

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora A (1 to 403 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50005429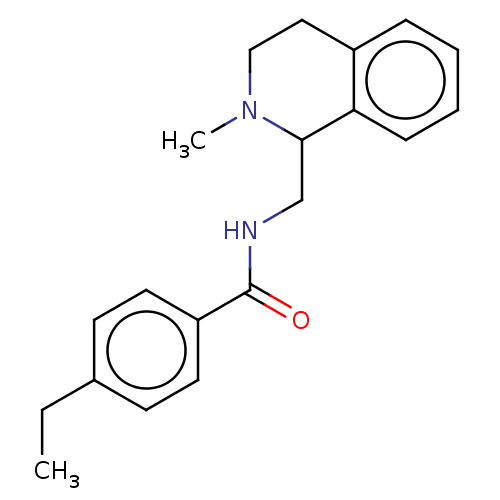 (CHEMBL4070288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora C (1 to 309 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50007985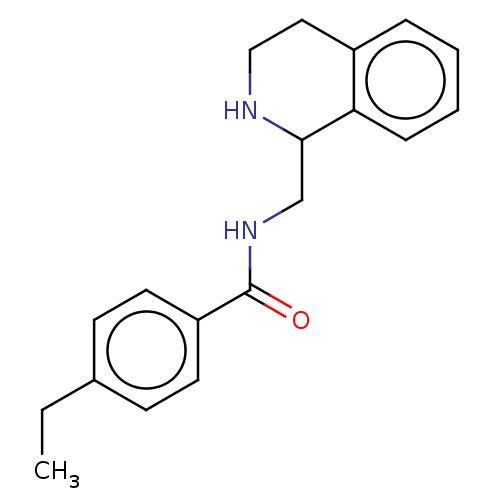 (CHEMBL4097865) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50042064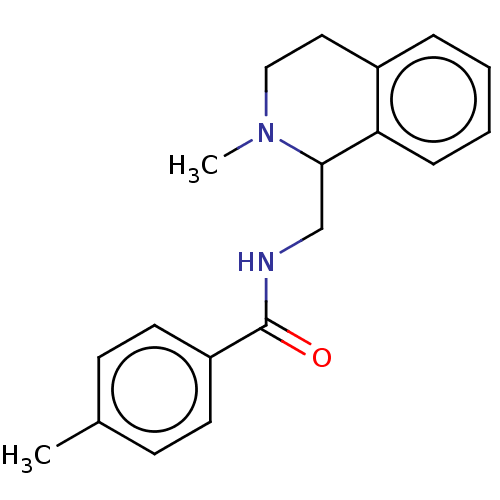 (CHEMBL4097466) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015426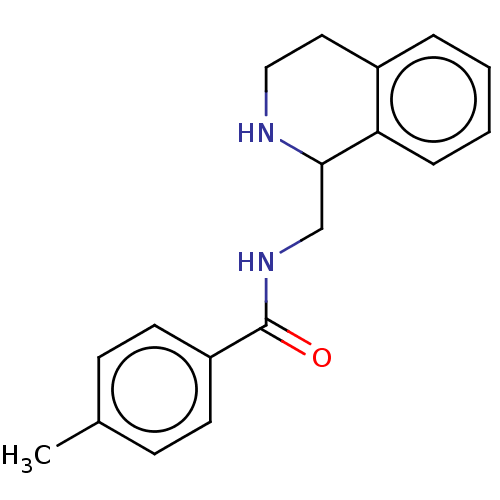 (CHEMBL4070750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239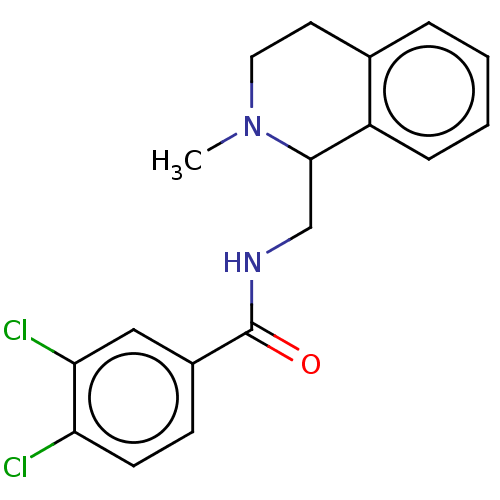 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora B (62 to 344 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008523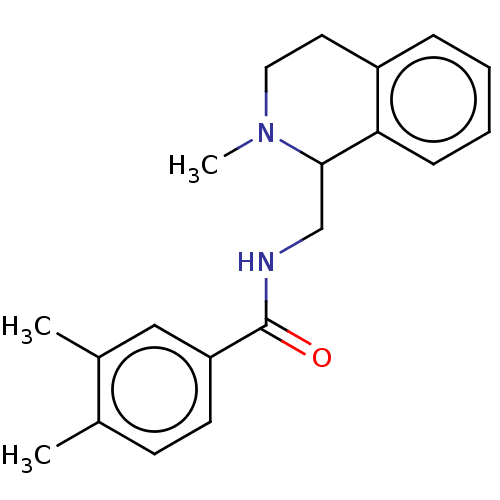 (CHEMBL4071332) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015433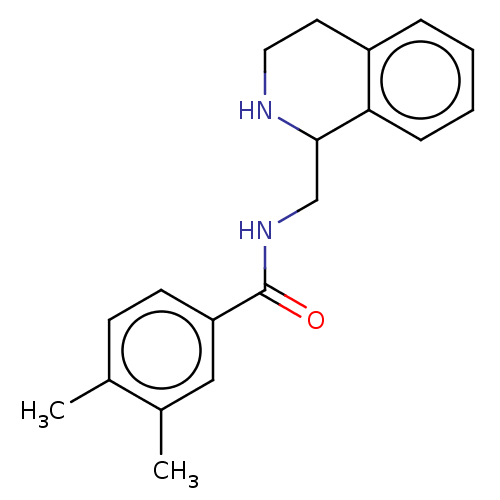 (CHEMBL4105599) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008003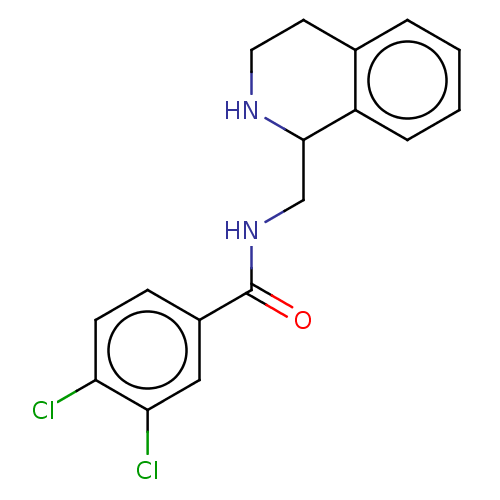 (CHEMBL4065924) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623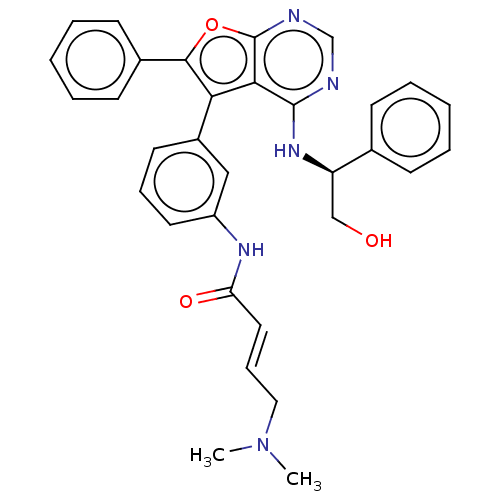 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50175305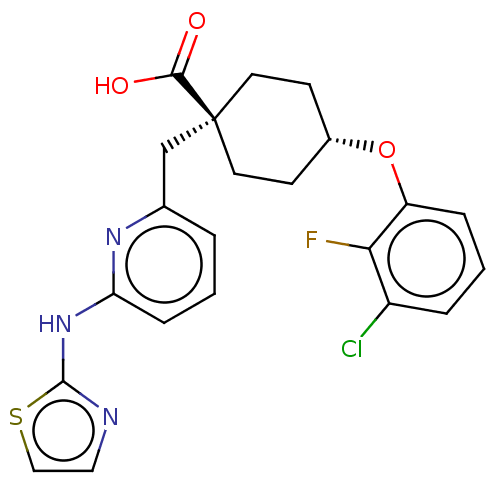 (CHEMBL3600873) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Aurora A expressed in Escherichia coli using RRR(GLRRASLG)4R-NH2 