

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM518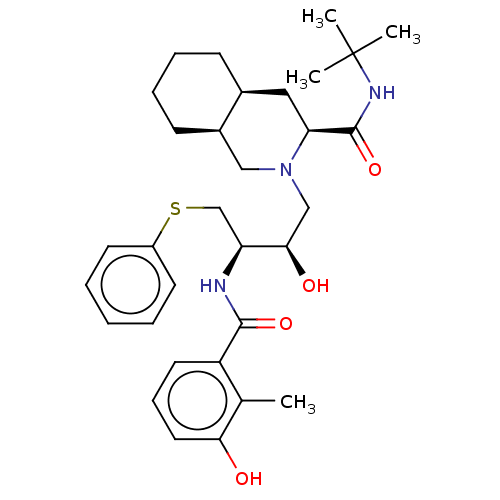 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | -62.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9206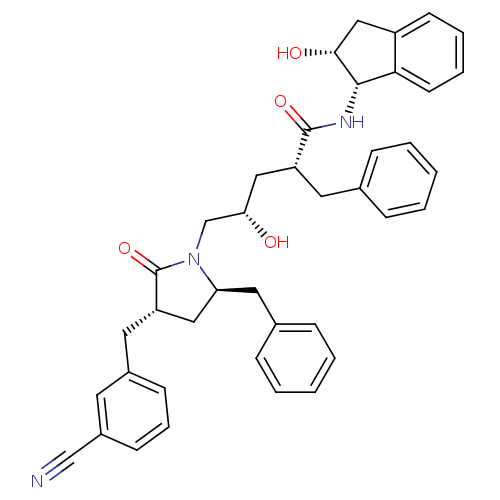 ((2R,4S)-2-benzyl-5-[(3S,5R)-5-benzyl-3-[(3-cyanoph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9183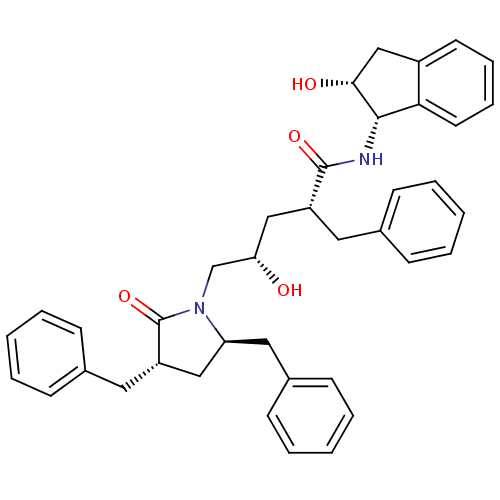 ((2R,4S)-2-benzyl-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9210 ((2R,4S)-2-benzyl-5-[(3S,5R)-5-benzyl-3-[(3-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9212 (3-{[(3S,5R)-5-benzyl-1-[(2S,4R)-2-hydroxy-4-{[(1S,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0700 | -58.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9214 ((2R,4S)-2-benzyl-5-[(3S,5R)-5-benzyl-3-{[3-(cyanom...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | -57.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9215 ((2R,4S)-2-benzyl-5-[(3S,5R)-5-benzyl-3-{[3-(carbam...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | -57.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9218 (Pyrrolidone scaffold 26 | ethyl 2-{[(3-{[(3S,5R)-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9208 ((2R,4S)-5-[(3S,5R)-3-[(3-aminophenyl)methyl]-5-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9213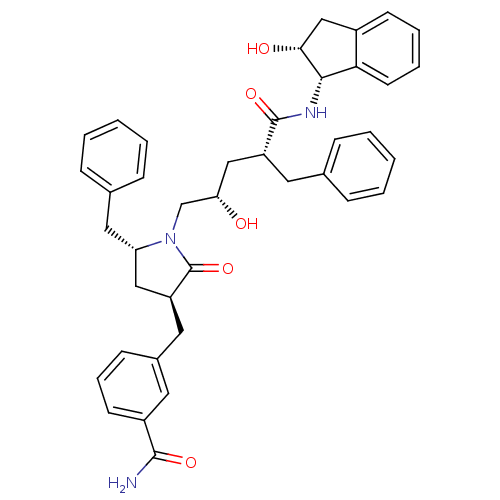 (3-{[(3S,5R)-5-benzyl-1-[(2S,4R)-4-benzyl-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9217 (3-{[(3S,5R)-5-benzyl-1-[(2S,4R)-4-benzyl-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -56.