 Found 681 hits with Last Name = 'keegan' and Initial = 's'
Found 681 hits with Last Name = 'keegan' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001577
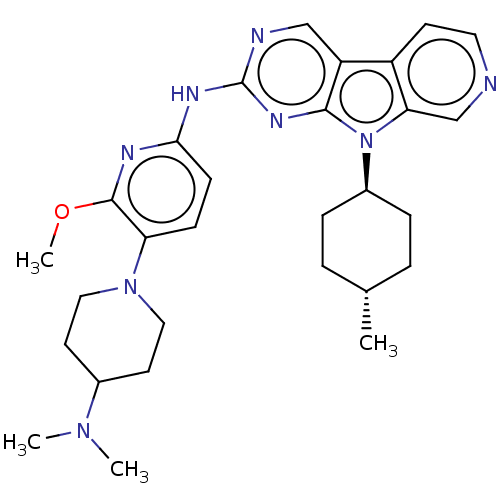
(CHEMBL3237712)Show SMILES COc1nc(Nc2ncc3c4ccncc4n([C@H]4CC[C@H](C)CC4)c3n2)ccc1N1CCC(CC1)N(C)C |r,wU:17.17,wD:20.21,(.78,-65.37,;2.1,-66.15,;3.44,-65.38,;3.44,-63.83,;4.77,-63.06,;4.76,-61.52,;6.09,-60.75,;6.08,-59.22,;7.4,-58.44,;8.75,-59.21,;10.22,-58.73,;10.84,-57.33,;12.37,-57.17,;13.28,-58.42,;12.65,-59.83,;11.12,-59.98,;10.21,-61.23,;10.69,-62.7,;12.2,-63.02,;12.67,-64.49,;11.63,-65.63,;12.1,-67.1,;10.13,-65.3,;9.66,-63.84,;8.74,-60.75,;7.42,-61.52,;6.1,-63.83,;6.11,-65.38,;4.77,-66.15,;4.77,-67.69,;3.43,-68.46,;3.43,-69.99,;4.76,-70.76,;6.09,-70,;6.1,-68.45,;4.75,-72.3,;3.41,-73.07,;6.08,-73.08,)| Show InChI InChI=1S/C29H38N8O/c1-19-5-7-21(8-6-19)37-25-18-30-14-11-22(25)23-17-31-29(34-27(23)37)33-26-10-9-24(28(32-26)38-4)36-15-12-20(13-16-36)35(2)3/h9-11,14,17-21H,5-8,12-13,15-16H2,1-4H3,(H,31,32,33,34)/t19-,21- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501402

(CHEMBL4071605)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H15ClN10O/c1-8(24-15-9(7-20)14(21)26-18(22)27-15)16-25-11-4-2-3-10(19)13(11)17(30)29(16)12-5-6-23-28-12/h2-6,8H,1H3,(H,23,28)(H5,21,22,24,26,27)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001541
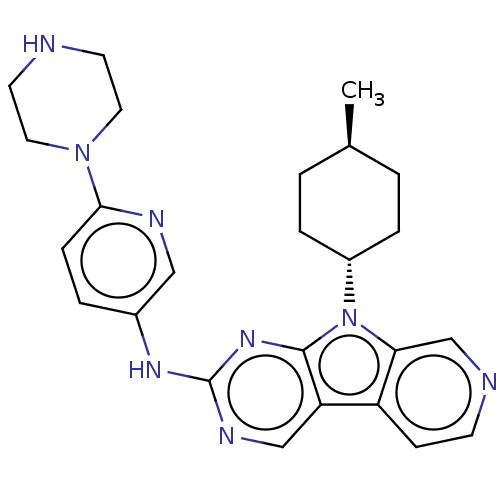
(CHEMBL3237706)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(nc3)N3CCNCC3)nc12 |r,wU:4.7,wD:1.0,(34.74,-35.4,;34.27,-33.94,;35.31,-32.8,;34.84,-31.33,;33.33,-31.01,;32.3,-32.15,;32.77,-33.61,;32.86,-29.55,;33.77,-28.3,;35.3,-28.14,;35.93,-26.73,;35.02,-25.48,;33.49,-25.65,;32.87,-27.05,;31.4,-27.52,;30.05,-26.75,;28.73,-27.53,;28.74,-29.06,;27.41,-29.84,;27.41,-31.38,;28.74,-32.13,;28.75,-33.66,;27.42,-34.45,;26.08,-33.68,;26.08,-32.14,;27.42,-35.99,;26.09,-36.76,;26.1,-38.29,;27.43,-39.06,;28.76,-38.29,;28.76,-36.74,;30.07,-29.83,;31.39,-29.07,)| Show InChI InChI=1S/C25H30N8/c1-17-2-5-19(6-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-18-4-7-23(28-14-18)32-12-10-26-11-13-32/h4,7-9,14-17,19,26H,2-3,5-6,10-13H2,1H3,(H,29,30,31)/t17-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501399

(CHEMBL4100135)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C21H16ClN7O2/c1-11(27-19-14(9-23)18(24)25-10-26-19)20-28-16-4-2-3-15(22)17(16)21(31)29(20)12-5-7-13(30)8-6-12/h2-8,10-11,30H,1H3,(H3,24,25,26,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168484

