 Found 210 hits with Last Name = 'kauffman' and Initial = 'rf'
Found 210 hits with Last Name = 'kauffman' and Initial = 'rf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50228247
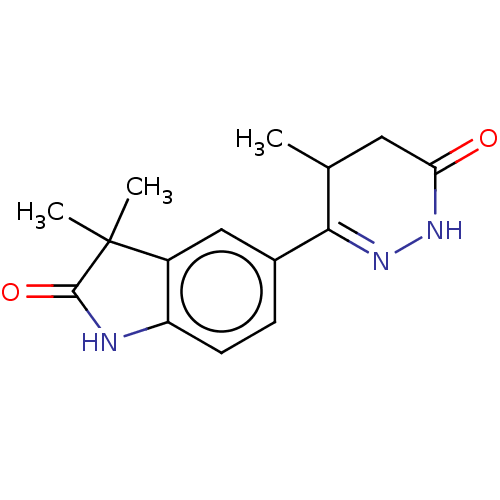
(CHEMBL38017 | LY-197055)Show SMILES CC1CC(=O)NN=C1c1ccc2NC(=O)C(C)(C)c2c1 |c:6| Show InChI InChI=1S/C15H17N3O2/c1-8-6-12(19)17-18-13(8)9-4-5-11-10(7-9)15(2,3)14(20)16-11/h4-5,7-8H,6H2,1-3H3,(H,16,20)(H,17,19) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement [3H]LY-186,126 from phosphodiesterase 4 of myocardial vesicles |
J Med Chem 32: 1476-80 (1989)
BindingDB Entry DOI: 10.7270/Q2P271B2 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50228246
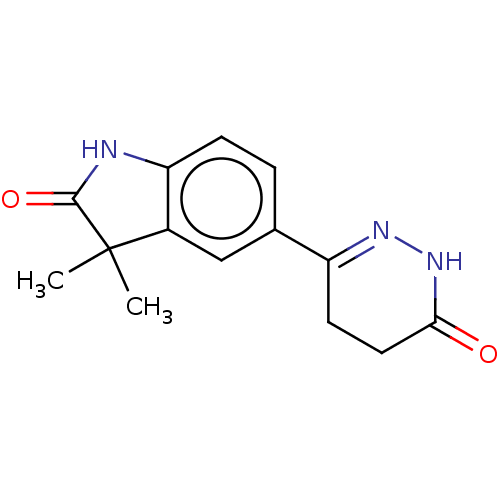
(Indolidan)Show SMILES CC1(C)C(=O)Nc2ccc(cc12)C1=NNC(=O)CC1 |t:14| Show InChI InChI=1S/C14H15N3O2/c1-14(2)9-7-8(3-4-11(9)15-13(14)19)10-5-6-12(18)17-16-10/h3-4,7H,5-6H2,1-2H3,(H,15,19)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement [3H]LY-186,126 from phosphodiesterase 4 of myocardial vesicles |
J Med Chem 32: 1476-80 (1989)
BindingDB Entry DOI: 10.7270/Q2P271B2 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50228245
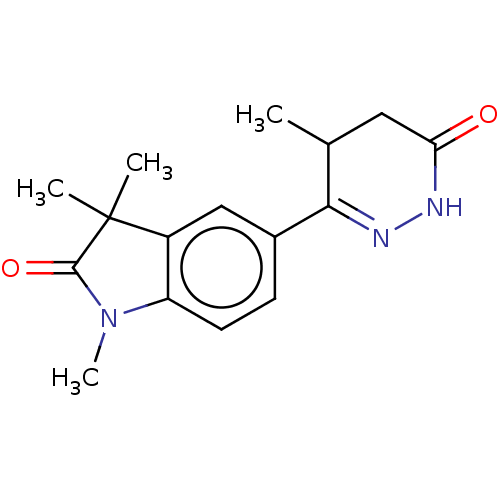
(CHEMBL34945 | LY-186126)Show SMILES CC1CC(=O)NN=C1c1ccc2N(C)C(=O)C(C)(C)c2c1 |c:6| Show InChI InChI=1S/C16H19N3O2/c1-9-7-13(20)17-18-14(9)10-5-6-12-11(8-10)16(2,3)15(21)19(12)4/h5-6,8-9H,7H2,1-4H3,(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement [3H]LY-186,126 from phosphodiesterase 4 of myocardial vesicles |
J Med Chem 32: 1476-80 (1989)
BindingDB Entry DOI: 10.7270/Q2P271B2 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50228248

