 Found 94 hits with Last Name = 'karoyan' and Initial = 'p'
Found 94 hits with Last Name = 'karoyan' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50070367
(Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Pro-Leu-Met-NH2 | ...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C66H102N18O13S/c1-39(2)36-47(58(90)75-43(55(71)87)29-35-98-3)80-62(94)52-24-15-34-84(52)65(97)49(38-41-18-8-5-9-19-41)81-59(91)48(37-40-16-6-4-7-17-40)79-57(89)44(25-27-53(69)85)76-56(88)45(26-28-54(70)86)77-60(92)51-23-14-33-83(51)64(96)46(21-10-11-30-67)78-61(93)50-22-13-32-82(50)63(95)42(68)20-12-31-74-66(72)73/h4-9,16-19,39,42-52H,10-15,20-38,67-68H2,1-3H3,(H2,69,85)(H2,70,86)(H2,71,87)(H,75,90)(H,76,88)(H,77,92)(H,78,93)(H,79,89)(H,80,94)(H,81,91)(H4,72,73,74)/t42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ P. et M. Curie
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards human tachykinin NK-1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 8: 1369-74 (1999)
BindingDB Entry DOI: 10.7270/Q2M32W9S |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ P. et M. Curie
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards human tachykinin NK-1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 8: 1369-74 (1999)
BindingDB Entry DOI: 10.7270/Q2M32W9S |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478359
(CHEMBL408871)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC(O)=O)Cc1ccccc1 Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)36(48-37(53)31(46-39(55)35(42)24(2)3)19-27-13-15-30(50)16-14-27)40(56)47-32(20-29-22-43-23-44-29)41(57)49-17-9-12-33(49)38(54)45-28(21-34(51)52)18-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,28,31-33,35-36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,54)(H,46,55)(H,47,56)(H,48,53)(H,51,52)/t25-,28-,31-,32-,33-,35-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50070371

(Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Pro-MePhe(pBz)-Trp...)Show SMILES CN([C@@H](Cc1ccc(Cc2ccccc2)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O)C(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)N1C2CC3CCC2(CS1(=O)=O)C3(C)C |THB:114:115:125:119.118| Show InChI InChI=1S/C93H123N19O15S/c1-92(2)63-42-43-93(92)56-128(126,127)112(77(93)54-63)75(30-17-45-100-91(98)99)90(125)110-47-19-32-73(110)85(120)104-68(29-15-16-44-94)87(122)109-46-18-31-72(109)84(119)103-67(39-41-79(96)114)81(116)102-66(38-40-78(95)113)82(117)106-70(50-58-23-9-5-10-24-58)83(118)107-71(51-59-25-11-6-12-26-59)88(123)111-48-20-33-74(111)89(124)108(3)76(52-61-36-34-60(35-37-61)49-57-21-7-4-8-22-57)86(121)105-69(80(97)115)53-62-55-101-65-28-14-13-27-64(62)65/h4-14,21-28,34-37,55,63,66-77,101H,15-20,29-33,38-54,56,94H2,1-3H3,(H2,95,113)(H2,96,114)(H2,97,115)(H,102,116)(H,103,119)(H,104,120)(H,105,121)(H,106,117)(H,107,118)(H4,98,99,100)/t63?,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77?,93?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ P. et M. Curie
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards human tachykinin NK-1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 8: 1369-74 (1999)
BindingDB Entry DOI: 10.7270/Q2M32W9S |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50415633

(CHEMBL259019)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CN)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C42H58N8O8/c1-5-26(4)37(49-39(55)33(19-28-13-15-31(51)16-14-28)47-38(54)32(22-43)25(2)3)41(57)48-34(20-30-23-44-24-45-30)42(58)50-17-9-12-35(50)40(56)46-29(21-36(52)53)18-27-10-7-6-8-11-27/h6-8,10-11,13-16,23-26,29,32-35,37,51H,5,9,12,17-22,43H2,1-4H3,(H,44,45)(H,46,56)(H,47,54)(H,48,57)(H,49,55)(H,52,53)/t26-,29-,32-,33-,34-,35-,37-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403652
(CHEMBL2110361 | GR-82334)Show SMILES CC(C)C[C@H](N1CC[C@@]2(CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)C2CCCN2)C1=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C69H93N15O15/c1-39(2)32-55(65(96)77-49(58(72)89)35-43-38-74-46-17-8-7-16-45(43)46)83-31-26-69(68(83)99)25-13-30-84(69)67(98)52(34-42-21-23-44(85)24-22-42)81-62(93)50(33-41-14-5-4-6-15-41)78-61(92)48(18-9-10-27-70)76-63(94)51(36-56(71)86)79-64(95)54-20-12-29-82(54)66(97)53(37-57(87)88)80-59(90)40(3)75-60(91)47-19-11-28-73-47/h4-8,14-17,21-24,38-40,47-55,73-74,85H,9-13,18-20,25-37,70H2,1-3H3,(H2,71,86)(H2,72,89)(H,75,91)(H,76,94)(H,77,96)(H,78,92)(H,79,95)(H,80,90)(H,81,93)(H,87,88)/t40-,47?,48-,49-,50-,51-,52-,53-,54-,55-,69-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ P. et M. Curie
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards human tachykinin NK-1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 8: 1369-74 (1999)
BindingDB Entry DOI: 10.7270/Q2M32W9S |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM85550

(Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H54N8O8/c1-5-24(4)34(47-35(50)29(44-37(52)33(41)23(2)3)18-26-13-15-28(49)16-14-26)38(53)45-30(20-27-21-42-22-43-27)39(54)48-17-9-12-32(48)36(51)46-31(40(55)56)19-25-10-7-6-8-11-25/h6-8,10-11,13-16,21-24,29-34,49H,5,9,12,17-20,41H2,1-4H3,(H,42,43)(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,55,56)/t24-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50070370
(Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Pro-Pro(trans-CH2-...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N1CCC(Cc2ccccc2)[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C78H105N19O13/c79-36-13-12-27-57(90-72(105)61-28-15-38-94(61)74(107)53(80)25-14-37-86-78(84)85)75(108)95-39-16-29-62(95)71(104)89-56(32-34-65(82)99)68(101)88-55(31-33-64(81)98)69(102)92-59(43-48-20-6-2-7-21-48)70(103)93-60(44-49-22-8-3-9-23-49)76(109)96-40-17-30-63(96)77(110)97-41-35-50(42-47-18-4-1-5-19-47)66(97)73(106)91-58(67(83)100)45-51-46-87-54-26-11-10-24-52(51)54/h1-11,18-24,26,46,50,53,55-63,66,87H,12-17,25,27-45,79-80H2,(H2,81,98)(H2,82,99)(H2,83,100)(H,88,101)(H,89,104)(H,90,105)(H,91,106)(H,92,102)(H,93,103)(H4,84,85,86)/t50?,53-,55-,56-,57-,58-,59-,60-,61-,62-,63-,66-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ P. et M. Curie
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards human tachykinin NK-1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 8: 1369-74 (1999)
BindingDB Entry DOI: 10.7270/Q2M32W9S |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478364
(CHEMBL248592)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCCC[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-36(51)30(45-38(53)34(42)24(2)3)19-27-14-16-29(50)17-15-27)39(54)46-31(21-28-22-43-23-44-28)40(55)49-18-10-9-13-33(49)37(52)47-32(41(56)57)20-26-11-7-6-8-12-26/h6-8,11-12,14-17,22-25,30-35,50H,5,9-10,13,18-21,42H2,1-4H3,(H,43,44)(H,45,53)(H,46,54)(H,47,52)(H,48,51)(H,56,57)/t25-,30-,31-,32-,33+,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50415634
(CHEMBL260622)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CN)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-37(52)31(18-27-13-15-29(50)16-14-27)45-36(51)30(21-42)24(2)3)39(54)46-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)47-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,54)(H,47,53)(H,48,52)(H,56,57)/t25-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478356
(CHEMBL408132)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)NC[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-36(51)31(46-38(53)34(42)24(2)3)19-27-13-15-30(50)16-14-27)39(54)47-32(20-29-22-43-23-45-29)40(55)49-17-9-12-33(49)37(52)44-21-28(41(56)57)18-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,28,31-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,45)(H,44,52)(H,46,53)(H,47,54)(H,48,51)(H,56,57)/t25-,28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50070369
(Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Pro-MePhe-Trp-NH2 ...)Show SMILES CN([C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O)C(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCN=C(N)N |wU:67.78,47.60,13.13,2.2,99.107,wD:29.31,76.81,92.98,36.48,83.95,58.69,(23.13,-7.36,;21.71,-6.76,;21.52,-5.23,;20.1,-4.63,;19.91,-3.11,;21.14,-2.18,;20.95,-.65,;19.53,-.05,;18.3,-.98,;18.49,-2.51,;22.75,-4.3,;24.17,-4.9,;22.56,-2.78,;23.79,-1.85,;23.6,-.32,;24.82,.61,;26.3,.16,;27.18,1.43,;26.25,2.65,;26.54,4.17,;25.38,5.17,;23.92,4.67,;23.63,3.16,;24.79,2.15,;25.2,-2.45,;25.39,-3.97,;26.43,-1.52,;20.48,-7.69,;19.06,-7.09,;20.67,-9.22,;22.02,-9.96,;21.73,-11.48,;20.2,-11.67,;19.55,-10.27,;18.04,-9.98,;17.53,-8.53,;17.03,-11.14,;17.53,-12.6,;16.52,-13.76,;15.01,-13.47,;14,-14.64,;14.51,-16.09,;16.02,-16.38,;17.03,-15.22,;15.52,-10.85,;14.51,-12.02,;14.06,-13.49,;13,-11.73,;12.49,-10.27,;13.5,-9.11,;15.01,-9.4,;16.02,-8.23,;15.52,-6.78,;14,-6.49,;13,-7.65,;11.99,-12.89,;10.47,-12.6,;9.97,-11.14,;9.47,-13.76,;9.97,-15.22,;8.96,-16.38,;9.47,-17.84,;10.98,-18.13,;8.46,-19,;7.95,-13.47,;6.95,-14.64,;7.45,-16.09,;5.43,-14.35,;4.93,-12.89,;3.42,-12.6,;2.91,-11.14,;3.92,-9.98,;1.4,-10.85,;4.43,-15.51,;2.91,-15.22,;2.41,-13.76,;1.91,-16.38,;2.25,-17.88,;.94,-18.68,;-.23,-17.67,;.37,-16.25,;-.42,-14.93,;.32,-13.58,;-1.96,-14.96,;-2.71,-16.31,;-1.91,-17.63,;-2.66,-18.98,;-1.86,-20.3,;-.32,-20.27,;-2.76,-13.64,;-4.3,-13.67,;-5.04,-15.02,;-5.1,-12.36,;-6.63,-12.22,;-6.98,-10.72,;-5.66,-9.93,;-4.5,-10.94,;-3,-10.59,;-1.94,-11.71,;-2.55,-9.11,;-3.6,-7.99,;-1.05,-8.76,;-.6,-7.29,;.9,-6.94,;1.35,-5.47,;2.85,-5.12,;3.29,-3.65,;3.9,-6.24,)| Show InChI InChI=1S/C76H103N19O13/c1-92(62(43-48-23-9-4-10-24-48)71(104)89-56(65(81)98)44-49-45-85-52-27-12-11-25-50(49)52)75(108)61-31-18-40-95(61)74(107)58(42-47-21-7-3-8-22-47)91-68(101)57(41-46-19-5-2-6-20-46)90-67(100)53(32-34-63(79)96)86-66(99)54(33-35-64(80)97)87-69(102)60-30-17-39-94(60)73(106)55(28-13-14-36-77)88-70(103)59-29-16-38-93(59)72(105)51(78)26-15-37-84-76(82)83/h2-12,19-25,27,45,51,53-62,85H,13-18,26,28-44,77-78H2,1H3,(H2,79,96)(H2,80,97)(H2,81,98)(H,86,99)(H,87,102)(H,88,103)(H,89,104)(H,90,100)(H,91,101)(H4,82,83,84)/t51-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ P. et M. Curie
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards human tachykinin NK-1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 8: 1369-74 (1999)
BindingDB Entry DOI: 10.7270/Q2M32W9S |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478366