as substrate after 40 mins in presence of... | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247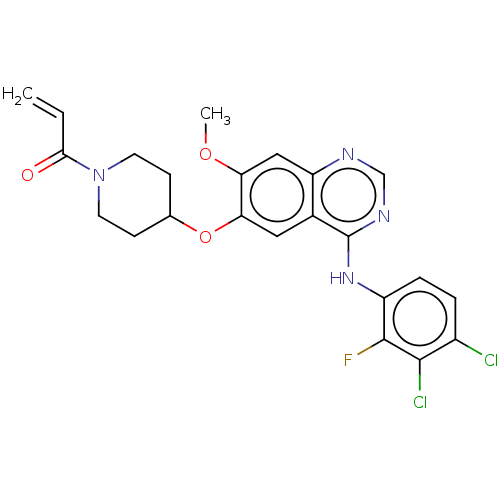 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50206389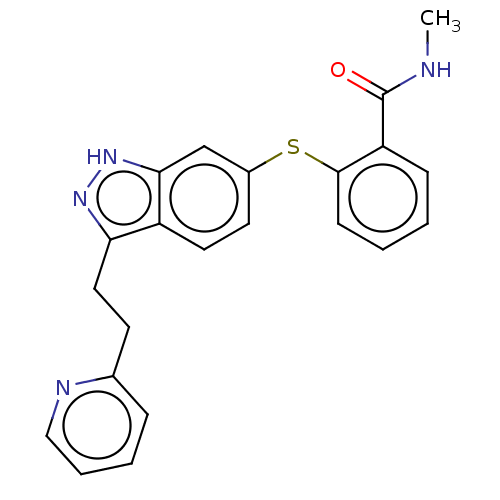 (CHEMBL3939307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR1 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50206389 (CHEMBL3939307) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR3 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618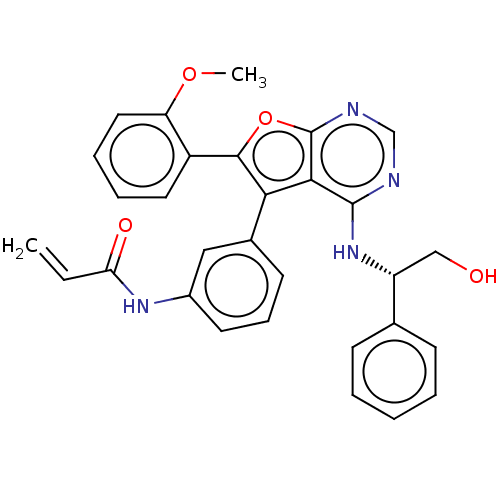 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50206389 (CHEMBL3939307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008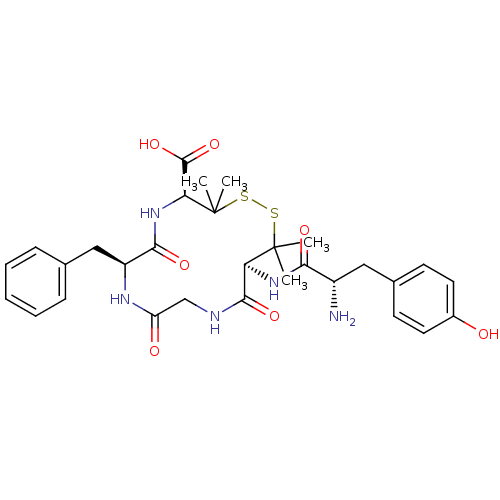 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens) | BDBM50241089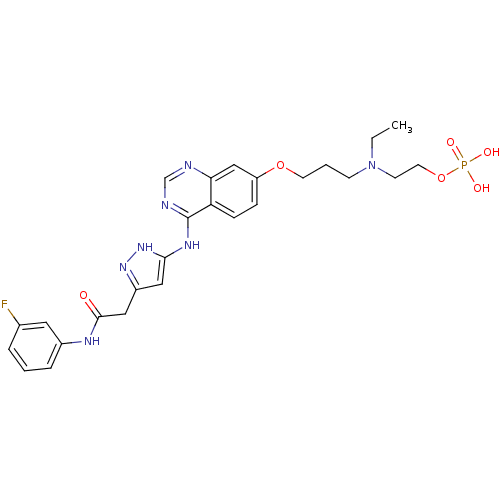 (2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant GST-tagged N-terminal truncated human Aurora A (123 to 401 residues) expressed in Sf9 insect cell using tetra-LRRASLG pepti... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01806 BindingDB Entry DOI: 10.7270/Q2Q2444S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II" Curated by ChEMBL | Assay Description Inhibition of recombinant human PDGFRbeta expressed in insect cells using Ulight-Poly GAT[EAY(1:1:1)]n as substrate after 30 mins by LANCE method | Eur J Med Chem 150: 491-505 (2018) Article DOI: 10.1016/j.ejmech.2018.02.080 BindingDB