(CHEMBL3805137 | US9765060, Compound Y)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-14(10-23)18(24)25-11-26-19)20-28-16-9-5-8-15(22)17(16)21(30)29(20)13-6-3-2-4-7-13/h2-9,11-12H,1H3,(H3,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001583
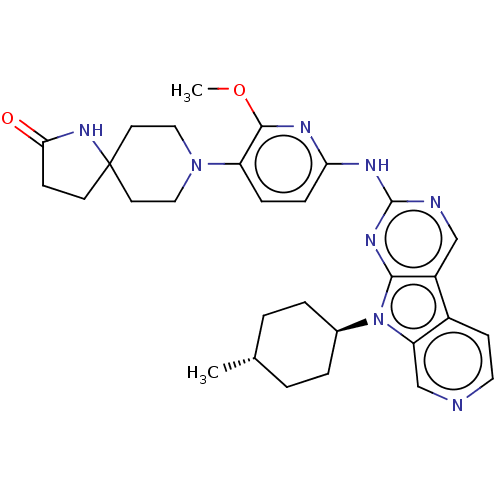
(CHEMBL3237718)Show SMILES COc1nc(Nc2ncc3c4ccncc4n([C@H]4CC[C@H](C)CC4)c3n2)ccc1N1CCC2(CCC(=O)N2)CC1 |r,wU:17.17,wD:20.21,(36.2,-55.73,;37.53,-56.52,;38.87,-55.76,;38.89,-54.23,;40.23,-53.48,;40.24,-51.95,;41.57,-51.18,;41.56,-49.64,;42.89,-48.87,;44.23,-49.63,;45.7,-49.16,;46.33,-47.76,;47.86,-47.59,;48.77,-48.84,;48.14,-50.25,;46.6,-50.41,;45.7,-51.66,;46.17,-53.12,;47.67,-53.44,;48.14,-54.91,;47.11,-56.05,;47.58,-57.52,;45.6,-55.72,;45.13,-54.26,;44.23,-51.18,;42.9,-51.95,;41.54,-54.25,;41.53,-55.78,;40.19,-56.54,;40.18,-58.07,;38.84,-58.83,;38.82,-60.36,;40.14,-61.15,;38.9,-62.06,;39.37,-63.52,;40.91,-63.52,;41.82,-64.77,;41.39,-62.06,;41.49,-60.4,;41.51,-58.85,)| Show InChI InChI=1S/C30H36N8O2/c1-19-3-5-20(6-4-19)38-24-18-31-14-10-21(24)22-17-32-29(35-27(22)38)34-25-8-7-23(28(33-25)40-2)37-15-12-30(13-16-37)11-9-26(39)36-30/h7-8,10,14,17-20H,3-6,9,11-13,15-16H2,1-2H3,(H,36,39)(H,32,33,34,35)/t19-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501403
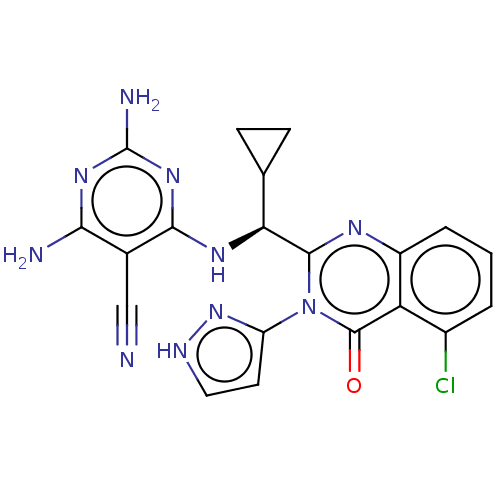
(CHEMBL4084645)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3cccc(Cl)c3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C20H17ClN10O/c21-11-2-1-3-12-14(11)19(32)31(13-6-7-25-30-13)18(26-12)15(9-4-5-9)27-17-10(8-22)16(23)28-20(24)29-17/h1-3,6-7,9,15H,4-5H2,(H,25,30)(H5,23,24,27,28,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501408
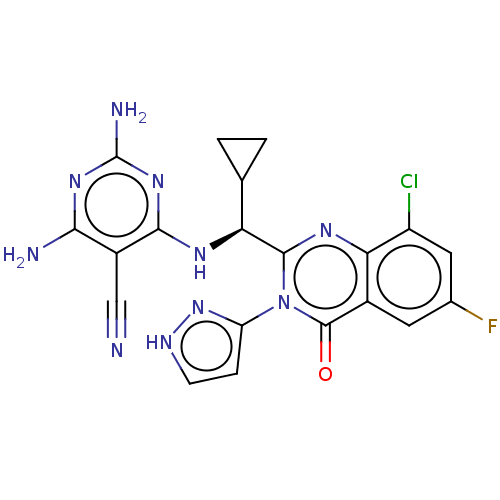
(CHEMBL4081433)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3c(Cl)cc(F)cc3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C20H16ClFN10O/c21-12-6-9(22)5-10-15(12)28-18(32(19(10)33)13-3-4-26-31-13)14(8-1-2-8)27-17-11(7-23)16(24)29-20(25)30-17/h3-6,8,14H,1-2H2,(H,26,31)(H5,24,25,27,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001576
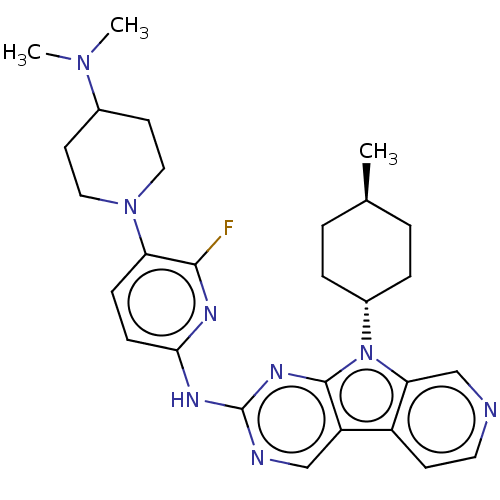
(CHEMBL3237711)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(N4CCC(CC4)N(C)C)c(F)n3)nc12 |r,wU:4.7,wD:1.0,(48.6,-50.76,;48.13,-49.29,;49.17,-48.15,;48.7,-46.68,;47.19,-46.36,;46.16,-47.5,;46.63,-48.96,;46.72,-44.9,;47.63,-43.65,;49.16,-43.49,;49.79,-42.08,;48.88,-40.83,;47.35,-40.99,;46.73,-42.4,;45.26,-42.87,;43.91,-42.1,;42.58,-42.88,;42.6,-44.41,;41.27,-45.19,;41.25,-46.72,;42.56,-47.49,;42.55,-49.02,;41.22,-49.78,;41.2,-51.31,;39.86,-52.07,;39.84,-53.6,;41.17,-54.39,;42.51,-53.63,;42.53,-52.09,;41.15,-55.93,;39.8,-56.68,;42.47,-56.72,;39.9,-49,;38.55,-49.76,;39.91,-47.47,;43.93,-45.18,;45.25,-44.42,)| Show InChI InChI=1S/C28H35FN8/c1-18-4-6-20(7-5-18)37-24-17-30-13-10-21(24)22-16-31-28(34-27(22)37)33-25-9-8-23(26(29)32-25)36-14-11-19(12-15-36)35(2)3/h8-10,13,16-20H,4-7,11-12,14-15H2,1-3H3,(H,31,32,33,34)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501400
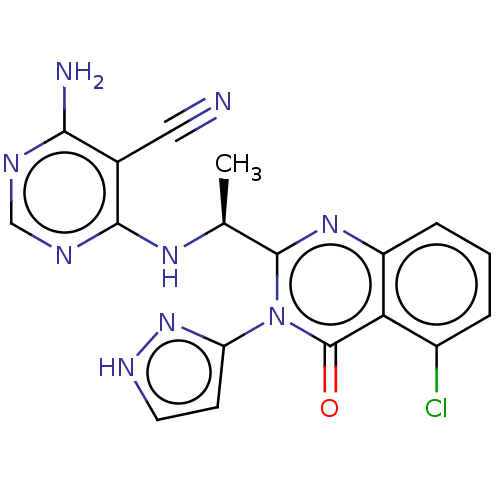
(CHEMBL4082130)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H14ClN9O/c1-9(25-16-10(7-20)15(21)22-8-23-16)17-26-12-4-2-3-11(19)14(12)18(29)28(17)13-5-6-24-27-13/h2-6,8-9H,1H3,(H,24,27)(H3,21,22,23,25)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112481
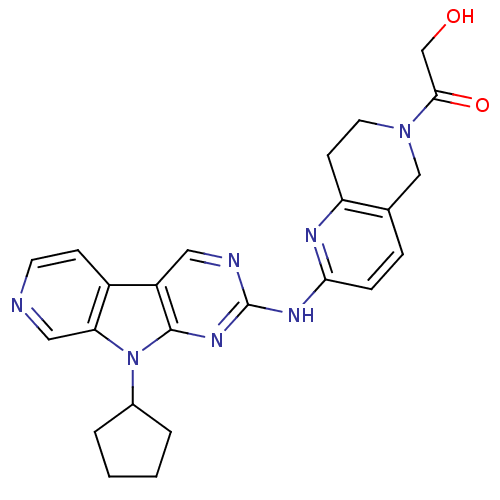
(US8623885, 22)Show SMILES OCC(=O)N1CCc2nc(Nc3ncc4c5ccncc5n(C5CCCC5)c4n3)ccc2C1 Show InChI InChI=1S/C24H25N7O2/c32-14-22(33)30-10-8-19-15(13-30)5-6-21(27-19)28-24-26-11-18-17-7-9-25-12-20(17)31(23(18)29-24)16-3-1-2-4-16/h5-7,9,11-12,16,32H,1-4,8,10,13-14H2,(H,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501408