(CHEMBL258480)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC[C@H](CC(C)C)C(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C42H57N7O9/c1-5-26(4)36(48-37(51)32(19-28-13-15-31(50)16-14-28)44-22-29(41(55)56)18-25(2)3)39(53)46-33(21-30-23-43-24-45-30)40(54)49-17-9-12-35(49)38(52)47-34(42(57)58)20-27-10-7-6-8-11-27/h6-8,10-11,13-16,23-26,29,32-36,44,50H,5,9,12,17-22H2,1-4H3,(H,43,45)(H,46,53)(H,47,52)(H,48,51)(H,55,56)(H,57,58)/t26-,29-,32-,33-,34-,35-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478362
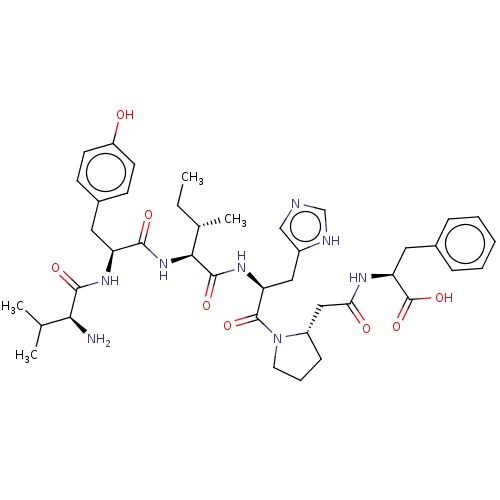
(CHEMBL261417)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1CC(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)36(48-37(52)31(46-38(53)35(42)24(2)3)18-27-13-15-30(50)16-14-27)39(54)47-32(20-28-22-43-23-44-28)40(55)49-17-9-12-29(49)21-34(51)45-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,29,31-33,35-36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,53)(H,47,54)(H,48,52)(H,56,57)/t25-,29-,31-,32-,33-,35-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478360
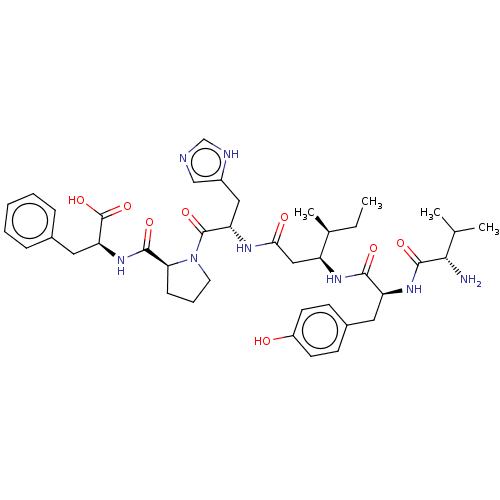
(CHEMBL261731)Show SMILES CC[C@H](C)[C@H](CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)30(46-37(52)31(47-39(54)36(42)24(2)3)18-27-13-15-29(50)16-14-27)21-35(51)45-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)48-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-34,36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,52)(H,47,54)(H,48,53)(H,56,57)/t25-,30-,31-,32-,33-,34-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478354