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9219 ((2R,4S)-2-benzyl-5-[(3S,5R)-5-benzyl-3-({3-[(ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9216 (3-{[(3S,5R)-5-benzyl-1-[(2S,4R)-4-benzyl-2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | -54.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9220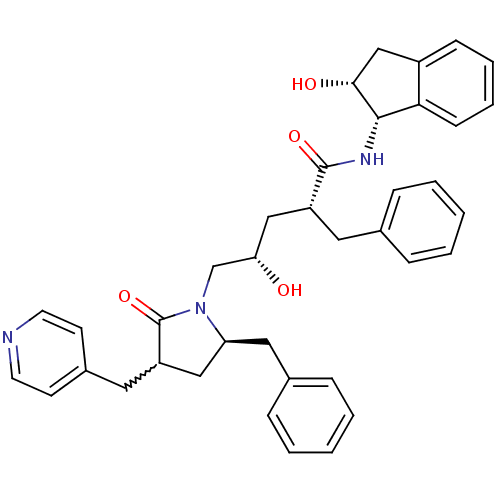 ((2R,4S)-2-benzyl-5-[(5R)-5-benzyl-2-oxo-3-(pyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | -54.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9207 ((2R,4S)-2-benzyl-5-[(5R)-5-benzyl-3-[(4-cyanopheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | -54.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9211 (Pyrrolidone scaffold 20b | methyl 3-{[(3S,5R)-5-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9225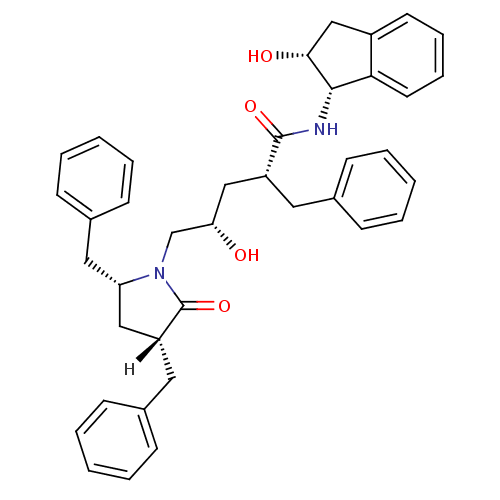 ((2R,4S)-2-benzyl-5-[(3R,5R)-3,5-dibenzyl-2-oxopyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >0.5 | >-53.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471981 (CHEMBL446629) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471983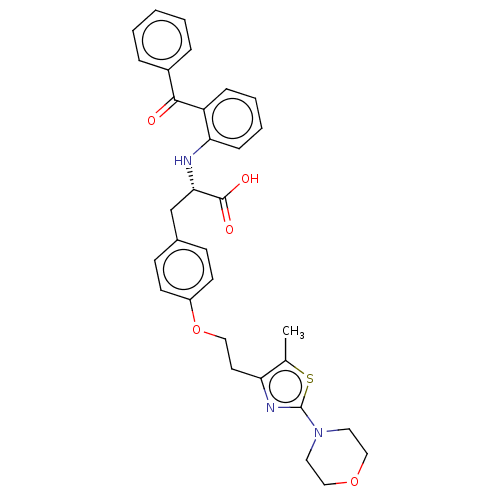 (CHEMBL149876) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9235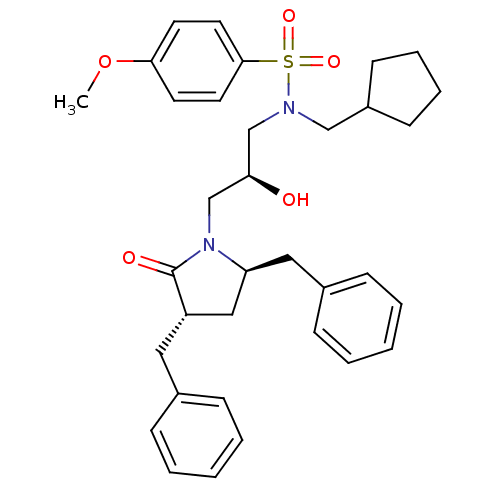 ((2S)-N-(cyclopentylmethyl)-3-[(3S,5R)-3,5-dibenzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 3431-3 (2002) Article DOI: 10.1016/s0960-894x(02)00733-3 BindingDB Entry DOI: 10.7270/Q2B27SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9229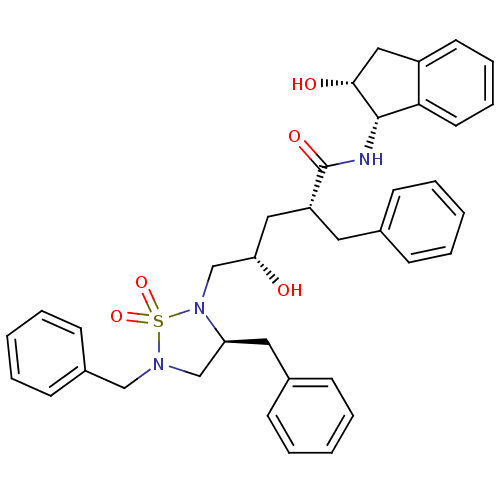 ((2R,4S)-2-benzyl-5-[(3S)-3,5-dibenzyl-1,1-dioxo-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471980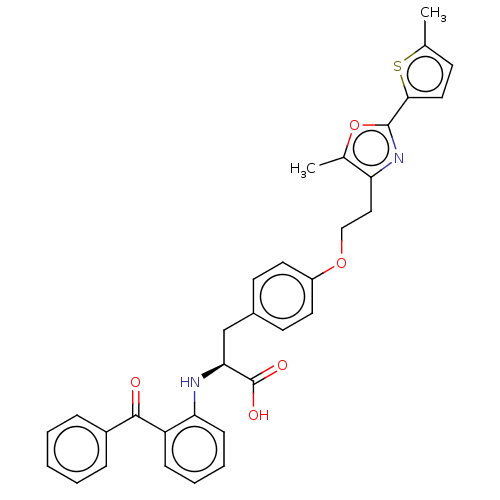 (CHEMBL147095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471978 (CHEMBL147090) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471951 (CHEMBL343210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471959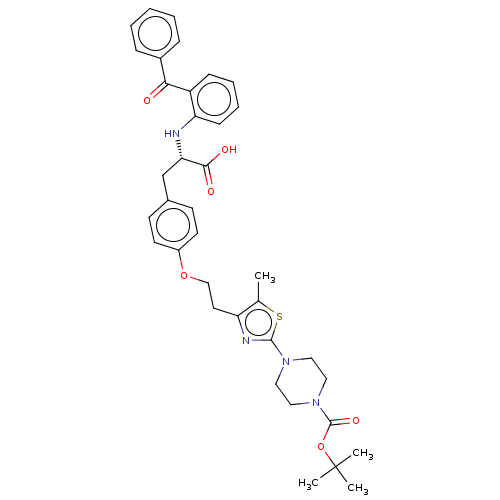 (CHEMBL146301) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085044 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471968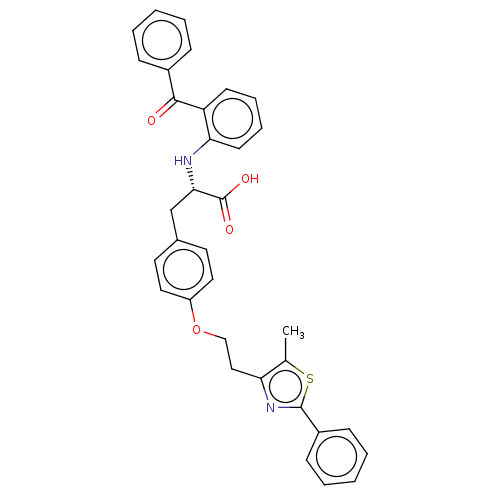 (CHEMBL358379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471953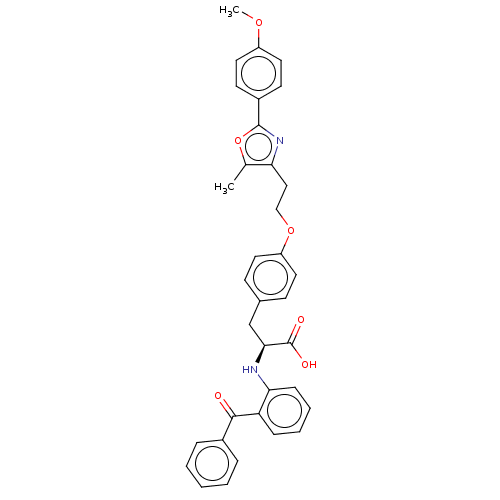 (CHEMBL356382) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9230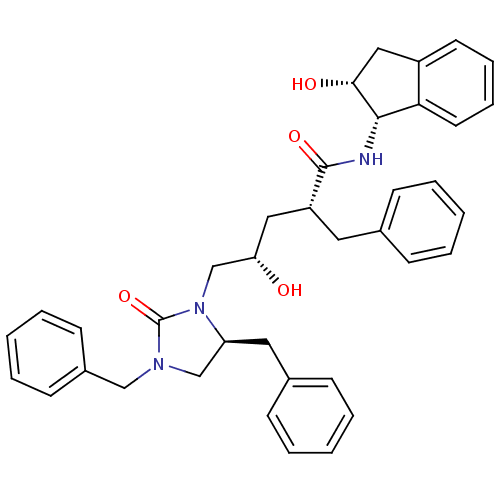 ((2R,4S)-2-benzyl-5-[(5S)-3,5-dibenzyl-2-oxoimidazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 10: 1159-62 (2000) Article DOI: 10.1016/s0960-894x(00)00163-3 BindingDB Entry