(CHEMBL4081433)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3c(Cl)cc(F)cc3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C20H16ClFN10O/c21-12-6-9(22)5-10-15(12)28-18(32(19(10)33)13-3-4-26-31-13)14(8-1-2-8)27-17-11(7-23)16(24)29-20(25)30-17/h3-6,8,14H,1-2H2,(H,26,31)(H5,24,25,27,29,30)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov... |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501399

(CHEMBL4100135)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C21H16ClN7O2/c1-11(27-19-14(9-23)18(24)25-10-26-19)20-28-16-4-2-3-15(22)17(16)21(31)29(20)12-5-7-13(30)8-6-12/h2-8,10-11,30H,1H3,(H3,24,25,26,27)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov... |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501402

(CHEMBL4071605)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H15ClN10O/c1-8(24-15-9(7-20)14(21)26-18(22)27-15)16-25-11-4-2-3-10(19)13(11)17(30)29(16)12-5-6-23-28-12/h2-6,8H,1H3,(H,23,28)(H5,21,22,24,26,27)/t8-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov... |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357333
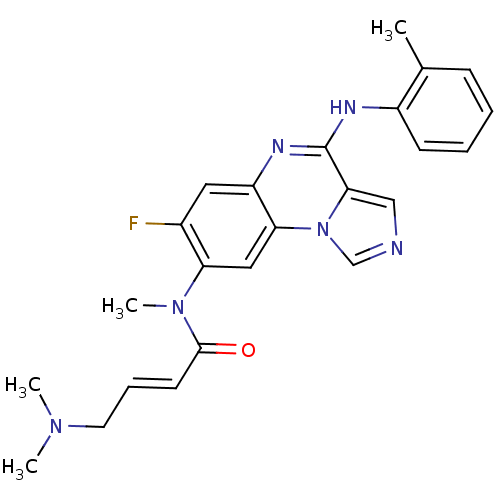
(CHEMBL1916891)Show SMILES CN(C)C\C=C\C(=O)N(C)c1cc2c(cc1F)nc(Nc1ccccc1C)c1cncn21 Show InChI InChI=1S/C24H25FN6O/c1-16-8-5-6-9-18(16)27-24-22-14-26-15-31(22)21-13-20(17(25)12-19(21)28-24)30(4)23(32)10-7-11-29(2)3/h5-10,12-15H,11H2,1-4H3,(H,27,28)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate preincubated for 60 mins measured after 60 mins by ... |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501411

(CHEMBL4068742)Show SMILES Cc1nc(N)nc(N[C@@H](C2CC2)c2nc3cccc(Cl)c3c(=O)n2-c2cc[nH]n2)c1C#N |r| Show InChI InChI=1S/C21H18ClN9O/c1-10-12(9-23)18(29-21(24)26-10)28-17(11-5-6-11)19-27-14-4-2-3-13(22)16(14)20(32)31(19)15-7-8-25-30-15/h2-4,7-8,11,17H,5-6H2,1H3,(H,25,30)(H3,24,26,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357314
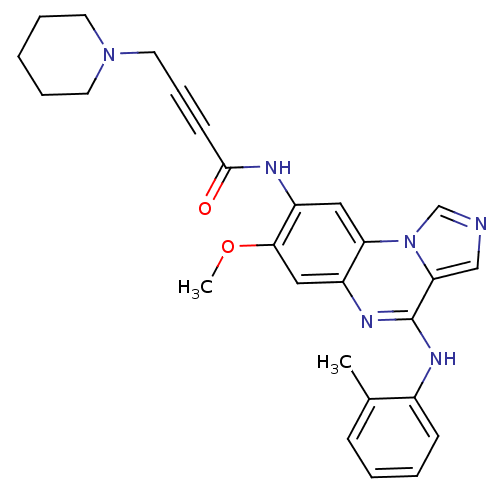
(CHEMBL1916713)Show SMILES COc1cc2nc(Nc3ccccc3C)c3cncn3c2cc1NC(=O)C#CCN1CCCCC1 Show InChI InChI=1S/C27H28N6O2/c1-19-9-4-5-10-20(19)30-27-24-17-28-18-33(24)23-15-22(25(35-2)16-21(23)31-27)29-26(34)11-8-14-32-12-6-3-7-13-32/h4-5,9-10,15-18H,3,6-7,12-14H2,1-2H3,(H,29,34)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.847 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357313
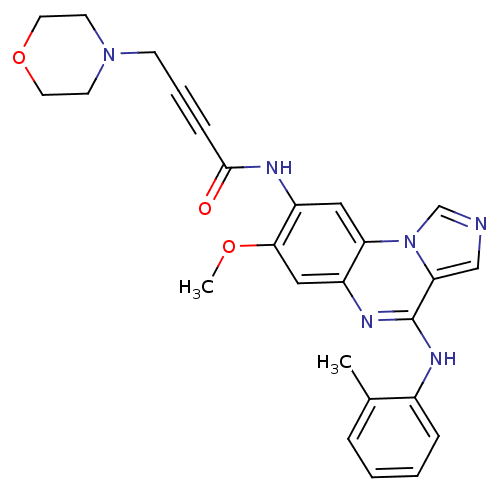
(CHEMBL1916712)Show SMILES COc1cc2nc(Nc3ccccc3C)c3cncn3c2cc1NC(=O)C#CCN1CCOCC1 Show InChI InChI=1S/C26H26N6O3/c1-18-6-3-4-7-19(18)29-26-23-16-27-17-32(23)22-14-21(24(34-2)15-20(22)30-26)28-25(33)8-5-9-31-10-12-35-13-11-31/h3-4,6-7,14-17H,9-13H2,1-2H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.847 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357316
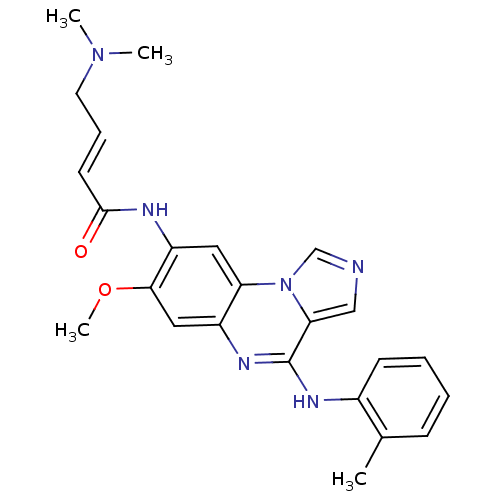
(CHEMBL1916715)Show SMILES COc1cc2nc(Nc3ccccc3C)c3cncn3c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C24H26N6O2/c1-16-8-5-6-9-17(16)27-24-21-14-25-15-30(21)20-12-19(22(32-4)13-18(20)28-24)26-23(31)10-7-11-29(2)3/h5-10,12-15H,11H2,1-4H3,(H,26,31)(H,27,28)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.884 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001543