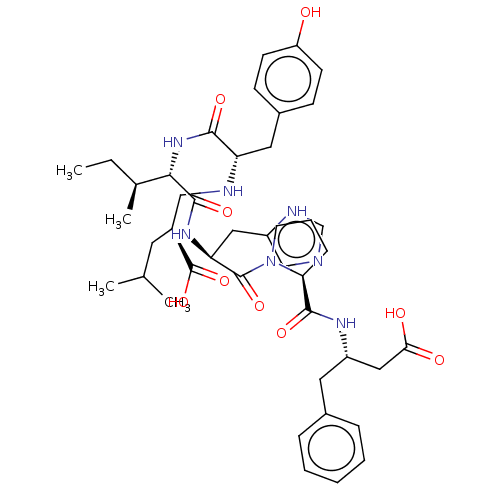
(CHEMBL261422)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC[C@H](CC(C)C)C(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC(O)=O)Cc1ccccc1 Show InChI InChI=1S/C43H59N7O9/c1-5-27(4)38(49-39(54)34(20-29-13-15-33(51)16-14-29)45-23-30(43(58)59)18-26(2)3)41(56)48-35(21-32-24-44-25-46-32)42(57)50-17-9-12-36(50)40(55)47-31(22-37(52)53)19-28-10-7-6-8-11-28/h6-8,10-11,13-16,24-27,30-31,34-36,38,45,51H,5,9,12,17-23H2,1-4H3,(H,44,46)(H,47,55)(H,48,56)(H,49,54)(H,52,53)(H,58,59)/t27-,30-,31-,34-,35-,36-,38-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478367
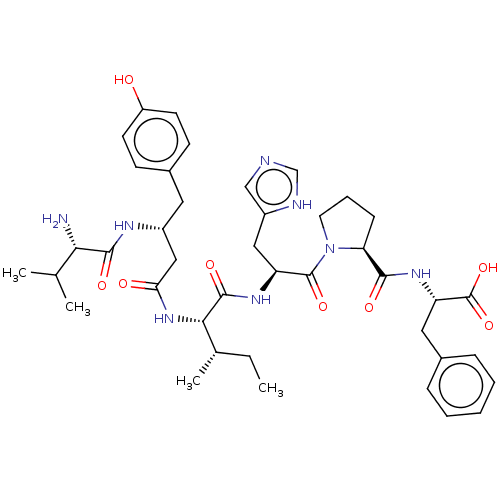
(CHEMBL259382)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)36(48-34(51)21-28(45-38(53)35(42)24(2)3)18-27-13-15-30(50)16-14-27)39(54)46-31(20-29-22-43-23-44-29)40(55)49-17-9-12-33(49)37(52)47-32(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,28,31-33,35-36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,53)(H,46,54)(H,47,52)(H,48,51)(H,56,57)/t25-,28+,31-,32-,33-,35-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478357
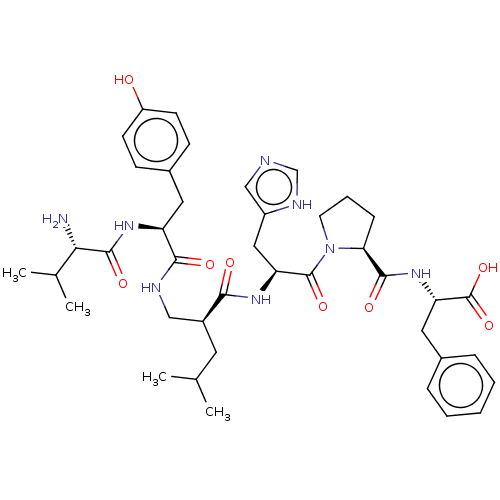
(CHEMBL428457)Show SMILES CC(C)C[C@@H](CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-24(2)17-28(21-44-37(52)31(46-39(54)35(42)25(3)4)18-27-12-14-30(50)15-13-27)36(51)47-32(20-29-22-43-23-45-29)40(55)49-16-8-11-34(49)38(53)48-33(41(56)57)19-26-9-6-5-7-10-26/h5-7,9-10,12-15,22-25,28,31-35,50H,8,11,16-21,42H2,1-4H3,(H,43,45)(H,44,52)(H,46,54)(H,47,51)(H,48,53)(H,56,57)/t28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478357

