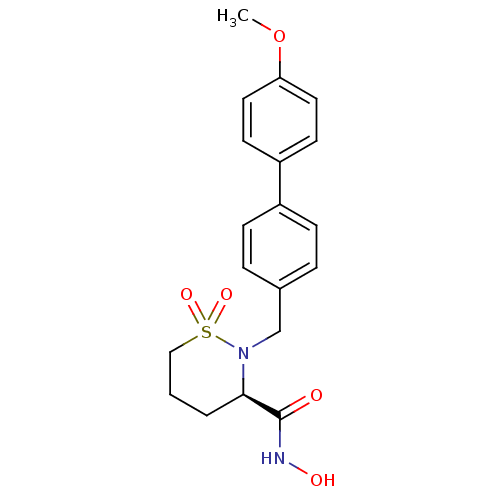
((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50160855

(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50160855

(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50356961
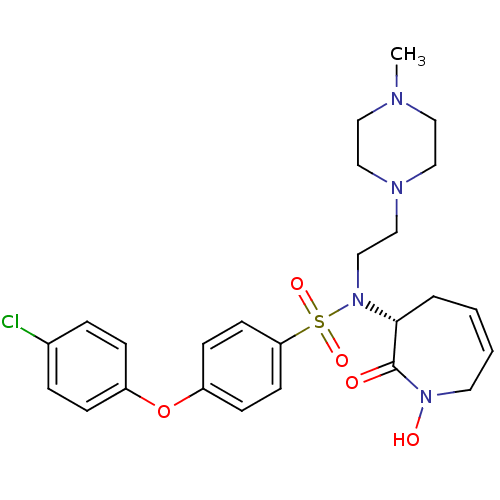
(CHEMBL1916212)Show SMILES CN1CCN(CCN([C@@H]2CC=CCN(O)C2=O)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CC1 |r,c:10| Show InChI InChI=1S/C25H31ClN4O5S/c1-27-14-16-28(17-15-27)18-19-30(24-4-2-3-13-29(32)25(24)31)36(33,34)23-11-9-22(10-12-23)35-21-7-5-20(26)6-8-21/h2-3,5-12,24,32H,4,13-19H2,1H3/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356946

(CHEMBL1916054)Show SMILES ON1CC(CN2CCOCC2)=CC[C@@H](NS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:11| Show InChI InChI=1S/C23H26ClN3O6S/c24-18-2-4-19(5-3-18)33-20-6-8-21(9-7-20)34(30,31)25-22-10-1-17(16-27(29)23(22)28)15-26-11-13-32-14-12-26/h1-9,22,25,29H,10-16H2/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50356960
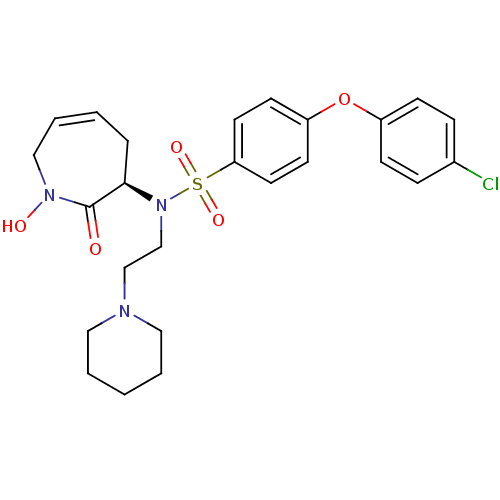
(CHEMBL1916211)Show SMILES ON1CC=CC[C@@H](N(CCN2CCCCC2)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C25H30ClN3O5S/c26-20-7-9-21(10-8-20)34-22-11-13-23(14-12-22)35(32,33)29(19-18-27-15-3-1-4-16-27)24-6-2-5-17-28(31)25(24)30/h2,5,7-14,24,31H,1,3-4,6,15-19H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50356959
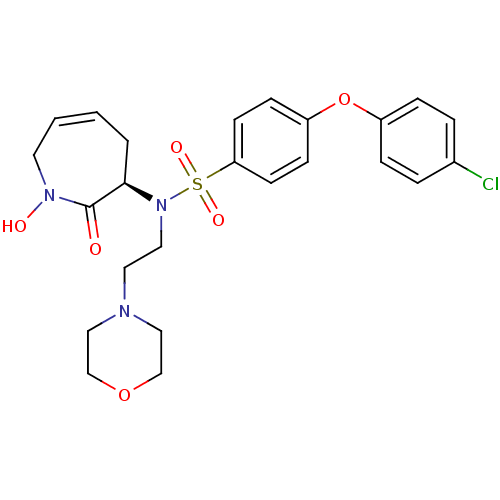
(CHEMBL1916210)Show SMILES ON1CC=CC[C@@H](N(CCN2CCOCC2)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C24H28ClN3O6S/c25-19-4-6-20(7-5-19)34-21-8-10-22(11-9-21)35(31,32)28(14-13-26-15-17-33-18-16-26)23-3-1-2-12-27(30)24(23)29/h1-2,4-11,23,30H,3,12-18H2/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50356959