(CHEMBL3237708)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCC(N)CC3)nc12 |r,wU:4.7,wD:1.0,(11.29,-49.99,;10.82,-48.52,;11.85,-47.38,;11.38,-45.91,;9.88,-45.59,;8.84,-46.74,;9.31,-48.2,;9.41,-44.13,;10.31,-42.88,;11.84,-42.73,;12.48,-41.32,;11.56,-40.07,;10.04,-40.23,;9.41,-41.63,;7.94,-42.1,;6.6,-41.34,;5.27,-42.12,;5.28,-43.65,;3.95,-44.42,;3.96,-45.96,;5.29,-46.72,;5.3,-48.25,;3.96,-49.03,;2.63,-48.27,;2.62,-46.73,;3.97,-50.58,;2.64,-51.35,;2.64,-52.89,;3.98,-53.66,;3.98,-55.2,;5.31,-52.88,;5.31,-51.34,;6.62,-44.42,;7.94,-43.65,)| Show InChI InChI=1S/C26H32N8/c1-17-2-4-19(5-3-17)34-23-16-28-11-8-21(23)22-15-30-26(32-25(22)34)31-24-7-6-20(14-29-24)33-12-9-18(27)10-13-33/h6-8,11,14-19H,2-5,9-10,12-13,27H2,1H3,(H,29,30,31,32)/t17-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001576

(CHEMBL3237711)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(N4CCC(CC4)N(C)C)c(F)n3)nc12 |r,wU:4.7,wD:1.0,(48.6,-50.76,;48.13,-49.29,;49.17,-48.15,;48.7,-46.68,;47.19,-46.36,;46.16,-47.5,;46.63,-48.96,;46.72,-44.9,;47.63,-43.65,;49.16,-43.49,;49.79,-42.08,;48.88,-40.83,;47.35,-40.99,;46.73,-42.4,;45.26,-42.87,;43.91,-42.1,;42.58,-42.88,;42.6,-44.41,;41.27,-45.19,;41.25,-46.72,;42.56,-47.49,;42.55,-49.02,;41.22,-49.78,;41.2,-51.31,;39.86,-52.07,;39.84,-53.6,;41.17,-54.39,;42.51,-53.63,;42.53,-52.09,;41.15,-55.93,;39.8,-56.68,;42.47,-56.72,;39.9,-49,;38.55,-49.76,;39.91,-47.47,;43.93,-45.18,;45.25,-44.42,)| Show InChI InChI=1S/C28H35FN8/c1-18-4-6-20(7-5-18)37-24-17-30-13-10-21(24)22-16-31-28(34-27(22)37)33-25-9-8-23(26(29)32-25)36-14-11-19(12-15-36)35(2)3/h8-10,13,16-20H,4-7,11-12,14-15H2,1-3H3,(H,31,32,33,34)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001539
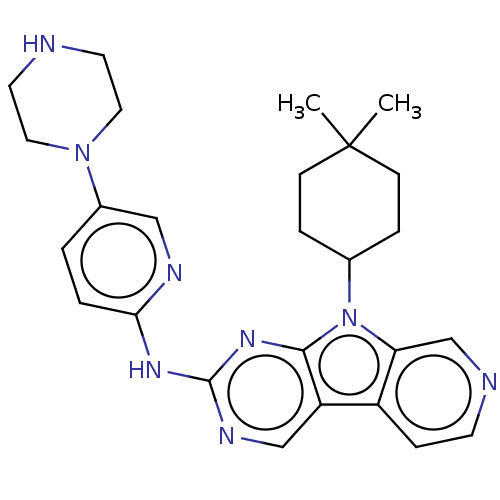
(CHEMBL3237704 | US8841312, 204)Show SMILES CC1(C)CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 Show InChI InChI=1S/C26H32N8/c1-26(2)8-5-18(6-9-26)34-22-17-28-10-7-20(22)21-16-30-25(32-24(21)34)31-23-4-3-19(15-29-23)33-13-11-27-12-14-33/h3-4,7,10,15-18,27H,5-6,8-9,11-14H2,1-2H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
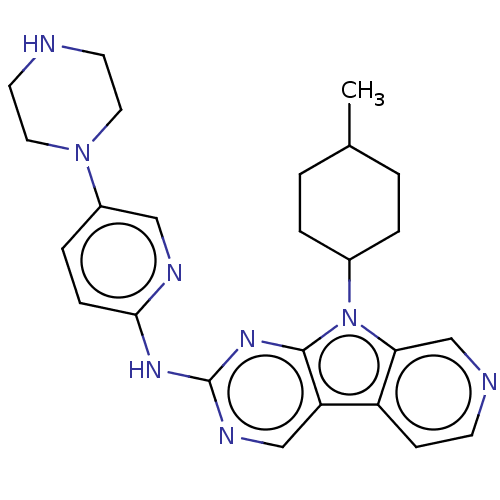
(Homo sapiens (Human)) | BDBM50001537
(CHEMBL3237702 | US8841312, 55)Show SMILES CC1CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 |(35.98,-56.71,;35.51,-55.25,;34.01,-54.92,;33.54,-53.46,;34.57,-52.32,;36.08,-52.63,;36.55,-54.1,;34.1,-50.85,;35.01,-49.6,;36.54,-49.45,;37.17,-48.04,;36.26,-46.79,;34.73,-46.95,;34.11,-48.35,;32.64,-48.83,;31.29,-48.06,;29.97,-48.84,;29.98,-50.37,;28.65,-51.14,;28.65,-52.68,;29.99,-53.45,;29.99,-55,;28.66,-55.77,;27.32,-55,;27.33,-53.45,;28.66,-57.31,;27.32,-58.08,;27.31,-59.61,;28.64,-60.38,;29.98,-59.62,;29.99,-58.07,;31.31,-51.14,;32.63,-50.37,)| Show InChI InChI=1S/C25H30N8/c1-17-2-4-18(5-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-23-7-6-19(14-28-23)32-12-10-26-11-13-32/h6-9,14-18,26H,2-5,10-13H2,1H3,(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112464
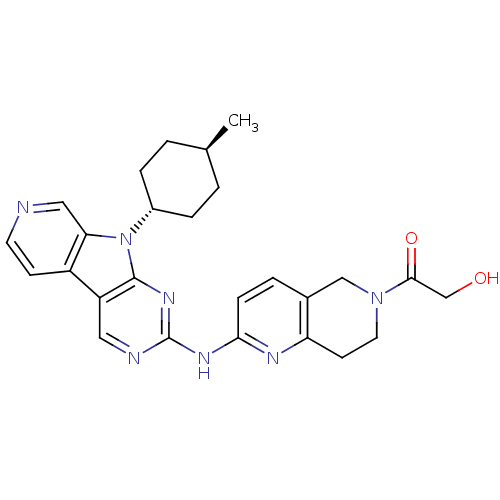
(US8623885, 5)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CN(CCc4n3)C(=O)CO)nc12 |r,wU:4.7,wD:1.0,(4.13,-3.21,;3.73,-1.73,;4.82,-.64,;4.42,.85,;2.93,1.25,;1.84,.16,;2.24,-1.33,;2.53,2.74,;3.44,3.98,;4.97,4.14,;5.6,5.55,;4.69,6.8,;3.16,6.64,;2.53,5.23,;1.07,4.75,;-.26,5.52,;-1.6,4.75,;-1.6,3.21,;-2.93,2.44,;-2.93,.9,;-1.6,.13,;-1.6,-1.41,;-2.93,-2.18,;-2.93,-3.72,;-4.26,-4.49,;-5.6,-3.72,;-5.6,-2.18,;-4.26,-1.41,;-4.26,.13,;-4.26,-6.03,;-5.6,-6.8,;-2.93,-6.8,;-1.6,-6.03,;-.26,2.44,;1.07,3.21,)| Show InChI InChI=1S/C26H29N7O2/c1-16-2-5-18(6-3-16)33-22-13-27-10-8-19(22)20-12-28-26(31-25(20)33)30-23-7-4-17-14-32(24(35)15-34)11-9-21(17)29-23/h4,7-8,10,12-13,16,18,34H,2-3,5-6,9,11,14-15H2,1H3,(H,28,29,30,31)/t16-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112460
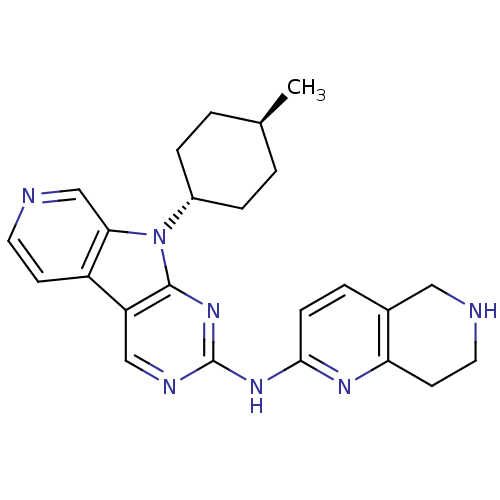
(US8623885, 1)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CNCCc4n3)nc12 |r,wU:4.7,wD:1.0,(4.13,-4.37,;3.73,-2.88,;4.82,-1.79,;4.42,-.3,;2.93,.09,;1.84,-.99,;2.24,-2.48,;2.53,1.58,;3.44,2.83,;4.97,2.99,;5.6,4.4,;4.69,5.64,;3.16,5.48,;2.53,4.07,;1.07,3.6,;-.26,4.37,;-1.6,3.6,;-1.6,2.06,;-2.93,1.29,;-2.93,-.25,;-1.6,-1.02,;-1.6,-2.56,;-2.93,-3.33,;-2.93,-4.87,;-4.26,-5.64,;-5.6,-4.87,;-5.6,-3.33,;-4.26,-2.56,;-4.26,-1.02,;-.26,1.29,;1.07,2.06,)| Show InChI InChI=1S/C24H27N7/c1-15-2-5-17(6-3-15)31-21-14-26-10-8-18(21)19-13-27-24(30-23(19)31)29-22-7-4-16-12-25-11-9-20(16)28-22/h4,7-8,10,13-15,17,25H,2-3,5-6,9,11-12H2,1H3,(H,27,28,29,30)/t15-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50357333