(CHEMBL428457)Show SMILES CC(C)C[C@@H](CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-24(2)17-28(21-44-37(52)31(46-39(54)35(42)25(3)4)18-27-12-14-30(50)15-13-27)36(51)47-32(20-29-22-43-23-45-29)40(55)49-16-8-11-34(49)38(53)48-33(41(56)57)19-26-9-6-5-7-10-26/h5-7,9-10,12-15,22-25,28,31-35,50H,8,11,16-21,42H2,1-4H3,(H,43,45)(H,44,52)(H,46,54)(H,47,51)(H,48,53)(H,56,57)/t28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM85550

(Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H54N8O8/c1-5-24(4)34(47-35(50)29(44-37(52)33(41)23(2)3)18-26-13-15-28(49)16-14-26)38(53)45-30(20-27-21-42-22-43-27)39(54)48-17-9-12-32(48)36(51)46-31(40(55)56)19-25-10-7-6-8-11-25/h6-8,10-11,13-16,21-24,29-34,49H,5,9,12,17-20,41H2,1-4H3,(H,42,43)(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,55,56)/t24-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]valsartan from human AT1 receptor expressed in CHO cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50415634

(CHEMBL260622)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CN)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-37(52)31(18-27-13-15-29(50)16-14-27)45-36(51)30(21-42)24(2)3)39(54)46-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)47-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,54)(H,47,53)(H,48,52)(H,56,57)/t25-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]valsartan from human AT1 receptor expressed in CHO cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001447
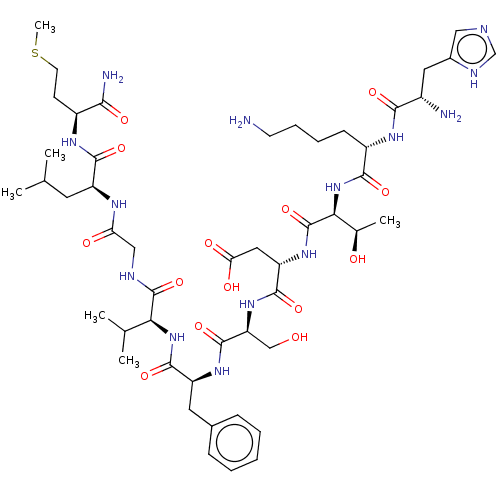
(CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)C(C)C)C(N)=O Show InChI InChI=1S/C50H80N14O14S/c1-26(2)18-34(45(73)58-32(42(53)70)15-17-79-6)57-38(67)23-55-49(77)40(27(3)4)63-47(75)35(19-29-12-8-7-9-13-29)60-48(76)37(24-65)62-46(74)36(21-39(68)69)61-50(78)41(28(5)66)64-44(72)33(14-10-11-16-51)59-43(71)31(52)20-30-22-54-25-56-30/h7-9,12-13,22,25-28,31-37,40-41,65-66H,10-11,14-21,23-24,51-52H2,1-6H3,(H2,53,70)(H,54,56)(H,55,77)(H,57,67)(H,58,73)(H,59,71)(H,60,76)(H,61,78)(H,62,74)(H,63,75)(H,64,72)(H,68,69)/t28-,31+,32+,33+,34+,35+,36+,37+,40+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ P. et M. Curie
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards human tachykinin NK-1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 8: 1369-74 (1999)
BindingDB Entry DOI: 10.7270/Q2M32W9S |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478363
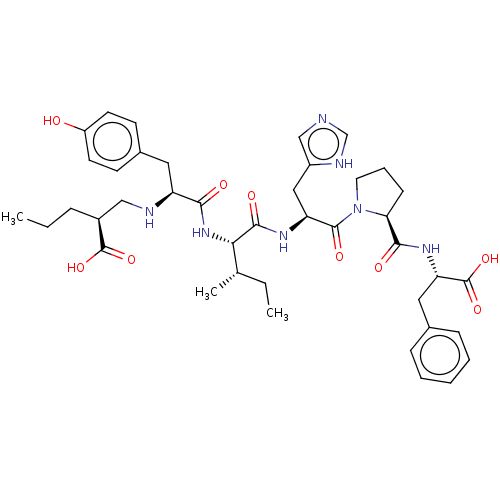
(CHEMBL403717)Show SMILES CCC[C@@H](CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(O)=O Show InChI InChI=1S/C41H55N7O9/c1-4-10-28(40(54)55)22-43-31(19-27-14-16-30(49)17-15-27)36(50)47-35(25(3)5-2)38(52)45-32(21-29-23-42-24-44-29)39(53)48-18-9-13-34(48)37(51)46-33(41(56)57)20-26-11-7-6-8-12-26/h6-8,11-12,14-17,23-25,28,31-35,43,49H,4-5,9-10,13,18-22H2,1-3H3,(H,42,44)(H,45,52)(H,46,51)(H,47,50)(H,54,55)(H,56,57)/t25-,28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM85550

(Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H54N8O8/c1-5-24(4)34(47-35(50)29(44-37(52)33(41)23(2)3)18-26-13-15-28(49)16-14-26)38(53)45-30(20-27-21-42-22-43-27)39(54)48-17-9-12-32(48)36(51)46-31(40(55)56)19-25-10-7-6-8-11-25/h6-8,10-11,13-16,21-24,29-34,49H,5,9,12,17-20,41H2,1-4H3,(H,42,43)(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,55,56)/t24-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 831 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478365
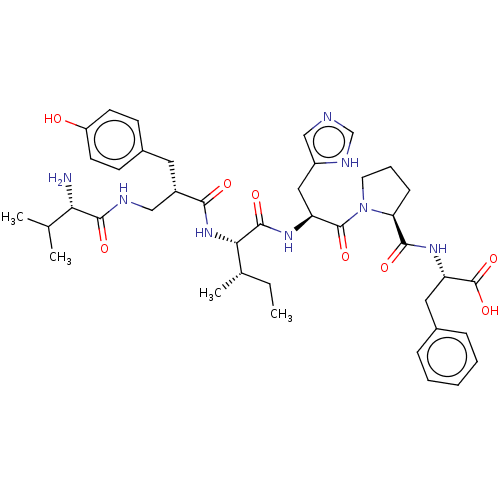
(CHEMBL427940)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CNC(=O)[C@@H](N)C(C)C)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-36(51)28(18-27-13-15-30(50)16-14-27)21-44-38(53)34(42)24(2)3)39(54)46-31(20-29-22-43-23-45-29)40(55)49-17-9-12-33(49)37(52)47-32(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,28,31-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,45)(H,44,53)(H,46,54)(H,47,52)(H,48,51)(H,56,57)/t25-,28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478366

(CHEMBL258480)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC[C@H](CC(C)C)C(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C42H57N7O9/c1-5-26(4)36(48-37(51)32(19-28-13-15-31(50)16-14-28)44-22-29(41(55)56)18-25(2)3)39(53)46-33(21-30-23-43-24-45-30)40(54)49-17-9-12-35(49)38(52)47-34(42(57)58)20-27-10-7-6-8-11-27/h6-8,10-11,13-16,23-26,29,32-36,44,50H,5,9,12,17-22H2,1-4H3,(H,43,45)(H,46,53)(H,47,52)(H,48,51)(H,55,56)(H,57,58)/t26-,29-,32-,33-,34-,35-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478355
(CHEMBL258642)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)C1(CN)CCCC1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C42H56N8O8/c1-3-26(2)35(49-36(52)31(20-28-13-15-30(51)16-14-28)48-41(58)42(24-43)17-7-8-18-42)38(54)46-32(22-29-23-44-25-45-29)39(55)50-19-9-12-34(50)37(53)47-33(40(56)57)21-27-10-5-4-6-11-27/h4-6,10-11,13-16,23,25-26,31-35,51H,3,7-9,12,17-22,24,43H2,1-2H3,(H,44,45)(H,46,54)(H,47,53)(H,48,58)(H,49,52)(H,56,57)/t26-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478356

(CHEMBL408132)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)NC[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-36(51)31(46-38(53)34(42)24(2)3)19-27-13-15-30(50)16-14-27)39(54)47-32(20-29-22-43-23-45-29)40(55)49-17-9-12-33(49)37(52)44-21-28(41(56)57)18-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,28,31-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,45)(H,44,52)(H,46,53)(H,47,54)(H,48,51)(H,56,57)/t25-,28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478359

(CHEMBL408871)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC(O)=O)Cc1ccccc1 Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)36(48-37(53)31(46-39(55)35(42)24(2)3)19-27-13-15-30(50)16-14-27)40(56)47-32(20-29-22-43-23-44-29)41(57)49-17-9-12-33(49)38(54)45-28(21-34(51)52)18-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,28,31-33,35-36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,54)(H,46,55)(H,47,56)(H,48,53)(H,51,52)/t25-,28-,31-,32-,33-,35-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478364

(CHEMBL248592)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCCC[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-36(51)30(45-38(53)34(42)24(2)3)19-27-14-16-29(50)17-15-27)39(54)46-31(21-28-22-43-23-44-28)40(55)49-18-10-9-13-33(49)37(52)47-32(41(56)57)20-26-11-7-6-8-12-26/h6-8,11-12,14-17,22-25,30-35,50H,5,9-10,13,18-21,42H2,1-4H3,(H,43,44)(H,45,53)(H,46,54)(H,47,52)(H,48,51)(H,56,57)/t25-,30-,31-,32-,33+,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50415634

(CHEMBL260622)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CN)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-37(52)31(18-27-13-15-29(50)16-14-27)45-36(51)30(21-42)24(2)3)39(54)46-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)47-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,54)(H,47,53)(H,48,52)(H,56,57)/t25-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478367

(CHEMBL259382)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)36(48-34(51)21-28(45-38(53)35(42)24(2)3)18-27-13-15-30(50)16-14-27)39(54)46-31(20-29-22-43-23-44-29)40(55)49-17-9-12-33(49)37(52)47-32(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,28,31-33,35-36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,53)(H,46,54)(H,47,52)(H,48,51)(H,56,57)/t25-,28+,31-,32-,33-,35-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478361

(CHEMBL258452)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)C[C@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)36(48-37(52)31(18-27-13-15-29(50)16-14-27)45-35(51)21-30(42)24(2)3)39(54)46-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)47-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-34,36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,54)(H,47,53)(H,48,52)(H,56,57)/t25-,30-,31-,32-,33-,34-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478362