(CHEMBL1916210)Show SMILES ON1CC=CC[C@@H](N(CCN2CCOCC2)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C24H28ClN3O6S/c25-19-4-6-20(7-5-19)34-21-8-10-22(11-9-21)35(31,32)28(14-13-26-15-17-33-18-16-26)23-3-1-2-12-27(30)24(23)29/h1-2,4-11,23,30H,3,12-18H2/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356942
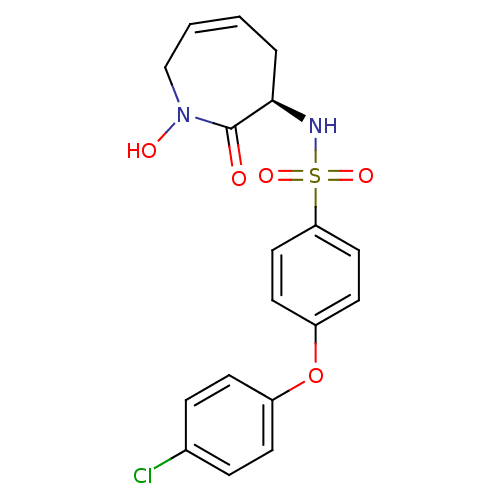
(CHEMBL1916050)Show SMILES ON1CC=CC[C@@H](NS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C18H17ClN2O5S/c19-13-4-6-14(7-5-13)26-15-8-10-16(11-9-15)27(24,25)20-17-3-1-2-12-21(23)18(17)22/h1-2,4-11,17,20,23H,3,12H2/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50356946

(CHEMBL1916054)Show SMILES ON1CC(CN2CCOCC2)=CC[C@@H](NS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:11| Show InChI InChI=1S/C23H26ClN3O6S/c24-18-2-4-19(5-3-18)33-20-6-8-21(9-7-20)34(30,31)25-22-10-1-17(16-27(29)23(22)28)15-26-11-13-32-14-12-26/h1-9,22,25,29H,10-16H2/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230525

((S)-3-(N-(4-(4-chlorophenoxy)phenyl)methan-2-ylsul...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(Cl)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C22H28ClN3O6S/c1-15-12-25(13-16(2)31-15)21(22(27)24-28)14-26(33(3,29)30)18-6-10-20(11-7-18)32-19-8-4-17(23)5-9-19/h4-11,15-16,21,28H,12-14H2,1-3H3,(H,24,27)/t15-,16+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50356961