DOI: 10.7270/Q2X63K5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471966 (CHEMBL147935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471974 (CHEMBL148950) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471982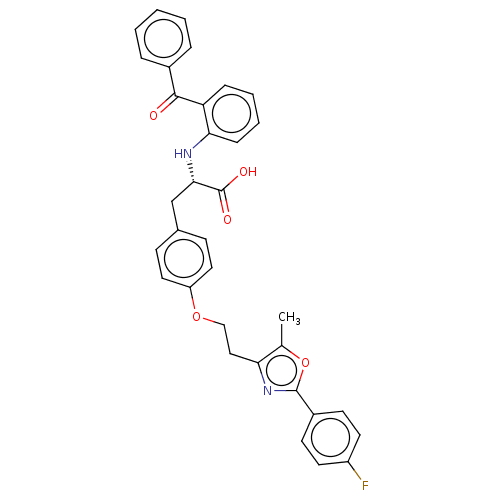 (CHEMBL147384) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9234 ((2S)-3-[(3S)-3-benzyl-5-oxo-1-oxa-4-azaspiro[5.5]u...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 3431-3 (2002) Article DOI: 10.1016/s0960-894x(02)00733-3 BindingDB Entry DOI: 10.7270/Q2B27SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471955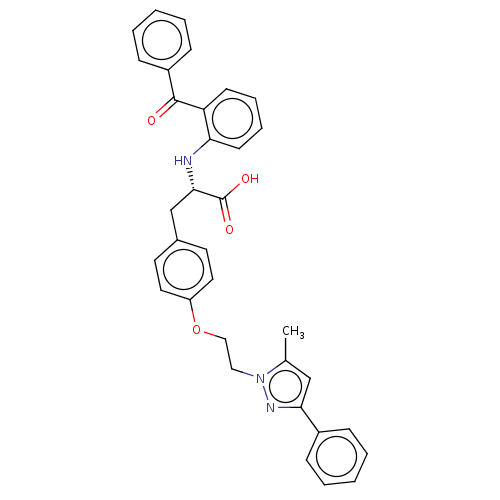 (CHEMBL148797) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471970 (CHEMBL148459) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13726 ((2S)-3-[(2R,5S)-5-benzyl-3-oxo-2-(prop-2-en-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 16: 5226-30 (2006) Article DOI: 10.1016/j.bmcl.2006.07.014 BindingDB Entry DOI: 10.7270/Q24Q7S7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9234 ((2S)-3-[(3S)-3-benzyl-5-oxo-1-oxa-4-azaspiro[5.5]u...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 16: 5226-30 (2006) Article DOI: 10.1016/j.bmcl.2006.07.014 BindingDB Entry DOI: 10.7270/Q24Q7S7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471948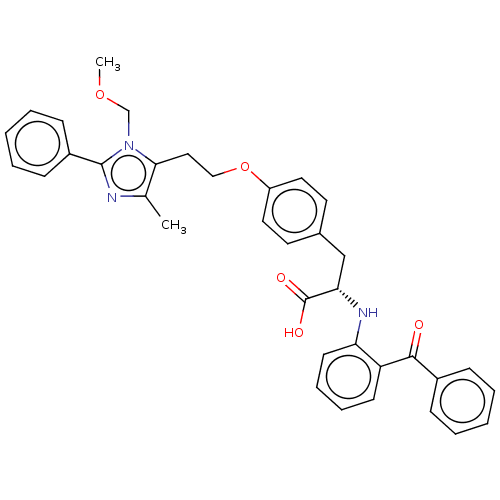 (CHEMBL149647) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471954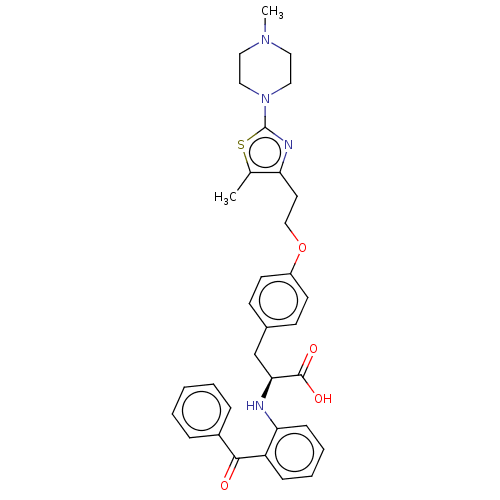 (CHEMBL358325) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9209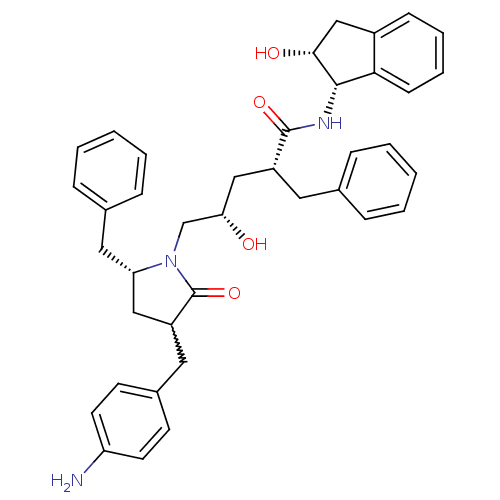 ((2R,4S)-5-[(5R)-3-[(4-aminophenyl)methyl]-5-benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | -49.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5689-92 (2004) Article DOI: 10.1016/j.bmcl.2004.08.039 BindingDB Entry DOI: 10.7270/Q21Z42M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471963 (CHEMBL359285) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471991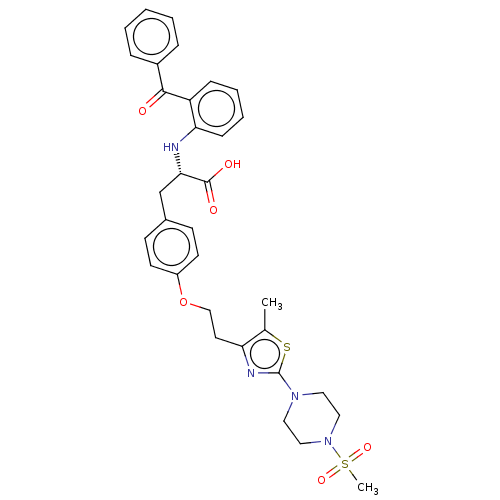 (CHEMBL146650) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471989 (CHEMBL149835) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9204 ((2R,4S)-2-benzyl-5-[(5S)-5-benzyl-3-[(3-fluorophen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | -48.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 14: 5685-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.038 BindingDB Entry DOI: 10.7270/Q25Q4T9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471973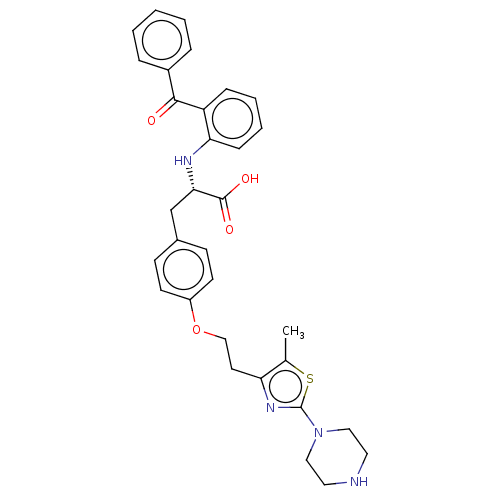 (CHEMBL146231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471967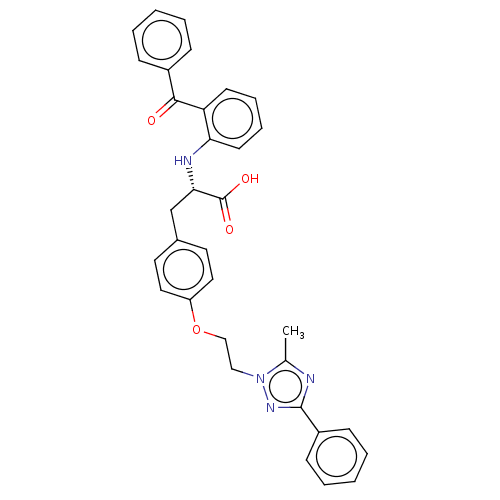 (CHEMBL342292) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 596 total ) | Next | Last >> |