(CHEMBL37981)Show InChI InChI=1S/C13H13N3O2/c1-16-13(18)5-4-11(15-16)8-2-3-10-9(6-8)7-12(17)14-10/h2-3,6H,4-5,7H2,1H3,(H,14,17) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement [3H]LY-186,126 from phosphodiesterase 4 of myocardial vesicles |
J Med Chem 32: 1476-80 (1989)
BindingDB Entry DOI: 10.7270/Q2P271B2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145721
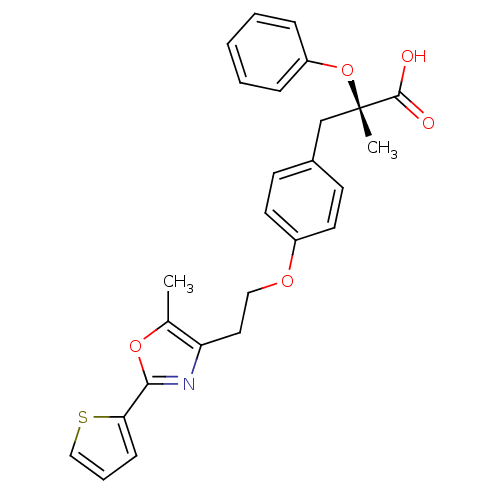
((S)-2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxa...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50135773
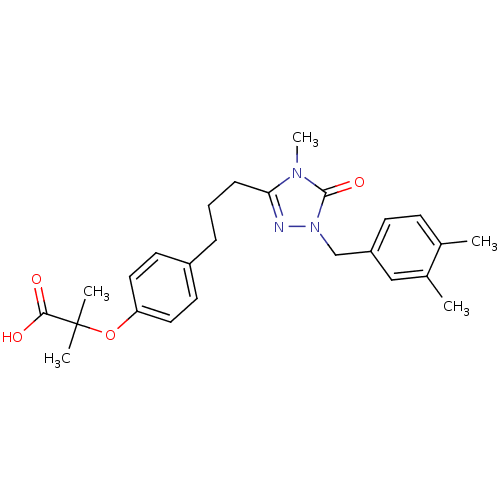
(2-(4-{3-[1-(3,4-Dimethyl-benzyl)-4-methyl-5-oxo-4,...)Show SMILES Cc1ccc(Cn2nc(CCCc3ccc(OC(C)(C)C(O)=O)cc3)n(C)c2=O)cc1C Show InChI InChI=1S/C25H31N3O4/c1-17-9-10-20(15-18(17)2)16-28-24(31)27(5)22(26-28)8-6-7-19-11-13-21(14-12-19)32-25(3,4)23(29)30/h9-15H,6-8,16H2,1-5H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145722
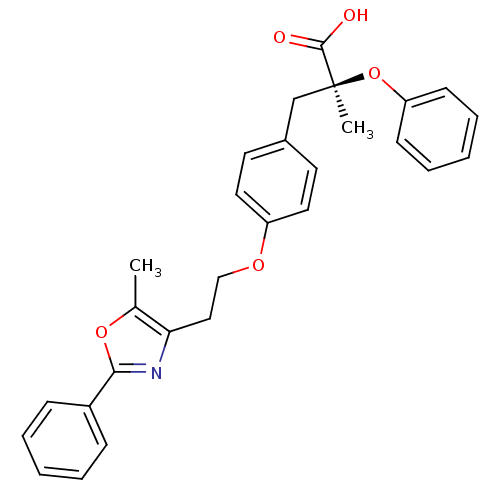
((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50064451
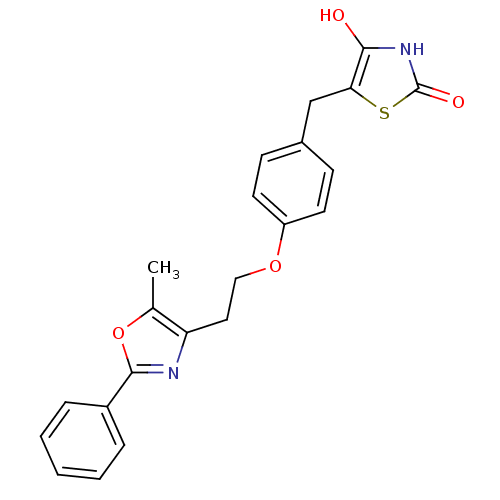
(5-{4-[2-(5-Methyl-2-phenyl-oxazol-4-yl)-ethoxy]-be...)Show SMILES Cc1oc(nc1CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1)-c1ccccc1 Show InChI InChI=1S/C22H20N2O4S/c1-14-18(23-21(28-14)16-5-3-2-4-6-16)11-12-27-17-9-7-15(8-10-17)13-19-20(25)24-22(26)29-19/h2-10,25H,11-13H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human peroxidase proliferator activated receptor gamma (hPPARgamma) |
J Med Chem 44: 2061-4 (2001)
BindingDB Entry DOI: 10.7270/Q2DZ07KH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50135777
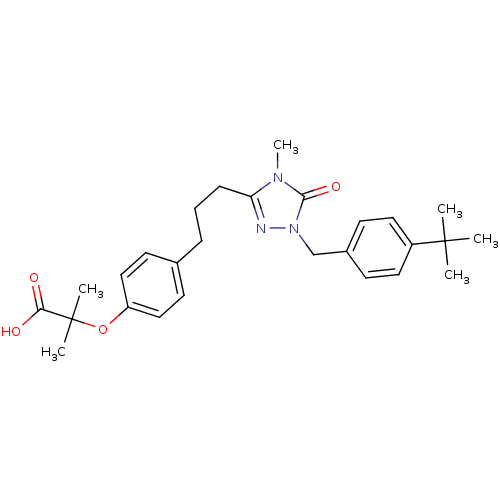
(2-(4-{3-[1-(4-tert-Butyl-benzyl)-4-methyl-5-oxo-4,...)Show SMILES Cn1c(CCCc2ccc(OC(C)(C)C(O)=O)cc2)nn(Cc2ccc(cc2)C(C)(C)C)c1=O Show InChI InChI=1S/C27H35N3O4/c1-26(2,3)21-14-10-20(11-15-21)18-30-25(33)29(6)23(28-30)9-7-8-19-12-16-22(17-13-19)34-27(4,5)24(31)32/h10-17H,7-9,18H2,1-6H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145719
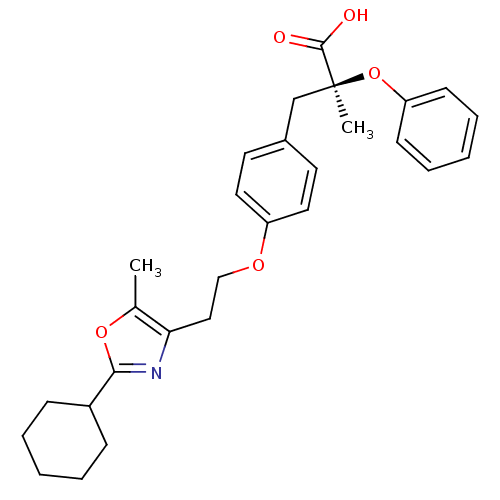
((S)-3-{4-[2-(2-Cyclohexyl-5-methyl-oxazol-4-yl)-et...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)C1CCCCC1 Show InChI InChI=1S/C28H33NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h4,7-8,11-16,22H,3,5-6,9-10,17-19H2,1-2H3,(H,30,31)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50135775
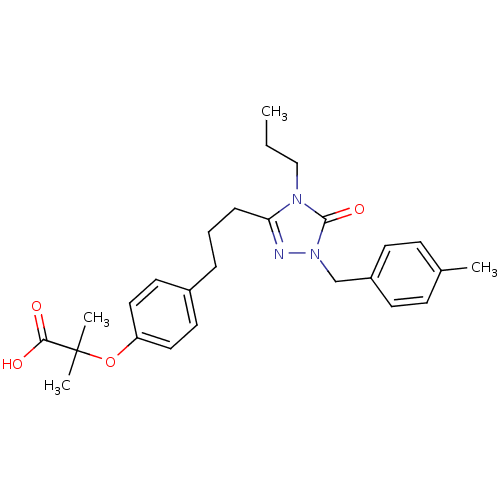
(2-Methyl-2-(4-{3-[1-(4-methyl-benzyl)-5-oxo-4-prop...)Show SMILES CCCn1c(CCCc2ccc(OC(C)(C)C(O)=O)cc2)nn(Cc2ccc(C)cc2)c1=O Show InChI InChI=1S/C26H33N3O4/c1-5-17-28-23(27-29(25(28)32)18-21-11-9-19(2)10-12-21)8-6-7-20-13-15-22(16-14-20)33-26(3,4)24(30)31/h9-16H,5-8,17-18H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145719