(CHEMBL261417)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1CC(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)36(48-37(52)31(46-38(53)35(42)24(2)3)18-27-13-15-30(50)16-14-27)39(54)47-32(20-28-22-43-23-44-28)40(55)49-17-9-12-29(49)21-34(51)45-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,29,31-33,35-36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,53)(H,47,54)(H,48,52)(H,56,57)/t25-,29-,31-,32-,33-,35-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478357

(CHEMBL428457)Show SMILES CC(C)C[C@@H](CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-24(2)17-28(21-44-37(52)31(46-39(54)35(42)25(3)4)18-27-12-14-30(50)15-13-27)36(51)47-32(20-29-22-43-23-45-29)40(55)49-16-8-11-34(49)38(53)48-33(41(56)57)19-26-9-6-5-7-10-26/h5-7,9-10,12-15,22-25,28,31-35,50H,8,11,16-21,42H2,1-4H3,(H,43,45)(H,44,52)(H,46,54)(H,47,51)(H,48,53)(H,56,57)/t28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478357

(CHEMBL428457)Show SMILES CC(C)C[C@@H](CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-24(2)17-28(21-44-37(52)31(46-39(54)35(42)25(3)4)18-27-12-14-30(50)15-13-27)36(51)47-32(20-29-22-43-23-45-29)40(55)49-16-8-11-34(49)38(53)48-33(41(56)57)19-26-9-6-5-7-10-26/h5-7,9-10,12-15,22-25,28,31-35,50H,8,11,16-21,42H2,1-4H3,(H,43,45)(H,44,52)(H,46,54)(H,47,51)(H,48,53)(H,56,57)/t28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50478358
(CHEMBL258494)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CN)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-37(52)31(18-27-13-15-29(50)16-14-27)45-36(51)30(21-42)24(2)3)39(54)46-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)47-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,54)(H,47,53)(H,48,52)(H,56,57)/t25-,30+,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50415633

(CHEMBL259019)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CN)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C42H58N8O8/c1-5-26(4)37(49-39(55)33(19-28-13-15-31(51)16-14-28)47-38(54)32(22-43)25(2)3)41(57)48-34(20-30-23-44-24-45-30)42(58)50-17-9-12-35(50)40(56)46-29(21-36(52)53)18-27-10-7-6-8-11-27/h6-8,10-11,13-16,23-26,29,32-35,37,51H,5,9,12,17-22,43H2,1-4H3,(H,44,45)(H,46,56)(H,47,54)(H,48,57)(H,49,55)(H,52,53)/t26-,29-,32-,33-,34-,35-,37-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478360

(CHEMBL261731)Show SMILES CC[C@H](C)[C@H](CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)30(46-37(52)31(47-39(54)36(42)24(2)3)18-27-13-15-29(50)16-14-27)21-35(51)45-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)48-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-34,36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,52)(H,47,54)(H,48,53)(H,56,57)/t25-,30-,31-,32-,33-,34-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478363

(CHEMBL403717)Show SMILES CCC[C@@H](CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O)C(O)=O Show InChI InChI=1S/C41H55N7O9/c1-4-10-28(40(54)55)22-43-31(19-27-14-16-30(49)17-15-27)36(50)47-35(25(3)5-2)38(52)45-32(21-29-23-42-24-44-29)39(53)48-18-9-13-34(48)37(51)46-33(41(56)57)20-26-11-7-6-8-12-26/h6-8,11-12,14-17,23-25,28,31-35,43,49H,4-5,9-10,13,18-22H2,1-3H3,(H,42,44)(H,45,52)(H,46,51)(H,47,50)(H,54,55)(H,56,57)/t25-,28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478365

(CHEMBL427940)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CNC(=O)[C@@H](N)C(C)C)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-36(51)28(18-27-13-15-30(50)16-14-27)21-44-38(53)34(42)24(2)3)39(54)46-31(20-29-22-43-23-45-29)40(55)49-17-9-12-33(49)37(52)47-32(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,28,31-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,45)(H,44,53)(H,46,54)(H,47,52)(H,48,51)(H,56,57)/t25-,28-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478355

(CHEMBL258642)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)C1(CN)CCCC1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C42H56N8O8/c1-3-26(2)35(49-36(52)31(20-28-13-15-30(51)16-14-28)48-41(58)42(24-43)17-7-8-18-42)38(54)46-32(22-29-23-44-25-45-29)39(55)50-19-9-12-34(50)37(53)47-33(40(56)57)21-27-10-5-4-6-11-27/h4-6,10-11,13-16,23,25-26,31-35,51H,3,7-9,12,17-22,24,43H2,1-2H3,(H,44,45)(H,46,54)(H,47,53)(H,48,58)(H,49,52)(H,56,57)/t26-,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478354

(CHEMBL261422)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC[C@H](CC(C)C)C(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC(O)=O)Cc1ccccc1 Show InChI InChI=1S/C43H59N7O9/c1-5-27(4)38(49-39(54)34(20-29-13-15-33(51)16-14-29)45-23-30(43(58)59)18-26(2)3)41(56)48-35(21-32-24-44-25-46-32)42(57)50-17-9-12-36(50)40(55)47-31(22-37(52)53)19-28-10-7-6-8-11-28/h6-8,10-11,13-16,24-27,30-31,34-36,38,45,51H,5,9,12,17-23H2,1-4H3,(H,44,46)(H,47,55)(H,48,56)(H,49,54)(H,52,53)(H,58,59)/t27-,30-,31-,34-,35-,36-,38-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478361