(CHEMBL1916212)Show SMILES CN1CCN(CCN([C@@H]2CC=CCN(O)C2=O)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CC1 |r,c:10| Show InChI InChI=1S/C25H31ClN4O5S/c1-27-14-16-28(17-15-27)18-19-30(24-4-2-3-13-29(32)25(24)31)36(33,34)23-11-9-22(10-12-23)35-21-7-5-20(26)6-8-21/h2-3,5-12,24,32H,4,13-19H2,1H3/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185884
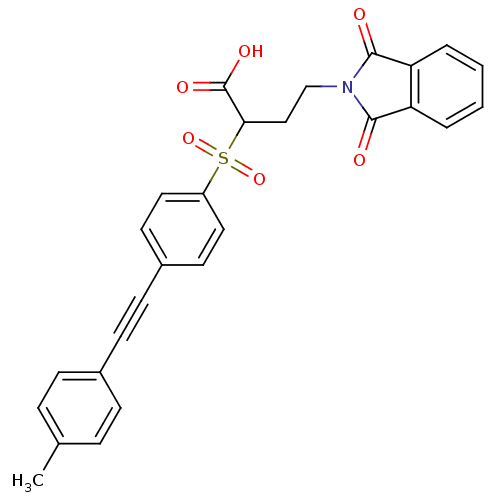
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO6S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)35(33,34)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230526

((S)-N-hydroxy-2-(isopropylamino)-3-(N-(4-(p-tolylo...)Show SMILES CC(C)N[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C20H27N3O5S/c1-14(2)21-19(20(24)22-25)13-23(29(4,26)27)16-7-11-18(12-8-16)28-17-9-5-15(3)6-10-17/h5-12,14,19,21,25H,13H2,1-4H3,(H,22,24)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230497
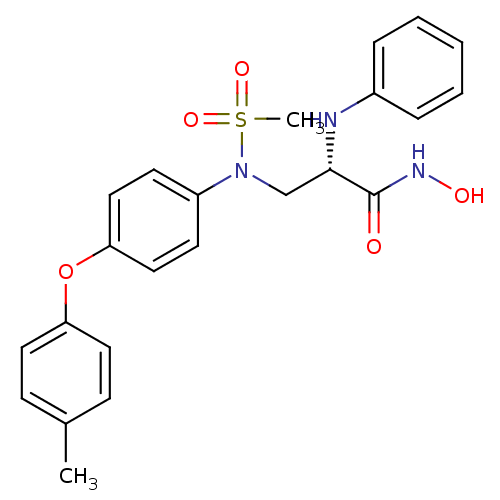
((S)-N-hydroxy-2-(phenylamino)-3-(N-(4-(p-tolyloxy)...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](Nc2ccccc2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H25N3O5S/c1-17-8-12-20(13-9-17)31-21-14-10-19(11-15-21)26(32(2,29)30)16-22(23(27)25-28)24-18-6-4-3-5-7-18/h3-15,22,24,28H,16H2,1-2H3,(H,25,27)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50356946

(CHEMBL1916054)Show SMILES ON1CC(CN2CCOCC2)=CC[C@@H](NS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:11| Show InChI InChI=1S/C23H26ClN3O6S/c24-18-2-4-19(5-3-18)33-20-6-8-21(9-7-20)34(30,31)25-22-10-1-17(16-27(29)23(22)28)15-26-11-13-32-14-12-26/h1-9,22,25,29H,10-16H2/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230508

((S)-N-hydroxy-2-((S)-1-phenylethylamino)-3-(N-(4-(...)Show SMILES C[C@H](N[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO)c1ccccc1 Show InChI InChI=1S/C25H29N3O5S/c1-18-9-13-22(14-10-18)33-23-15-11-21(12-16-23)28(34(3,31)32)17-24(25(29)27-30)26-19(2)20-7-5-4-6-8-20/h4-16,19,24,26,30H,17H2,1-3H3,(H,27,29)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50356960

(CHEMBL1916211)Show SMILES ON1CC=CC[C@@H](N(CCN2CCCCC2)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C25H30ClN3O5S/c26-20-7-9-21(10-8-20)34-22-11-13-23(14-12-22)35(32,33)29(19-18-27-15-3-1-4-16-27)24-6-2-5-17-28(31)25(24)30/h2,5,7-14,24,31H,1,3-4,6,15-19H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50356942

(CHEMBL1916050)Show SMILES ON1CC=CC[C@@H](NS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C18H17ClN2O5S/c19-13-4-6-14(7-5-13)26-15-8-10-16(11-9-15)27(24,25)20-17-3-1-2-12-21(23)18(17)22/h1-2,4-11,17,20,23H,3,12H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50356942