((S)-3-{4-[2-(2-Cyclohexyl-5-methyl-oxazol-4-yl)-et...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)C1CCCCC1 Show InChI InChI=1S/C28H33NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h4,7-8,11-16,22H,3,5-6,9-10,17-19H2,1-2H3,(H,30,31)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARalpha/delta agonist from human Peroxisome proliferator activated receptor alpha |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145712
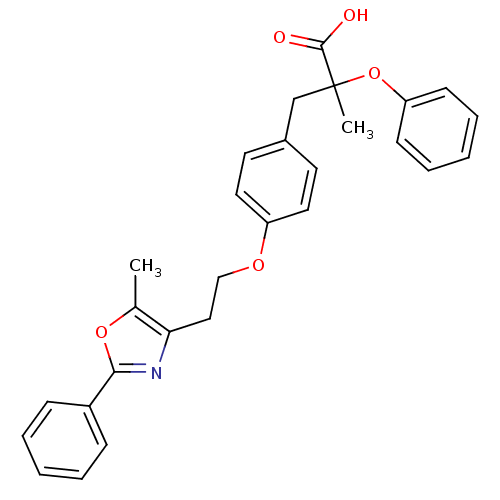
(2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-e...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50135782

(2-(4-{3-[1-(3-Methoxy-benzyl)-5-oxo-4-propyl-4,5-d...)Show SMILES CCCn1c(CCCc2ccc(OC(C)(C)C(O)=O)cc2)nn(Cc2cccc(OC)c2)c1=O Show InChI InChI=1S/C26H33N3O5/c1-5-16-28-23(27-29(25(28)32)18-20-9-6-10-22(17-20)33-4)11-7-8-19-12-14-21(15-13-19)34-26(2,3)24(30)31/h6,9-10,12-15,17H,5,7-8,11,16,18H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145722