(CHEMBL258452)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)C[C@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)36(48-37(52)31(18-27-13-15-29(50)16-14-27)45-35(51)21-30(42)24(2)3)39(54)46-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)47-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-34,36,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,54)(H,47,53)(H,48,52)(H,56,57)/t25-,30-,31-,32-,33-,34-,36-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <9.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50478358

(CHEMBL258494)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CN)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C41H56N8O8/c1-5-25(4)35(48-37(52)31(18-27-13-15-29(50)16-14-27)45-36(51)30(21-42)24(2)3)39(54)46-32(20-28-22-43-23-44-28)40(55)49-17-9-12-34(49)38(53)47-33(41(56)57)19-26-10-7-6-8-11-26/h6-8,10-11,13-16,22-25,30-35,50H,5,9,12,17-21,42H2,1-4H3,(H,43,44)(H,45,51)(H,46,54)(H,47,53)(H,48,52)(H,56,57)/t25-,30+,31-,32-,33-,34-,35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant APN expressed in HEK293 cells |
J Med Chem 51: 2291-6 (2008)
Article DOI: 10.1021/jm701490g
BindingDB Entry DOI: 10.7270/Q25M68HP |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50495479
(CHEMBL3109084)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NSc1ncccc1[N+]([O-])=O |r| Show InChI InChI=1S/C16H14N4O4S/c21-16(22)13(8-10-9-18-12-5-2-1-4-11(10)12)19-25-15-14(20(23)24)6-3-7-17-15/h1-7,9,13,18-19H,8H2,(H,21,22)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie-Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNMT1 expressed in H19 cells assessed as inhibition of tritiated methyl incorporation from [3H]-labeled AdoMet into h... |
J Med Chem 57: 421-34 (2014)
Article DOI: 10.1021/jm401419p
BindingDB Entry DOI: 10.7270/Q2QN69Q3 |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50495477
(CHEMBL3109085)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NSc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O |r| Show InChI InChI=1S/C17H14N4O6S/c22-17(23)14(7-10-9-18-13-4-2-1-3-12(10)13)19-28-16-6-5-11(20(24)25)8-15(16)21(26)27/h1-6,8-9,14,18-19H,7H2,(H,22,23)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie-Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNMT1 expressed in H19 cells assessed as inhibition of tritiated methyl incorporation from [3H]-labeled AdoMet into h... |
J Med Chem 57: 421-34 (2014)
Article DOI: 10.1021/jm401419p
BindingDB Entry DOI: 10.7270/Q2QN69Q3 |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50495478

(CHEMBL3109076)Show SMILES OC(=O)[C@H]1[C@@H](Cc2c[nH]c3ccccc23)CCN1C(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C25H22N2O3/c28-24(19-10-9-16-5-1-2-6-17(16)13-19)27-12-11-18(23(27)25(29)30)14-20-15-26-22-8-4-3-7-21(20)22/h1-10,13,15,18,23,26H,11-12,14H2,(H,29,30)/t18-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie-Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNMT1 expressed in H19 cells assessed as inhibition of tritiated methyl incorporation from [3H]-labeled AdoMet into h... |
J Med Chem 57: 421-34 (2014)
Article DOI: 10.1021/jm401419p
BindingDB Entry DOI: 10.7270/Q2QN69Q3 |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50495476
(CHEMBL3109078)Show SMILES OC(=O)[C@H]1[C@@H](Cc2c[nH]c3ccccc23)CCN1C(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H20N2O3/c24-20(14-6-2-1-3-7-14)23-11-10-15(19(23)21(25)26)12-16-13-22-18-9-5-4-8-17(16)18/h1-9,13,15,19,22H,10-12H2,(H,25,26)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie-Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNMT1 expressed in H19 cells assessed as inhibition of tritiated methyl incorporation from [3H]-labeled AdoMet into h... |
J Med Chem 57: 421-34 (2014)
Article DOI: 10.1021/jm401419p
BindingDB Entry DOI: 10.7270/Q2QN69Q3 |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50495480
(CHEMBL3109079)Show SMILES OC(=O)[C@@H]1[C@H](Cc2c[nH]c3ccccc23)CCN1Sc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O |r| Show InChI InChI=1S/C20H18N4O6S/c25-20(26)19-12(9-13-11-21-16-4-2-1-3-15(13)16)7-8-22(19)31-18-6-5-14(23(27)28)10-17(18)24(29)30/h1-6,10-12,19,21H,7-9H2,(H,25,26)/t12-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie-Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNMT1 expressed in H19 cells assessed as inhibition of tritiated methyl incorporation from [3H]-labeled AdoMet into h... |
J Med Chem 57: 421-34 (2014)
Article DOI: 10.1021/jm401419p
BindingDB Entry DOI: 10.7270/Q2QN69Q3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data