(CHEMBL1916050)Show SMILES ON1CC=CC[C@@H](NS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C18H17ClN2O5S/c19-13-4-6-14(7-5-13)26-15-8-10-16(11-9-15)27(24,25)20-17-3-1-2-12-21(23)18(17)22/h1-2,4-11,17,20,23H,3,12H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185896

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-tr...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H18F3NO5S/c28-27(29,30)36-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)37-23(26(34)35)15-16-31-24(32)21-3-1-2-4-22(21)25(31)33/h1-4,7-14,23H,15-16H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185883
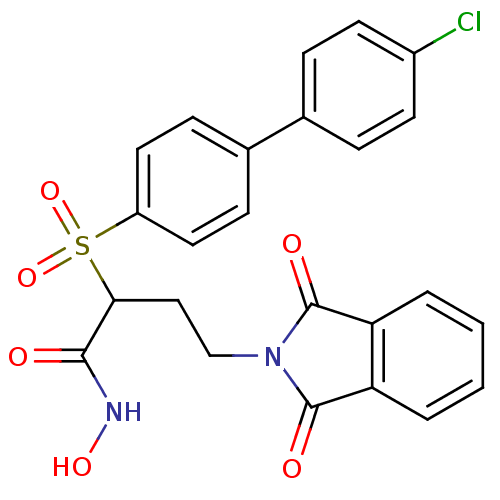
(2-(4'-chloro-biphenyl-4-sulfonyl)-4-(1,3-dioxo-1,3...)Show SMILES ONC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H19ClN2O6S/c25-17-9-5-15(6-10-17)16-7-11-18(12-8-16)34(32,33)21(22(28)26-31)13-14-27-23(29)19-3-1-2-4-20(19)24(27)30/h1-12,21,31H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230496

((S)-N-hydroxy-2-(3-methoxyphenylamino)-3-(N-(4-(p-...)Show SMILES COc1cccc(N[C@@H](CN(c2ccc(Oc3ccc(C)cc3)cc2)S(C)(=O)=O)C(=O)NO)c1 Show InChI InChI=1S/C24H27N3O6S/c1-17-7-11-20(12-8-17)33-21-13-9-19(10-14-21)27(34(3,30)31)16-23(24(28)26-29)25-18-5-4-6-22(15-18)32-2/h4-15,23,25,29H,16H2,1-3H3,(H,26,28)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230512
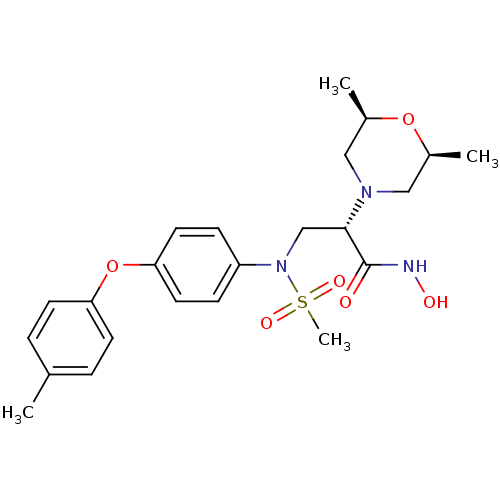
((S)-2-(cis-2,6-dimethylmorpholino)-N-hydroxy-3-(N-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-5-9-20(10-6-16)32-21-11-7-19(8-12-21)26(33(4,29)30)15-22(23(27)24-28)25-13-17(2)31-18(3)14-25/h5-12,17-18,22,28H,13-15H2,1-4H3,(H,24,27)/t17-,18+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185871

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(5-fl...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1nc2ccc(F)cc2[nH]1 Show InChI InChI=1S/C25H18FN3O6S/c26-15-7-10-19-20(13-15)28-22(27-19)14-5-8-16(9-6-14)36(34,35)21(25(32)33)11-12-29-23(30)17-3-1-2-4-18(17)24(29)31/h1-10,13,21H,11-12H2,(H,27,28)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356959

