

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50190307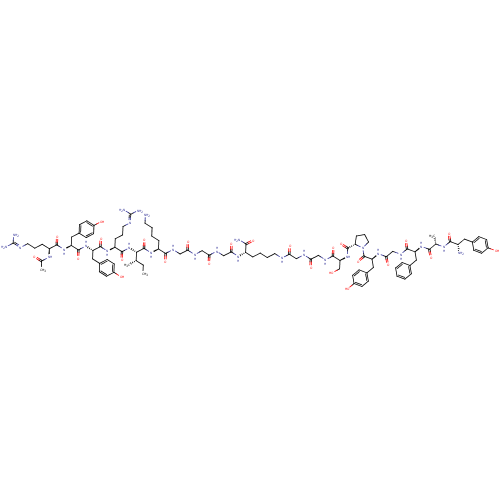 (Ac-RYYRIK-GGG-K-(NH2)-YAFGYPS-GG | CHEMBL414736) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00236 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain homogenate | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50190307 (Ac-RYYRIK-GGG-K-(NH2)-YAFGYPS-GG | CHEMBL414736) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0464 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]NOC from human ORL1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50190308 (Ac-RYYRIK-GGG-K-(NH2)-YRFB-GGGGG | CHEMBL441930) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0477 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain homogenate | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50190308 (Ac-RYYRIK-GGG-K-(NH2)-YRFB-GGGGG | CHEMBL441930) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0803 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]NOC from human ORL1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50190306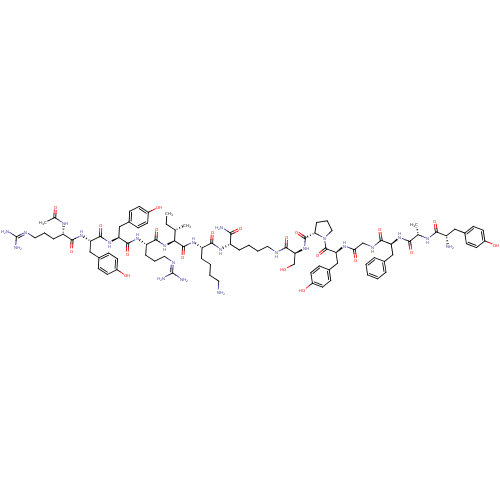 (Ac-RYYRIK-K-(NH2)-YAFGYPS | CHEMBL404792) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain homogenate | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085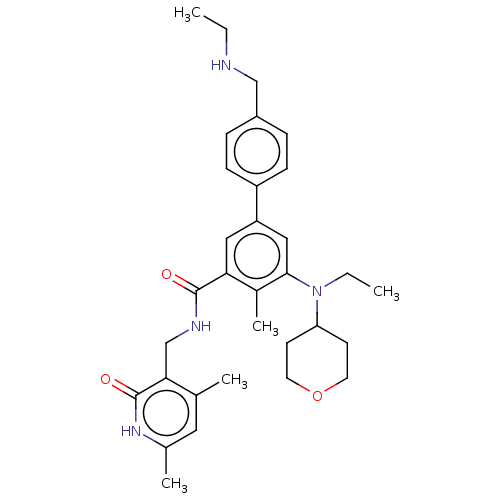 