Entry DOI: 10.7270/Q2B27XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530615 (CHEMBL4534719) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530615 (CHEMBL4534719) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II" Curated by ChEMBL | Assay Description Inhibition of recombinant human KDR expressed in Sf9 insect cells using Ulight-CAGAGAIETDKEYYTVKD as substrate after 60 mins by LANCE method | Eur J Med Chem 150: 491-505 (2018) Article DOI: 10.1016/j.ejmech.2018.02.080 BindingDB Entry DOI: 10.7270/Q2B27XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens) | BDBM31837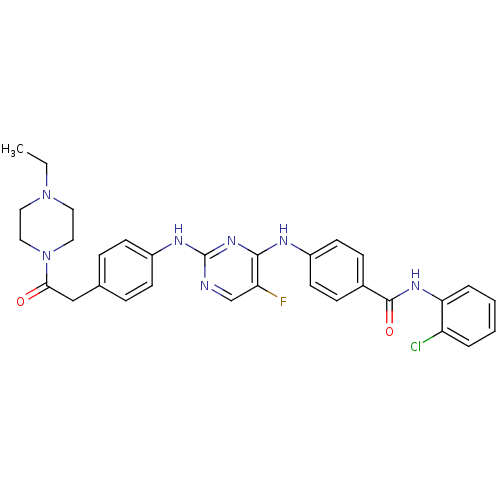 (2,4-Bisanilinopyrimidine, 10 | Aurora Inhibitor, 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant GST-tagged N-terminal truncated human Aurora A (123 to 401 residues) expressed in Sf9 insect cell using tetra-LRRASLG pepti... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01806 BindingDB Entry DOI: 10.7270/Q2Q2444S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II" Curated by ChEMBL | Assay Description Inhibition of recombinant human c-KIT using Ulight-TK peptide as substrate after 30 mins by LANCE method | Eur J Med Chem 150: 491-505 (2018) Article DOI: 10.1016/j.ejmech.2018.02.080 BindingDB Entry DOI: 10.7270/Q2B27XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II" Curated by ChEMBL | Assay Description Inhibition of recombinant human Flt4 using Ulight-CAGAGAIETDKEYYTVKD as