(CHEMBL1916210)Show SMILES ON1CC=CC[C@@H](N(CCN2CCOCC2)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C24H28ClN3O6S/c25-19-4-6-20(7-5-19)34-21-8-10-22(11-9-21)35(31,32)28(14-13-26-15-17-33-18-16-26)23-3-1-2-12-27(30)24(23)29/h1-2,4-11,23,30H,3,12-18H2/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185884

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO6S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)35(33,34)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185900

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES CSc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S2/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185888
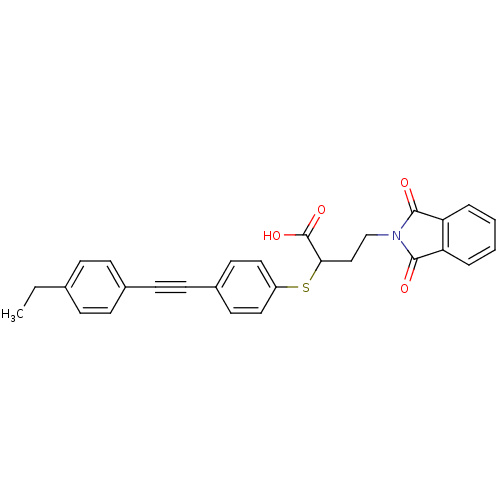
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-et...)Show SMILES CCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C28H23NO4S/c1-2-19-7-9-20(10-8-19)11-12-21-13-15-22(16-14-21)34-25(28(32)33)17-18-29-26(30)23-5-3-4-6-24(23)27(29)31/h3-10,13-16,25H,2,17-18H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50356964

(CHEMBL1916215)Show SMILES Cc1ccc(cc1)C#Cc1ccc(s1)S(=O)(=O)N(CCN1CCOCC1)[C@@H]1CC=CCN(O)C1=O |r,c:31| Show InChI InChI=1S/C25H29N3O5S2/c1-20-5-7-21(8-6-20)9-10-22-11-12-24(34-22)35(31,32)28(15-14-26-16-18-33-19-17-26)23-4-2-3-13-27(30)25(23)29/h2-3,5-8,11-12,23,30H,4,13-19H2,1H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50185871

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(5-fl...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1nc2ccc(F)cc2[nH]1 Show InChI InChI=1S/C25H18FN3O6S/c26-15-7-10-19-20(13-15)28-22(27-19)14-5-8-16(9-6-14)36(34,35)21(25(32)33)11-12-29-23(30)17-3-1-2-4-18(17)24(29)31/h1-10,13,21H,11-12H2,(H,27,28)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185875

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO7S/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374486
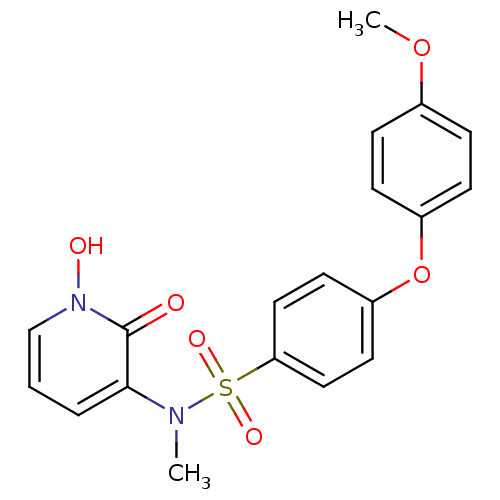
(CHEMBL408366)Show SMILES COc1ccc(Oc2ccc(cc2)S(=O)(=O)N(C)c2cccn(O)c2=O)cc1 Show InChI InChI=1S/C19H18N2O6S/c1-20(18-4-3-13-21(23)19(18)22)28(24,25)17-11-9-16(10-12-17)27-15-7-5-14(26-2)6-8-15/h3-13,23H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230507