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059841 ((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.538 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain homogenate | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163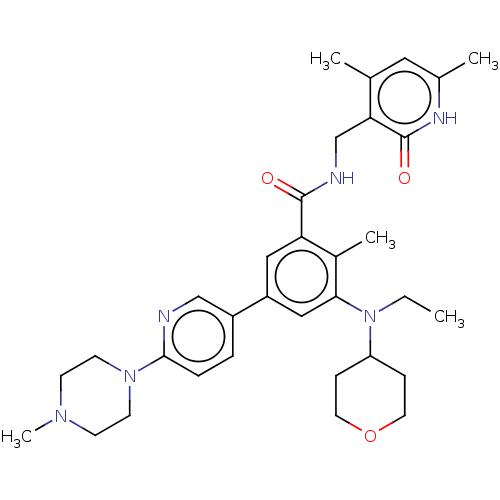 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50190305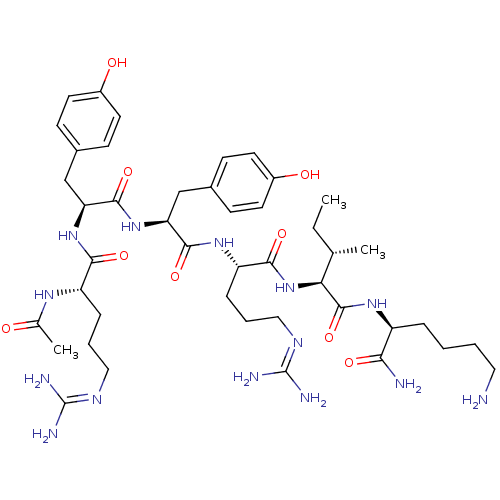 (Ac-RYYRIK-NH2 | CHEMBL437723) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.767 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]NOC from human ORL1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50190309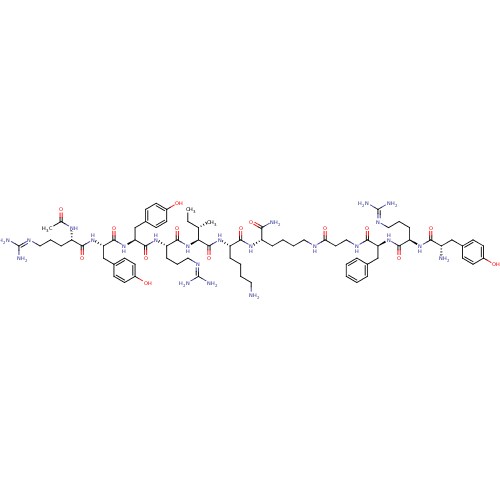 (Ac-RYYRIK-K-(NH2)-YRFB | CHEMBL412939) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.789 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]NOC from human ORL1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087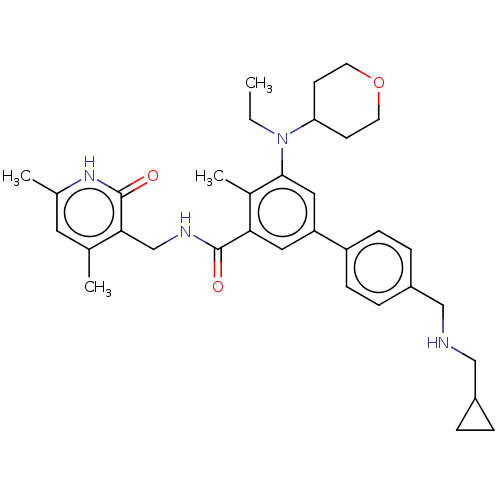 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176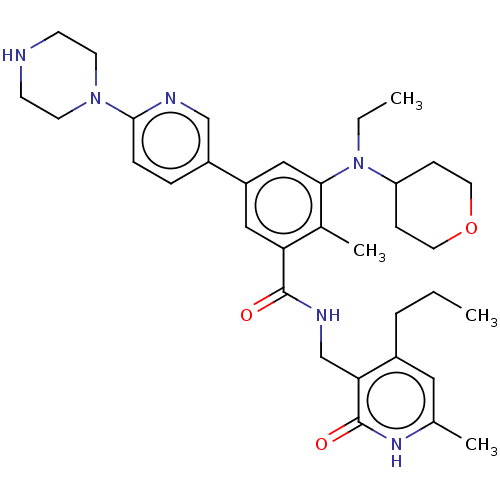 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172080 (US10155002, Compound 86 | US11052093, Compound 86 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172080 (US10155002, Compound 86 | US11052093, Compound 86 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172080 (US10155002, Compound 86 | US11052093, Compound 86 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50190304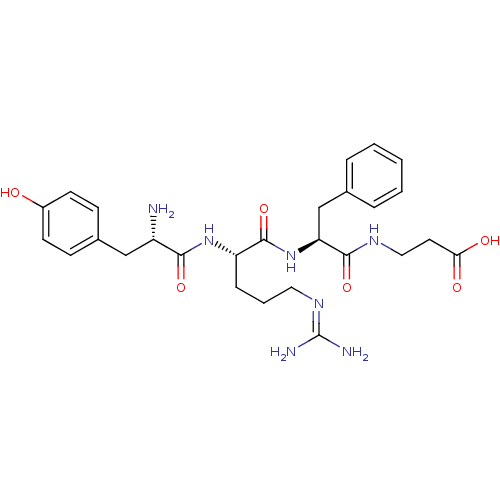 (CHEMBL215513 | YRFB) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain homogenate | Bioorg Med Chem Lett 16: 4839-41 (2006) Article DOI: 10.1016/j.bmcl.2006.06.060 BindingDB Entry DOI: 10.7270/Q2319WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138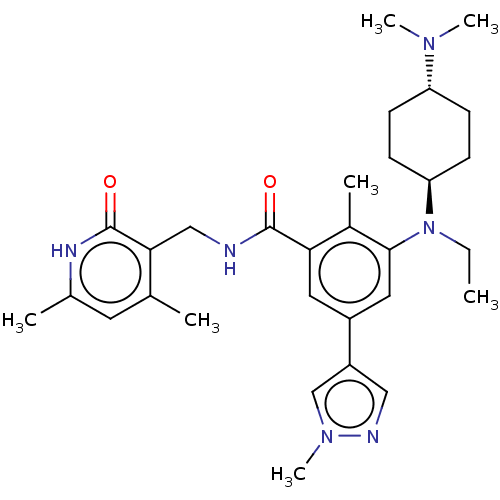 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172135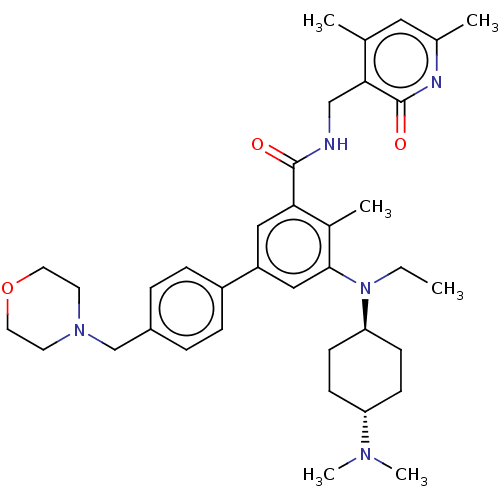 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172135 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172135 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172089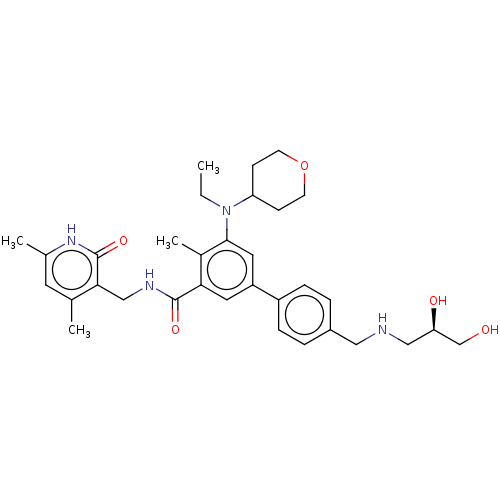 (US10155002, Compound 95 | US11052093, Compound 95 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172089 (US10155002, Compound 95 | US11052093, Compound 95 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172089 (US10155002, Compound 95 | US11052093, Compound 95 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172162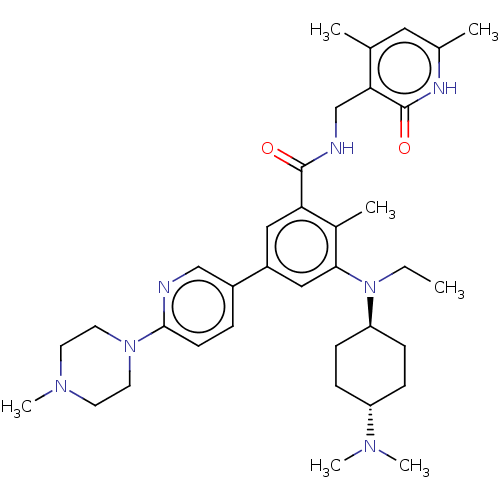 (US10155002, Compound 170 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172162 (US10155002, Compound 170 