substrate after 90 mins by LANCE method | Eur J Med Chem 150: 491-505 (2018) Article DOI: 10.1016/j.ejmech.2018.02.080 BindingDB Entry DOI: 10.7270/Q2B27XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II" Curated by ChEMBL | Assay Description Inhibition of recombinant human Src expressed in insect cells using Ulight-Poly GAT[EAY(1:1:1)]n as substrate after 10 mins by LANCE method | Eur J Med Chem 150: 491-505 (2018) Article DOI: 10.1016/j.ejmech.2018.02.080 BindingDB Entry DOI: 10.7270/Q2B27XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50370502 (CHEMBL4161600) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human recombinant Aurora A kinase domain (123 to 401 residues) expressed in Sf9 cells after 120 mins by kinase-glo luminesce... | Eur J Med Chem 151: 533-545 (2018) Article DOI: 10.1016/j.ejmech.2018.03.064 BindingDB Entry DOI: 10.7270/Q2D2216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM50175305 (CHEMBL3600873) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length N-terminal GST-tagged Aurora C (1 to 275 end residues) expressed in baculovirus expression system using G... | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530632 (CHEMBL4458437) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR kinase domain (696 to 1022 residues) using poly(Glu, Tyr) 4:1 substrate incubated for 60 mins by kinase-Glo plus ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 520 total ) | Next | Last >> |