((S)-2-(3-fluorophenylamino)-N-hydroxy-3-(N-(4-(p-t...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](Nc2cccc(F)c2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H24FN3O5S/c1-16-6-10-20(11-7-16)32-21-12-8-19(9-13-21)27(33(2,30)31)15-22(23(28)26-29)25-18-5-3-4-17(24)14-18/h3-14,22,25,29H,15H2,1-2H3,(H,26,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185900

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES CSc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S2/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185880

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-isop...)Show SMILES CC(C)c1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H25NO6S/c1-17(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)35(33,34)24(27(31)32)15-16-28-25(29)22-5-3-4-6-23(22)26(28)30/h3-14,17,24H,15-16H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50356950

(CHEMBL1916201)Show SMILES COc1ccc(Oc2ccc(cc2)S(=O)(=O)N[C@@H]2CC=CCN(O)C2=O)cc1 |r,c:20| Show InChI InChI=1S/C19H20N2O6S/c1-26-14-5-7-15(8-6-14)27-16-9-11-17(12-10-16)28(24,25)20-18-4-2-3-13-21(23)19(18)22/h2-3,5-12,18,20,23H,4,13H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50356949

(CHEMBL1916057)Show SMILES ON1CC=CC[C@@H](NS(=O)(=O)c2ccc(Oc3ccccc3)cc2)C1=O |r,c:3| Show InChI InChI=1S/C18H18N2O5S/c21-18-17(8-4-5-13-20(18)22)19-26(23,24)16-11-9-15(10-12-16)25-14-6-2-1-3-7-14/h1-7,9-12,17,19,22H,8,13H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230518

((S)-2-(cis-2,6-dimethylmorpholino)-N-hydroxy-3-(N-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C23H28F3N3O6S/c1-15-12-28(13-16(2)34-15)21(22(30)27-31)14-29(36(3,32)33)18-6-10-20(11-7-18)35-19-8-4-17(5-9-19)23(24,25)26/h4-11,15-16,21,31H,12-14H2,1-3H3,(H,27,30)/t15-,16+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185890
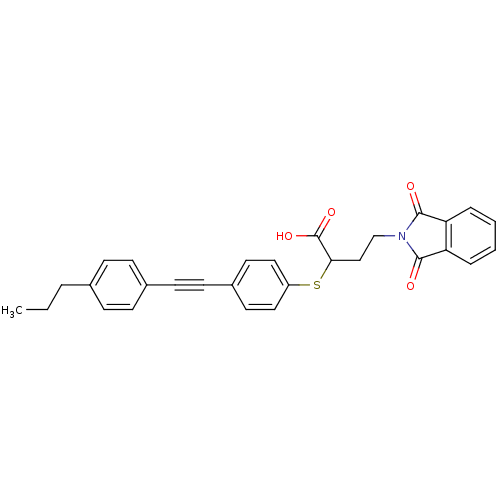
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-pr...)Show SMILES CCCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C29H25NO4S/c1-2-5-20-8-10-21(11-9-20)12-13-22-14-16-23(17-15-22)35-26(29(33)34)18-19-30-27(31)24-6-3-4-7-25(24)28(30)32/h3-4,6-11,14-17,26H,2,5,18-19H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185899
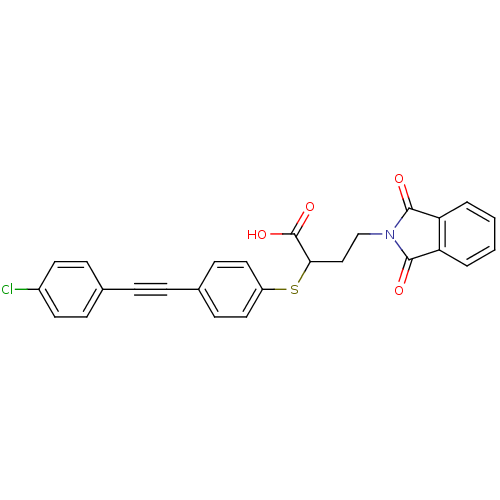
(2-[4-(4-chloro-phenylethynyl)-phenylsulfanyl]-4-(1...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(Cl)cc1 Show InChI InChI=1S/C26H18ClNO4S/c27-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)33-23(26(31)32)15-16-28-24(29)21-3-1-2-4-22(21)25(28)30/h1-4,7-14,23H,15-16H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185877
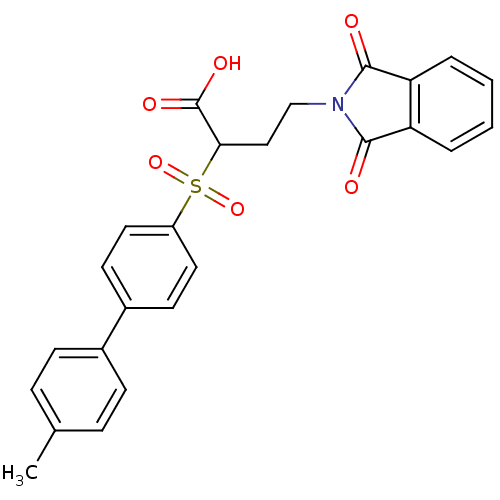
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S/c1-16-6-8-17(9-7-16)18-10-12-19(13-11-18)33(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50356945