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172162 (US10155002, Compound 170 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172181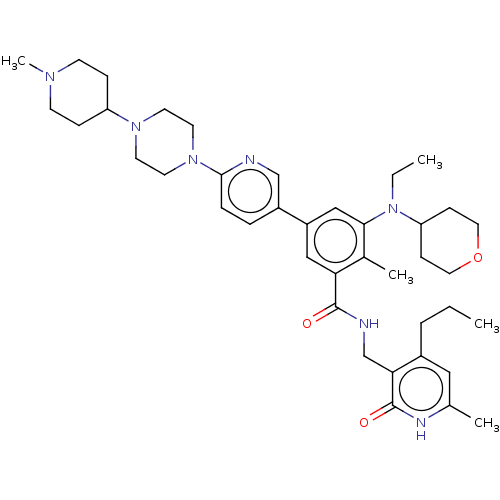 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172181 (US10155002, Compound 189 | US11052093, Compound 18...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172135 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172135 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172135 (EPZ007210 | US10155002, Compound 141 | US11052093,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172084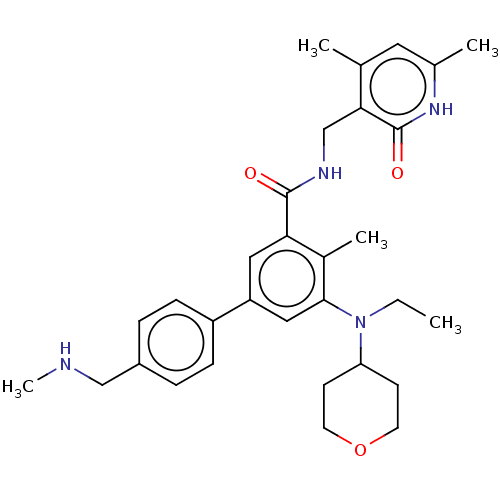 (US10155002, Compound 90 | US11052093, Compound 90 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172084 (US10155002, Compound 90 | US11052093, Compound 90 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172084 (US10155002, Compound 90 | US11052093, Compound 90 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172088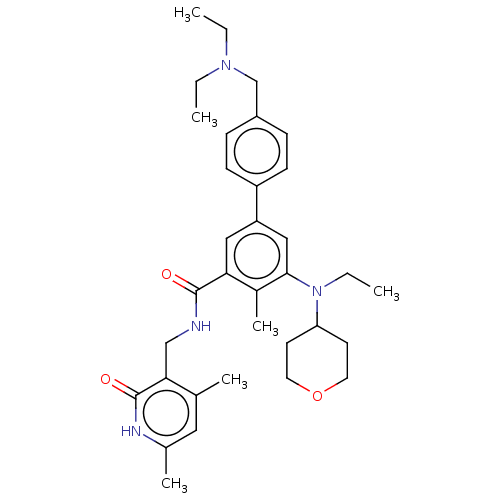 (US10155002, Compound 94 | US11052093, Compound 94 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172088 (US10155002, Compound 94 | US11052093, Compound 94 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.07 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1016 total ) | Next | Last >> |