(CHEMBL1916891)Show SMILES CN(C)C\C=C\C(=O)N(C)c1cc2c(cc1F)nc(Nc1ccccc1C)c1cncn21 Show InChI InChI=1S/C24H25FN6O/c1-16-8-5-6-9-18(16)27-24-22-14-26-15-31(22)21-13-20(17(25)12-19(21)28-24)30(4)23(32)10-7-11-29(2)3/h5-10,12-15H,11H2,1-4H3,(H,27,28)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR preincubated for 60 mins measured after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112475
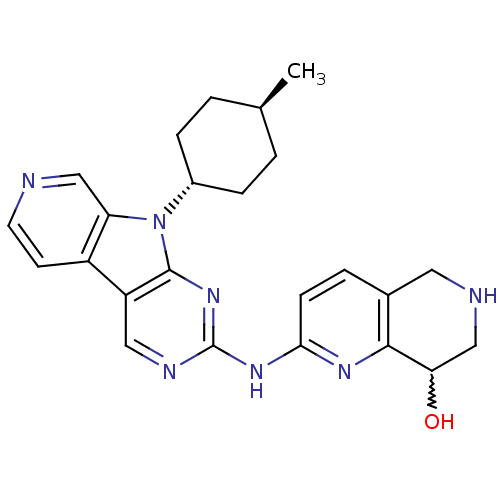
(US8623885, 16)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CNCC(O)c4n3)nc12 |r,w:26.28,wU:4.7,wD:1.0,(4.8,-4.37,;4.4,-2.88,;5.49,-1.79,;5.09,-.3,;3.6,.09,;2.51,-.99,;2.91,-2.48,;3.2,1.58,;4.11,2.83,;5.64,2.99,;6.26,4.4,;5.36,5.64,;3.83,5.48,;3.2,4.07,;1.74,3.6,;.4,4.37,;-.93,3.6,;-.93,2.06,;-2.26,1.29,;-2.26,-.25,;-.93,-1.02,;-.93,-2.56,;-2.26,-3.33,;-2.26,-4.87,;-3.6,-5.64,;-4.93,-4.87,;-4.93,-3.33,;-6.26,-2.56,;-3.6,-2.56,;-3.6,-1.02,;.4,1.29,;1.74,2.06,)| Show InChI InChI=1S/C24H27N7O/c1-14-2-5-16(6-3-14)31-19-12-25-9-8-17(19)18-11-27-24(30-23(18)31)29-21-7-4-15-10-26-13-20(32)22(15)28-21/h4,7-9,11-12,14,16,20,26,32H,2-3,5-6,10,13H2,1H3,(H,27,28,29,30)/t14-,16-,20? | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112464

(US8623885, 5)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CN(CCc4n3)C(=O)CO)nc12 |r,wU:4.7,wD:1.0,(4.13,-3.21,;3.73,-1.73,;4.82,-.64,;4.42,.85,;2.93,1.25,;1.84,.16,;2.24,-1.33,;2.53,2.74,;3.44,3.98,;4.97,4.14,;5.6,5.55,;4.69,6.8,;3.16,6.64,;2.53,5.23,;1.07,4.75,;-.26,5.52,;-1.6,4.75,;-1.6,3.21,;-2.93,2.44,;-2.93,.9,;-1.6,.13,;-1.6,-1.41,;-2.93,-2.18,;-2.93,-3.72,;-4.26,-4.49,;-5.6,-3.72,;-5.6,-2.18,;-4.26,-1.41,;-4.26,.13,;-4.26,-6.03,;-5.6,-6.8,;-2.93,-6.8,;-1.6,-6.03,;-.26,2.44,;1.07,3.21,)| Show InChI InChI=1S/C26H29N7O2/c1-16-2-5-18(6-3-16)33-22-13-27-10-8-19(22)20-12-28-26(31-25(20)33)30-23-7-4-17-14-32(24(35)15-34)11-9-21(17)29-23/h4,7-8,10,12-13,16,18,34H,2-3,5-6,9,11,14-15H2,1H3,(H,28,29,30,31)/t16-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50357333