(CHEMBL1916053)Show SMILES CC1=CC[C@@H](NS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)C(=O)N(O)C1 |r,t:1| Show InChI InChI=1S/C19H19ClN2O5S/c1-13-2-11-18(19(23)22(24)12-13)21-28(25,26)17-9-7-16(8-10-17)27-15-5-3-14(20)4-6-15/h2-10,18,21,24H,11-12H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... |
Bioorg Med Chem Lett 21: 6485-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.068
BindingDB Entry DOI: 10.7270/Q2BP0353 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230499

((S)-N-hydroxy-2-(isobutylamino)-3-(N-(4-(p-tolylox...)Show SMILES CC(C)CN[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C21H29N3O5S/c1-15(2)13-22-20(21(25)23-26)14-24(30(4,27)28)17-7-11-19(12-8-17)29-18-9-5-16(3)6-10-18/h5-12,15,20,22,26H,13-14H2,1-4H3,(H,23,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230516
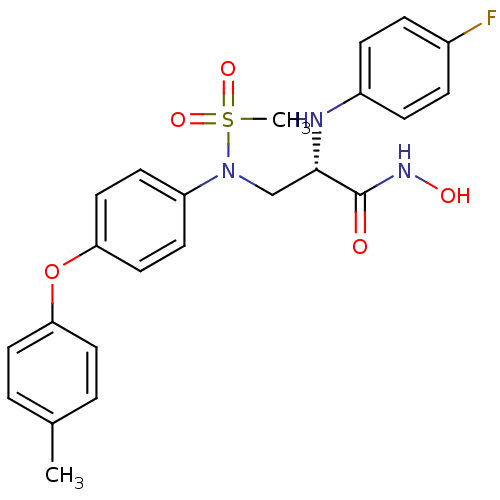
((S)-2-(4-fluorophenylamino)-N-hydroxy-3-(N-(4-(p-t...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](Nc2ccc(F)cc2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H24FN3O5S/c1-16-3-11-20(12-4-16)32-21-13-9-19(10-14-21)27(33(2,30)31)15-22(23(28)26-29)25-18-7-5-17(24)6-8-18/h3-14,22,25,29H,15H2,1-2H3,(H,26,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185896

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-tr...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H18F3NO5S/c28-27(29,30)36-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)37-23(26(34)35)15-16-31-24(32)21-3-1-2-4-22(21)25(31)33/h1-4,7-14,23H,15-16H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374494

(CHEMBL271736)Show SMILES CN1CCN(CCN(c2cccn(O)c2=O)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CC1 Show InChI InChI=1S/C24H27ClN4O5S/c1-26-13-15-27(16-14-26)17-18-29(23-3-2-12-28(31)24(23)30)35(32,33)22-10-8-21(9-11-22)34-20-6-4-19(25)5-7-20/h2-12,31H,13-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data