((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145722

((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARalpha/delta agonist from human Peroxisome proliferator activated receptor alpha |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50135780
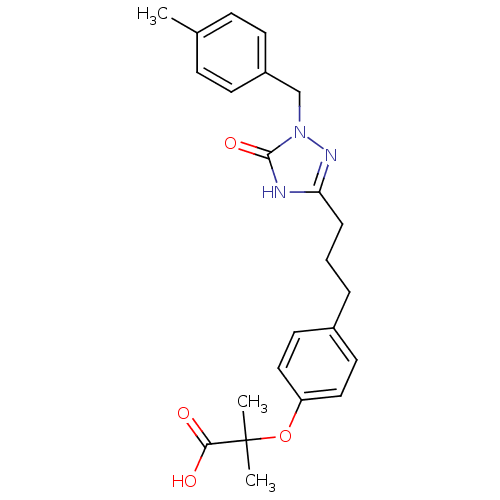
(2-Methyl-2-(4-{3-[1-(4-methyl-benzyl)-5-oxo-4,5-di...)Show SMILES Cc1ccc(Cn2nc(CCCc3ccc(OC(C)(C)C(O)=O)cc3)[nH]c2=O)cc1 Show InChI InChI=1S/C23H27N3O4/c1-16-7-9-18(10-8-16)15-26-22(29)24-20(25-26)6-4-5-17-11-13-19(14-12-17)30-23(2,3)21(27)28/h7-14H,4-6,15H2,1-3H3,(H,27,28)(H,24,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145710
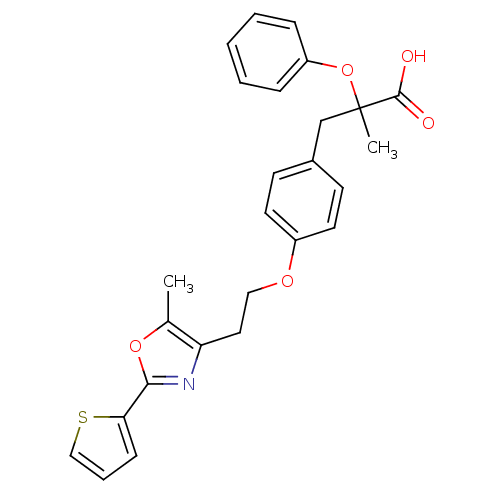
(2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxazol-...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50135779
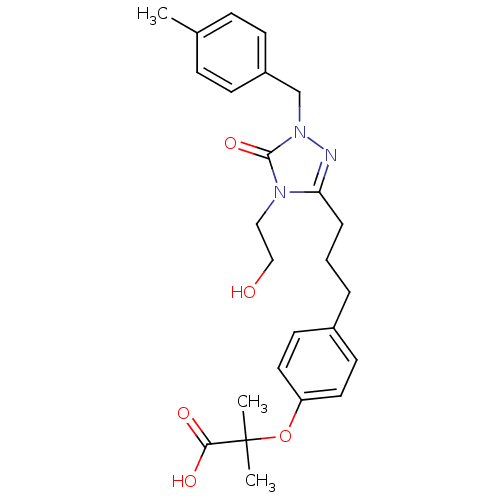
(2-(4-{3-[4-(2-Hydroxy-ethyl)-1-(4-methyl-benzyl)-5...)Show SMILES Cc1ccc(Cn2nc(CCCc3ccc(OC(C)(C)C(O)=O)cc3)n(CCO)c2=O)cc1 Show InChI InChI=1S/C25H31N3O5/c1-18-7-9-20(10-8-18)17-28-24(32)27(15-16-29)22(26-28)6-4-5-19-11-13-21(14-12-19)33-25(2,3)23(30)31/h7-14,29H,4-6,15-17H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145721

((S)-2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxa...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARalpha/delta agonist from human Peroxisome proliferator activated receptor alpha |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145712

(2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-e...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARalpha/delta agonist from human Peroxisome proliferator activated receptor alpha |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145712

(2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-e...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145710

(2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxazol-...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARalpha/delta agonist from human Peroxisome proliferator activated receptor alpha |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human peroxidase proliferator activated receptor gamma (hPPARgamma) |
J Med Chem 44: 2061-4 (2001)
BindingDB Entry DOI: 10.7270/Q2DZ07KH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145718

(3-{4-[2-(2-Cyclohexyl-5-methyl-oxazol-4-yl)-ethoxy...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)C1CCCCC1 Show InChI InChI=1S/C28H33NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h4,7-8,11-16,22H,3,5-6,9-10,17-19H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50156525
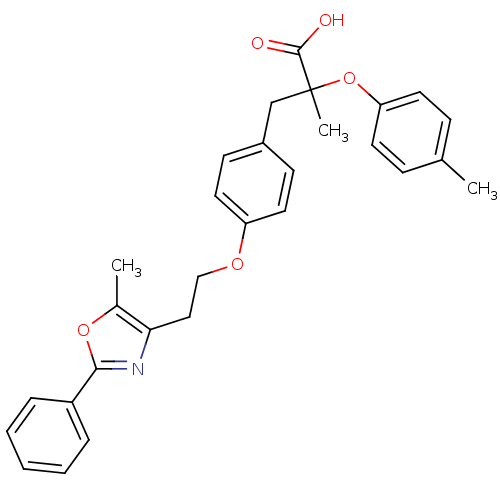
(2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-e...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(C)cc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C29H29NO5/c1-20-9-13-25(14-10-20)35-29(3,28(31)32)19-22-11-15-24(16-12-22)33-18-17-26-21(2)34-27(30-26)23-7-5-4-6-8-23/h4-16H,17-19H2,1-3H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145723
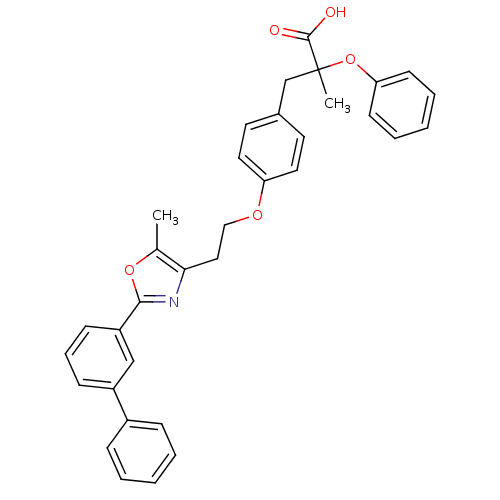
(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C34H31NO5/c1-24-31(35-32(39-24)28-13-9-12-27(22-28)26-10-5-3-6-11-26)20-21-38-29-18-16-25(17-19-29)23-34(2,33(36)37)40-30-14-7-4-8-15-30/h3-19,22H,20-21,23H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145718