(CHEMBL1916891)Show SMILES CN(C)C\C=C\C(=O)N(C)c1cc2c(cc1F)nc(Nc1ccccc1C)c1cncn21 Show InChI InChI=1S/C24H25FN6O/c1-16-8-5-6-9-18(16)27-24-22-14-26-15-31(22)21-13-20(17(25)12-19(21)28-24)30(4)23(32)10-7-11-29(2)3/h5-10,12-15H,11H2,1-4H3,(H,27,28)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BMX relative to control |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50357321

(CHEMBL1916879)Show SMILES CN(C)C\C=C\C(=O)N(C)c1ccc2nc(Nc3cc(NC(=O)c4cccc(c4)C(F)(F)F)ccc3C)c3cncn3c2c1 Show InChI InChI=1S/C32H30F3N7O2/c1-20-10-11-23(37-31(44)21-7-5-8-22(15-21)32(33,34)35)16-26(20)39-30-28-18-36-19-42(28)27-17-24(12-13-25(27)38-30)41(4)29(43)9-6-14-40(2)3/h5-13,15-19H,14H2,1-4H3,(H,37,44)(H,38,39)/b9-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LYN-A expressed in Sf9 cells after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357330
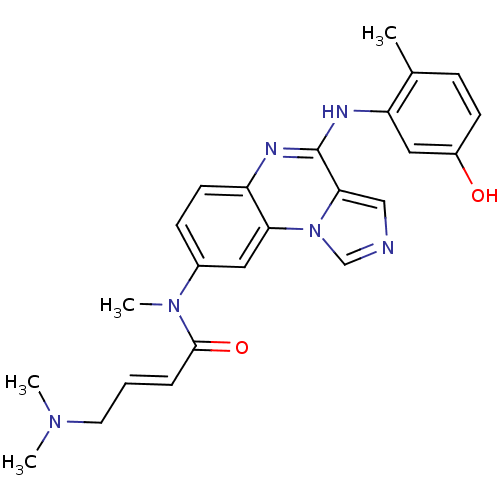
(CHEMBL1916888)Show SMILES CN(C)C\C=C\C(=O)N(C)c1ccc2nc(Nc3cc(O)ccc3C)c3cncn3c2c1 Show InChI InChI=1S/C24H26N6O2/c1-16-7-9-18(31)13-20(16)27-24-22-14-25-15-30(22)21-12-17(8-10-19(21)26-24)29(4)23(32)6-5-11-28(2)3/h5-10,12-15,31H,11H2,1-4H3,(H,26,27)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112477
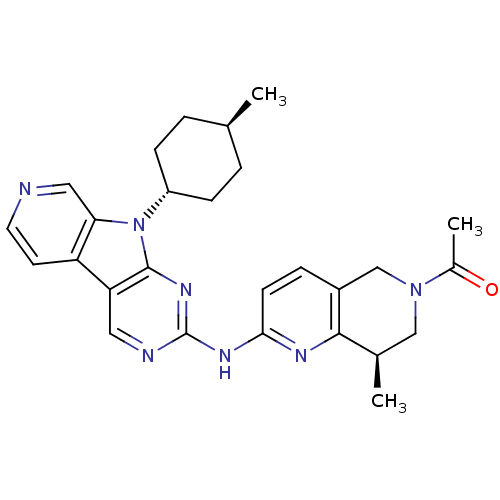
(US8623885, 18a)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CN(C[C@@H](C)c4n3)C(C)=O)nc12 |r,wU:4.7,26.28,wD:1.0,(4.8,5.44,;4.4,6.93,;5.49,8.02,;5.09,9.51,;3.6,9.91,;2.51,8.82,;2.91,7.33,;3.2,11.39,;4.11,12.64,;5.64,12.8,;6.26,14.21,;5.36,15.45,;3.83,15.29,;3.2,13.89,;1.74,13.41,;.4,14.18,;-.93,13.41,;-.93,11.87,;-2.26,11.1,;-2.26,9.56,;-.93,8.79,;-.93,7.25,;-2.26,6.48,;-2.26,4.94,;-3.6,4.17,;-4.93,4.94,;-4.93,6.48,;-6.26,7.25,;-3.6,7.25,;-3.6,8.79,;-3.6,2.63,;-2.26,1.86,;-4.93,1.86,;.4,11.1,;1.74,11.87,)| Show InChI InChI=1S/C27H31N7O/c1-16-4-7-20(8-5-16)34-23-13-28-11-10-21(23)22-12-29-27(32-26(22)34)31-24-9-6-19-15-33(18(3)35)14-17(2)25(19)30-24/h6,9-13,16-17,20H,4-5,7-8,14-15H2,1-3H3,(H,29,30,31,32)/t16-,17-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501403

(CHEMBL4084645)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3cccc(Cl)c3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C20H17ClN10O/c21-11-2-1-3-12-14(11)19(32)31(13-6-7-25-30-13)18(26-12)15(9-4-5-9)27-17-10(8-22)16(23)28-20(24)29-17/h1-3,6-7,9,15H,4-5H2,(H,25,30)(H5,23,24,27,28,29)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov... |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112469
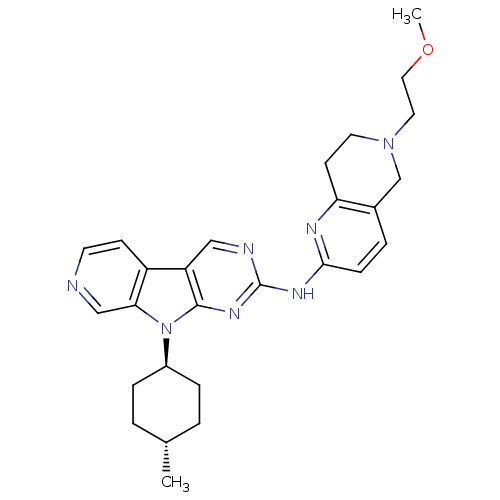
(US8623885, 10)Show SMILES COCCN1CCc2nc(Nc3ncc4c5ccncc5n([C@H]5CC[C@H](C)CC5)c4n3)ccc2C1 |r,wU:22.22,wD:25.26,(-1.6,-9.11,;-2.93,-8.34,;-2.93,-6.8,;-4.26,-6.03,;-4.26,-4.49,;-5.6,-3.72,;-5.6,-2.18,;-4.26,-1.41,;-4.26,.13,;-2.93,.9,;-2.93,2.44,;-1.6,3.21,;-1.6,4.75,;-.26,5.52,;1.07,4.75,;2.53,5.23,;3.16,6.64,;4.69,6.8,;5.6,5.55,;4.97,4.14,;3.44,3.98,;2.53,2.74,;2.93,1.25,;4.42,.85,;4.82,-.64,;3.73,-1.73,;4.13,-3.21,;2.24,-1.33,;1.84,.16,;1.07,3.21,;-.26,2.44,;-1.6,.13,;-1.6,-1.41,;-2.93,-2.18,;-2.93,-3.72,)| Show InChI InChI=1S/C27H33N7O/c1-18-3-6-20(7-4-18)34-24-16-28-11-9-21(24)22-15-29-27(32-26(22)34)31-25-8-5-19-17-33(13-14-35-2)12-10-23(19)30-25/h5,8-9,11,15-16,18,20H,3-4,6-7,10,12-14,17H2,1-2H3,(H,29,30,31,32)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501411