(3-{4-[2-(2-Cyclohexyl-5-methyl-oxazol-4-yl)-ethoxy...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)C1CCCCC1 Show InChI InChI=1S/C28H33NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h4,7-8,11-16,22H,3,5-6,9-10,17-19H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARalpha/delta agonist from human Peroxisome proliferator activated receptor alpha |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28799
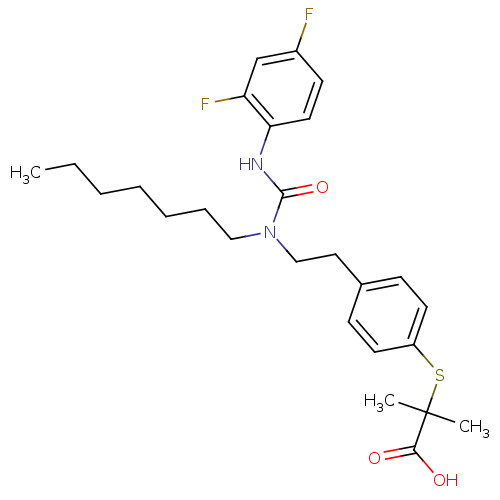
(2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)a...)Show SMILES CCCCCCCN(CCc1ccc(SC(C)(C)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H34F2N2O3S/c1-4-5-6-7-8-16-30(25(33)29-23-14-11-20(27)18-22(23)28)17-15-19-9-12-21(13-10-19)34-26(2,3)24(31)32/h9-14,18H,4-8,15-17H2,1-3H3,(H,29,33)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50228245

(CHEMBL34945 | LY-186126)Show SMILES CC1CC(=O)NN=C1c1ccc2N(C)C(=O)C(C)(C)c2c1 |c:6| Show InChI InChI=1S/C16H19N3O2/c1-9-7-13(20)17-18-14(9)10-5-6-12-11(8-10)16(2,3)15(21)19(12)4/h5-6,8-9H,7H2,1-4H3,(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of sarcoplasmic reticulum Phosphodiesterase 4 |
J Med Chem 32: 1476-80 (1989)
BindingDB Entry DOI: 10.7270/Q2P271B2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145713
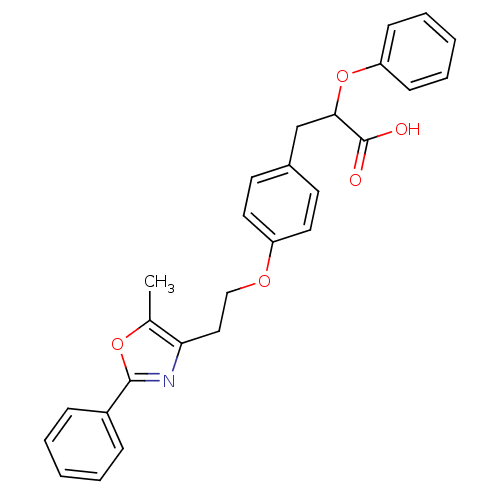
(3-{4-[2-(5-Methyl-2-phenyl-oxazol-4-yl)-ethoxy]-ph...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C27H25NO5/c1-19-24(28-26(32-19)21-8-4-2-5-9-21)16-17-31-22-14-12-20(13-15-22)18-25(27(29)30)33-23-10-6-3-7-11-23/h2-15,25H,16-18H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50156523
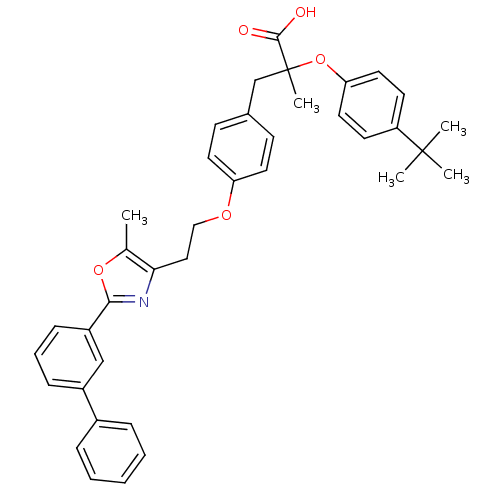
(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C38H39NO5/c1-26-34(39-35(43-26)30-13-9-12-29(24-30)28-10-7-6-8-11-28)22-23-42-32-18-14-27(15-19-32)25-38(5,36(40)41)44-33-20-16-31(17-21-33)37(2,3)4/h6-21,24H,22-23,25H2,1-5H3,(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50156523

(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C38H39NO5/c1-26-34(39-35(43-26)30-13-9-12-29(24-30)28-10-7-6-8-11-28)22-23-42-32-18-14-27(15-19-32)25-38(5,36(40)41)44-33-20-16-31(17-21-33)37(2,3)4/h6-21,24H,22-23,25H2,1-5H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50156527

(2-(4-tert-Butyl-phenoxy)-2-methyl-3-{4-[2-(5-methy...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C32H35NO5/c1-22-28(33-29(37-22)24-9-7-6-8-10-24)19-20-36-26-15-11-23(12-16-26)21-32(5,30(34)35)38-27-17-13-25(14-18-27)31(2,3)4/h6-18H,19-21H2,1-5H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50228246

(Indolidan)Show SMILES CC1(C)C(=O)Nc2ccc(cc12)C1=NNC(=O)CC1 |t:14| Show InChI InChI=1S/C14H15N3O2/c1-14(2)9-7-8(3-4-11(9)15-13(14)19)10-5-6-12(18)17-16-10/h3-4,7H,5-6H2,1-2H3,(H,15,19)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of sarcoplasmic reticulum Phosphodiesterase 4 |
J Med Chem 32: 1476-80 (1989)
BindingDB Entry DOI: 10.7270/Q2P271B2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145723

(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C34H31NO5/c1-24-31(35-32(39-24)28-13-9-12-27(22-28)26-10-5-3-6-11-26)20-21-38-29-18-16-25(17-19-29)23-34(2,33(36)37)40-30-14-7-4-8-15-30/h3-19,22H,20-21,23H2,1-2H3,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145723

(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C34H31NO5/c1-24-31(35-32(39-24)28-13-9-12-27(22-28)26-10-5-3-6-11-26)20-21-38-29-18-16-25(17-19-29)23-34(2,33(36)37)40-30-14-7-4-8-15-30/h3-19,22H,20-21,23H2,1-2H3,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARalpha/delta agonist from human Peroxisome proliferator activated receptor alpha |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145714
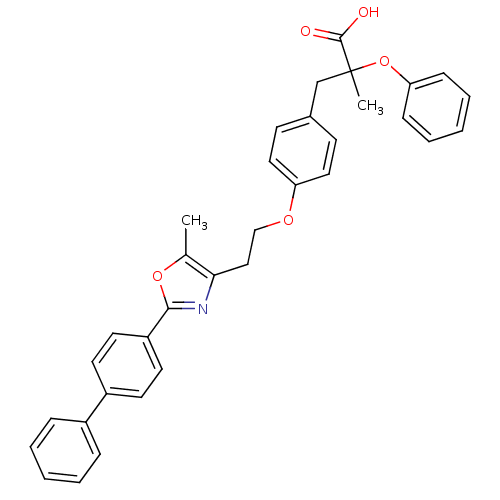
(3-{4-[2-(2-Biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C34H31NO5/c1-24-31(35-32(39-24)28-17-15-27(16-18-28)26-9-5-3-6-10-26)21-22-38-29-19-13-25(14-20-29)23-34(2,33(36)37)40-30-11-7-4-8-12-30/h3-20H,21-23H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50100442
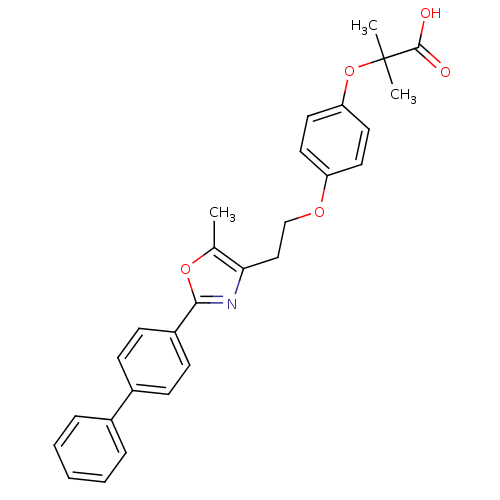
(2-{4-[2-(2-Biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(OC(C)(C)C(O)=O)cc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-19-25(17-18-32-23-13-15-24(16-14-23)34-28(2,3)27(30)31)29-26(33-19)22-11-9-21(10-12-22)20-7-5-4-6-8-20/h4-16H,17-18H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxidase proliferator activated receptor alpha (hPPARalpha) |
J Med Chem 44: 2061-4 (2001)
BindingDB Entry DOI: 10.7270/Q2DZ07KH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50156526
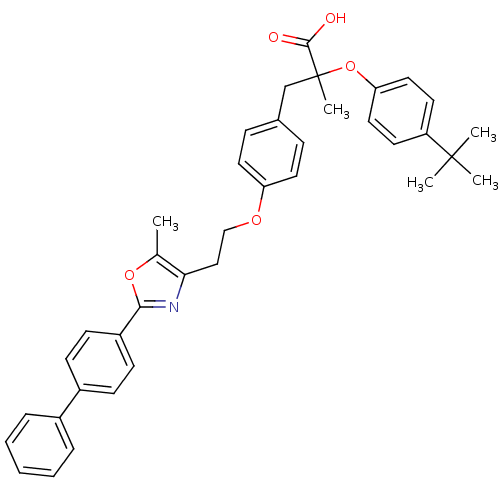
(3-{4-[2-(2-Biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C38H39NO5/c1-26-34(39-35(43-26)30-15-13-29(14-16-30)28-9-7-6-8-10-28)23-24-42-32-19-11-27(12-20-32)25-38(5,36(40)41)44-33-21-17-31(18-22-33)37(2,3)4/h6-22H,23-25H2,1-5H3,(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50135781
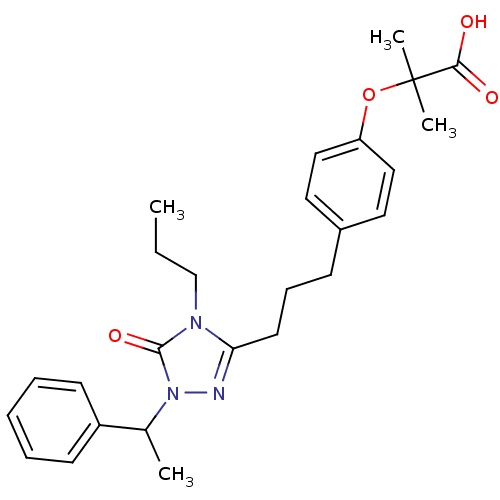
(2-Methyl-2-(4-{3-[5-oxo-1-(1-phenyl-ethyl)-4-propy...)Show SMILES CCCn1c(CCCc2ccc(OC(C)(C)C(O)=O)cc2)nn(C(C)c2ccccc2)c1=O Show InChI InChI=1S/C26H33N3O4/c1-5-18-28-23(27-29(25(28)32)19(2)21-11-7-6-8-12-21)13-9-10-20-14-16-22(17-15-20)33-26(3,4)24(30)31/h6-8,11-12,14-17,19H,5,9-10,13,18H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50100444
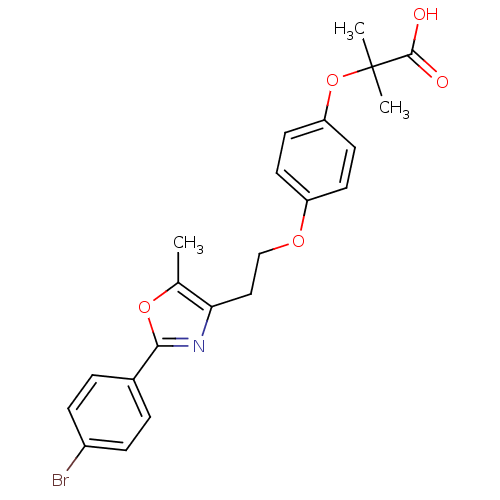