(CHEMBL4068742)Show SMILES Cc1nc(N)nc(N[C@@H](C2CC2)c2nc3cccc(Cl)c3c(=O)n2-c2cc[nH]n2)c1C#N |r| Show InChI InChI=1S/C21H18ClN9O/c1-10-12(9-23)18(29-21(24)26-10)28-17(11-5-6-11)19-27-14-4-2-3-13(22)16(14)20(32)31(19)15-7-8-25-30-15/h2-4,7-8,11,17H,5-6H2,1H3,(H,25,30)(H3,24,26,28,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov... |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112462

(US8623885, 3)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CN(CCO)CCc4n3)nc12 |r,wU:4.7,wD:1.0,(4.13,-3.21,;3.73,-1.73,;4.82,-.64,;4.42,.85,;2.93,1.25,;1.84,.16,;2.24,-1.33,;2.53,2.74,;3.44,3.98,;4.97,4.14,;5.6,5.55,;4.69,6.8,;3.16,6.64,;2.53,5.23,;1.07,4.75,;-.26,5.52,;-1.6,4.75,;-1.6,3.21,;-2.93,2.44,;-2.93,.9,;-1.6,.13,;-1.6,-1.41,;-2.93,-2.18,;-2.93,-3.72,;-4.26,-4.49,;-4.26,-6.03,;-2.93,-6.8,;-1.6,-6.03,;-5.6,-3.72,;-5.6,-2.18,;-4.26,-1.41,;-4.26,.13,;-.26,2.44,;1.07,3.21,)| Show InChI InChI=1S/C26H31N7O/c1-17-2-5-19(6-3-17)33-23-15-27-10-8-20(23)21-14-28-26(31-25(21)33)30-24-7-4-18-16-32(12-13-34)11-9-22(18)29-24/h4,7-8,10,14-15,17,19,34H,2-3,5-6,9,11-13,16H2,1H3,(H,28,29,30,31)/t17-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
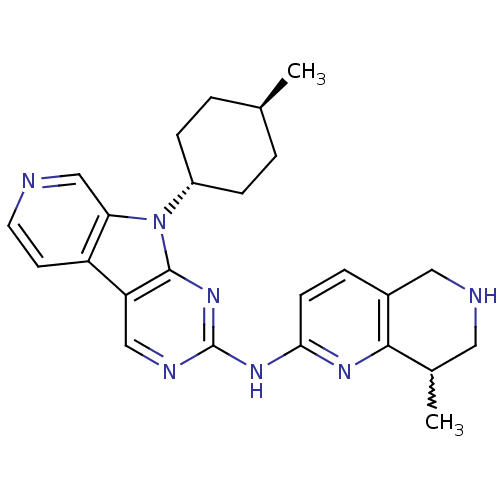
(Homo sapiens (Human)) | BDBM112472
(US8623885, 13)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CNCC(C)c4n3)nc12 |r,w:26.28,wU:4.7,wD:1.0,(4.8,-4.37,;4.4,-2.88,;5.49,-1.79,;5.09,-.3,;3.6,.09,;2.51,-.99,;2.91,-2.48,;3.2,1.58,;4.11,2.83,;5.64,2.99,;6.26,4.4,;5.36,5.64,;3.83,5.48,;3.2,4.07,;1.74,3.6,;.4,4.37,;-.93,3.6,;-.93,2.06,;-2.26,1.29,;-2.26,-.25,;-.93,-1.02,;-.93,-2.56,;-2.26,-3.33,;-2.26,-4.87,;-3.6,-5.64,;-4.93,-4.87,;-4.93,-3.33,;-6.26,-2.56,;-3.6,-2.56,;-3.6,-1.02,;.4,1.29,;1.74,2.06,)| Show InChI InChI=1S/C25H29N7/c1-15-3-6-18(7-4-15)32-21-14-26-10-9-19(21)20-13-28-25(31-24(20)32)30-22-8-5-17-12-27-11-16(2)23(17)29-22/h5,8-10,13-16,18,27H,3-4,6-7,11-12H2,1-2H3,(H,28,29,30,31)/t15-,16?,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
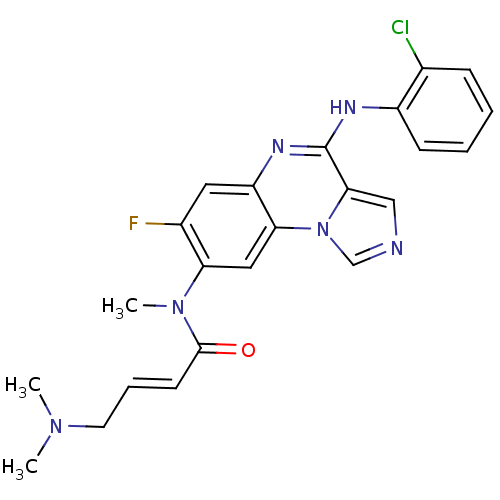
(Homo sapiens (Human)) | BDBM50357334
(CHEMBL1916892)Show SMILES CN(C)C\C=C\C(=O)N(C)c1cc2c(cc1F)nc(Nc1ccccc1Cl)c1cncn21 Show InChI InChI=1S/C23H22ClFN6O/c1-29(2)10-6-9-22(32)30(3)19-12-20-18(11-16(19)25)28-23(21-13-26-14-31(20)21)27-17-8-5-4-7-15(17)24/h4-9,11-14H,10H2,1-3H3,(H,27,28)/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
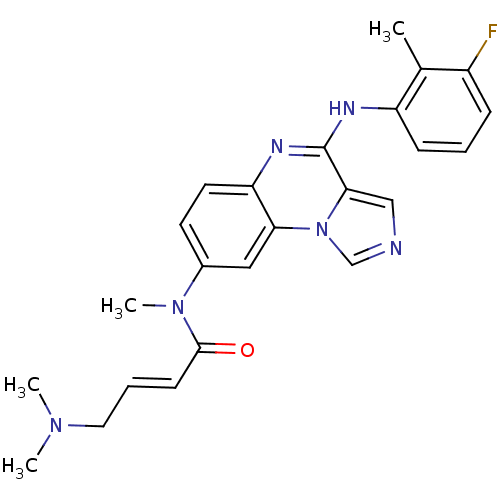
(Homo sapiens (Human)) | BDBM50357324
(CHEMBL1916882)Show SMILES CN(C)C\C=C\C(=O)N(C)c1ccc2nc(Nc3cccc(F)c3C)c3cncn3c2c1 Show InChI InChI=1S/C24H25FN6O/c1-16-18(25)7-5-8-19(16)27-24-22-14-26-15-31(22)21-13-17(10-11-20(21)28-24)30(4)23(32)9-6-12-29(2)3/h5-11,13-15H,12H2,1-4H3,(H,27,28)/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501407