(2-(4-{2-[2-(4-Bromo-phenyl)-5-methyl-oxazol-4-yl]-...)Show SMILES Cc1oc(nc1CCOc1ccc(OC(C)(C)C(O)=O)cc1)-c1ccc(Br)cc1 Show InChI InChI=1S/C22H22BrNO5/c1-14-19(24-20(28-14)15-4-6-16(23)7-5-15)12-13-27-17-8-10-18(11-9-17)29-22(2,3)21(25)26/h4-11H,12-13H2,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxidase proliferator activated receptor alpha (hPPARalpha) |
J Med Chem 44: 2061-4 (2001)
BindingDB Entry DOI: 10.7270/Q2DZ07KH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50135774
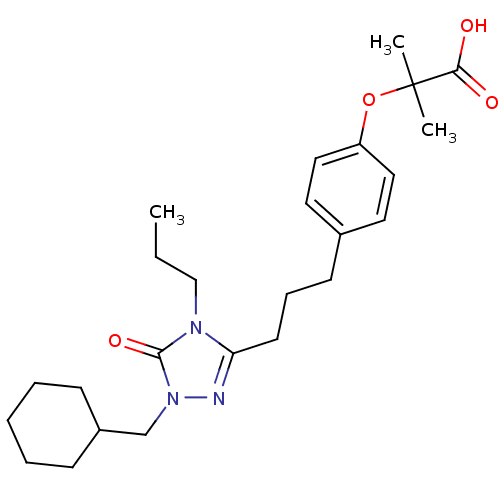
(2-{4-[3-(1-Cyclohexylmethyl-5-oxo-4-propyl-4,5-dih...)Show SMILES CCCn1c(CCCc2ccc(OC(C)(C)C(O)=O)cc2)nn(CC2CCCCC2)c1=O Show InChI InChI=1S/C25H37N3O4/c1-4-17-27-22(26-28(24(27)31)18-20-9-6-5-7-10-20)12-8-11-19-13-15-21(16-14-19)32-25(2,3)23(29)30/h13-16,20H,4-12,17-18H2,1-3H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Peroxisome proliferator activated receptor alpha |
J Med Chem 46: 5121-4 (2003)
Article DOI: 10.1021/jm034173l
BindingDB Entry DOI: 10.7270/Q2D50MB0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145716
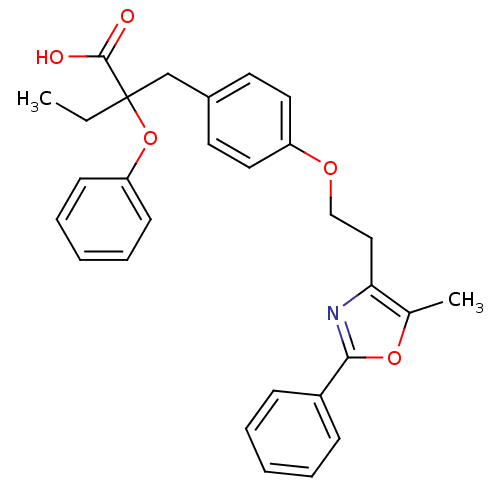
(2-{4-[2-(5-Methyl-2-phenyl-oxazol-4-yl)-ethoxy]-be...)Show SMILES CCC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)(Oc1ccccc1)C(O)=O Show InChI InChI=1S/C29H29NO5/c1-3-29(28(31)32,35-25-12-8-5-9-13-25)20-22-14-16-24(17-15-22)33-19-18-26-21(2)34-27(30-26)23-10-6-4-7-11-23/h4-17H,3,18-20H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145717
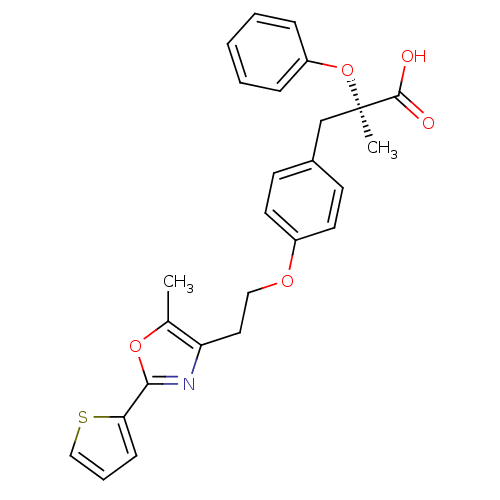
((R)-2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxa...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@](C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50156524
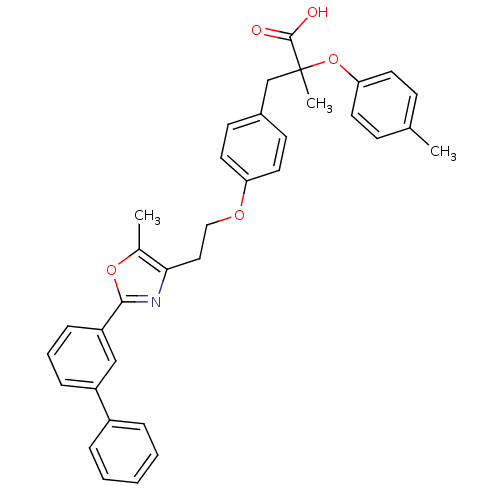
(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(C)cc2)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C35H33NO5/c1-24-12-16-31(17-13-24)41-35(3,34(37)38)23-26-14-18-30(19-15-26)39-21-20-32-25(2)40-33(36-32)29-11-7-10-28(22-29)27-8-5-4-6-9-27/h4-19,22H,20-21,23H2,1-3H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50156522
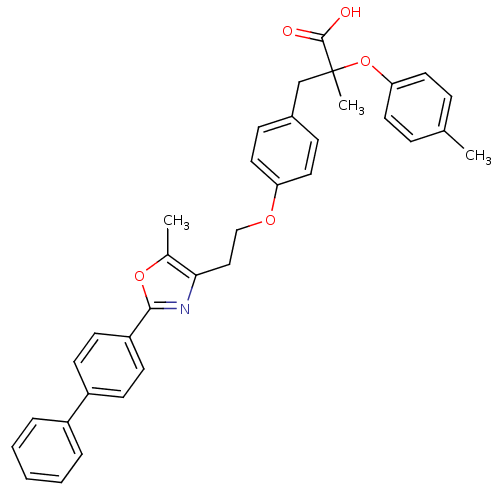
(3-{4-[2-(2-Biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(C)cc2)C(O)=O)cc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C35H33NO5/c1-24-9-17-31(18-10-24)41-35(3,34(37)38)23-26-11-19-30(20-12-26)39-22-21-32-25(2)40-33(36-32)29-15-13-28(14-16-29)27-7-5-4-6-8-27/h4-20H,21-23H2,1-3H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50145715
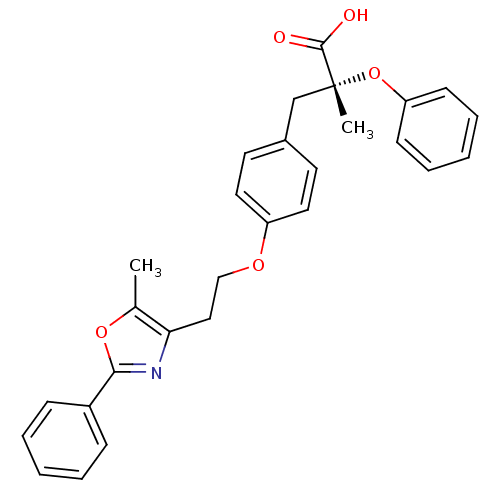
((R)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@](C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31)/t28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of PPARgamma agonist from human PPAR gamma receptor |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50145723

(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C34H31NO5/c1-24-31(35-32(39-24)28-13-9-12-27(22-28)26-10-5-3-6-11-26)20-21-38-29-18-16-25(17-19-29)23-34(2,33(36)37)40-30-14-7-4-8-15-30/h3-19,22H,20-21,23H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 404 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50075315
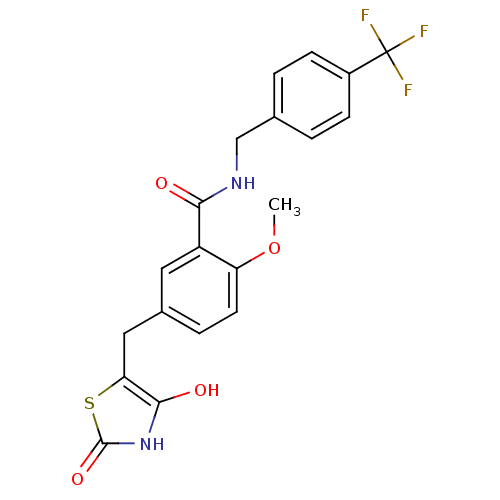
(5-(2,4-Dioxo-thiazolidin-5-ylmethyl)-2-methoxy-N-(...)Show SMILES COc1ccc(Cc2sc(=O)[nH]c2O)cc1C(=O)NCc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H17F3N2O4S/c1-29-15-7-4-12(9-16-18(27)25-19(28)30-16)8-14(15)17(26)24-10-11-2-5-13(6-3-11)20(21,22)23/h2-8,27H,9-10H2,1H3,(H,24,26)(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human peroxidase proliferator activated receptor gamma (hPPARgamma) |
J Med Chem 44: 2061-4 (2001)
BindingDB Entry DOI: 10.7270/Q2DZ07KH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data