(CHEMBL4059461)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1ccon1 |r| Show InChI InChI=1S/C17H14Cl2N8O2/c1-7(22-14-12(19)13(20)24-17(21)25-14)15-23-9-4-2-3-8(18)11(9)16(28)27(15)10-5-6-29-26-10/h2-7H,1H3,(H5,20,21,22,24,25)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357333

(CHEMBL1916891)Show SMILES CN(C)C\C=C\C(=O)N(C)c1cc2c(cc1F)nc(Nc1ccccc1C)c1cncn21 Show InChI InChI=1S/C24H25FN6O/c1-16-8-5-6-9-18(16)27-24-22-14-26-15-31(22)21-13-20(17(25)12-19(21)28-24)30(4)23(32)10-7-11-29(2)3/h5-10,12-15H,11H2,1-4H3,(H,27,28)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay |
Bioorg Med Chem Lett 21: 6258-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.008
BindingDB Entry DOI: 10.7270/Q21836XD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368657

(US10227350, Compound 105 | US10227350, Compound 10...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(N)c1F)-c1nnc[nH]1 |(-5.25,-.24,;-3.71,-.24,;-3.24,-1.7,;-1.7,-1.7,;-.67,-2.85,;.84,-2.53,;1.32,-1.06,;.28,.08,;-1.22,-.24,;-2.47,.67,;-2.47,2.21,;-1.13,2.98,;-1.13,4.52,;-2.47,5.29,;-3.8,4.52,;-5.13,5.29,;-5.13,6.83,;-6.47,4.52,;-6.47,2.98,;-5.13,2.21,;-5.13,.67,;-3.8,2.98,;2.8,-.66,;3.2,.82,;4.69,1.22,;5.78,.13,;5.38,-1.35,;6.47,-2.44,;3.89,-1.75,;3.49,-3.24,;-1.07,-4.33,;-.16,-5.58,;-1.07,-6.83,;-2.53,-6.35,;-2.53,-4.81,)| Show InChI InChI=1S/C24H15F3N8/c1-11-33-21-14(24-31-10-32-34-24)8-12(13-4-6-30-23(28)20(13)27)9-18(21)35(11)17-5-7-29-22-16(26)3-2-15(25)19(17)22/h2-10H,1H3,(H2,28,30)(H,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His6-tagged p110beta/human recombinant full-length untagged p85alpha expressed in baculovirus ... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001545

(CHEMBL3237710)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(nn3)N3CCC(CC3)N(C)C)nc12 |r,wU:4.7,wD:1.0,(36.03,-51.17,;35.57,-49.71,;36.6,-48.56,;36.13,-47.09,;34.63,-46.77,;33.59,-47.92,;34.06,-49.38,;34.16,-45.31,;35.06,-44.06,;36.59,-43.9,;37.22,-42.5,;36.31,-41.25,;34.79,-41.41,;34.16,-42.81,;32.69,-43.28,;31.34,-42.52,;30.02,-43.3,;30.03,-44.83,;28.7,-45.6,;28.68,-47.13,;30,-47.91,;29.99,-49.43,;28.65,-50.19,;27.33,-49.41,;27.35,-47.88,;28.64,-51.73,;27.29,-52.48,;27.28,-54.01,;28.6,-54.8,;29.94,-54.05,;29.96,-52.5,;28.58,-56.34,;27.24,-57.1,;29.91,-57.13,;31.36,-45.6,;32.69,-44.83,)| Show InChI InChI=1S/C27H35N9/c1-18-4-6-20(7-5-18)36-23-17-28-13-10-21(23)22-16-29-27(31-26(22)36)30-24-8-9-25(33-32-24)35-14-11-19(12-15-35)34(2)3/h8-10,13,16-20H,4-7,11-12,14-15H2,1-3H3,(H,29,30,31,32)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001537

(CHEMBL3237702 | US8841312, 55)Show SMILES CC1CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 |(35.98,-56.71,;35.51,-55.25,;34.01,-54.92,;33.54,-53.46,;34.57,-52.32,;36.08,-52.63,;36.55,-54.1,;34.1,-50.85,;35.01,-49.6,;36.54,-49.45,;37.17,-48.04,;36.26,-46.79,;34.73,-46.95,;34.11,-48.35,;32.64,-48.83,;31.29,-48.06,;29.97,-48.84,;29.98,-50.37,;28.65,-51.14,;28.65,-52.68,;29.99,-53.45,;29.99,-55,;28.66,-55.77,;27.32,-55,;27.33,-53.45,;28.66,-57.31,;27.32,-58.08,;27.31,-59.61,;28.64,-60.38,;29.98,-59.62,;29.99,-58.07,;31.31,-51.14,;32.63,-50.37,)| Show InChI InChI=1S/C25H30N8/c1-17-2-4-18(5-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-23-7-6-19(14-28-23)32-12-10-26-11-13-32/h6-9,14-18,26H,2-5,10-13H2,1H3,(H,28,29,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
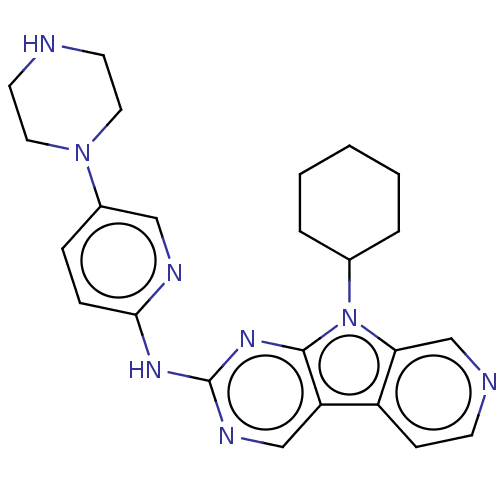
(Homo sapiens (Human)) | BDBM50001536
(CHEMBL3237451 | US8841312, 23)Show SMILES C1CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 Show InChI InChI=1S/C24H28N8/c1-2-4-17(5-3-1)32-21-16-26-9-8-19(21)20-15-28-24(30-23(20)32)29-22-7-6-18(14-27-22)31-12-10-25-11-13-31/h6-9,14-17,25H,1-5,10-13H2,(H,27,28,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112463

(US8623885, 4)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CN(CCCO)CCc4n3)nc12 |r,wU:4.7,wD:1.0,(4.13,-3.21,;3.73,-1.73,;4.82,-.64,;4.42,.85,;2.93,1.25,;1.84,.16,;2.24,-1.33,;2.53,2.74,;3.44,3.98,;4.97,4.14,;5.6,5.55,;4.69,6.8,;3.16,6.64,;2.53,5.23,;1.07,4.75,;-.26,5.52,;-1.6,4.75,;-1.6,3.21,;-2.93,2.44,;-2.93,.9,;-1.6,.13,;-1.6,-1.41,;-2.93,-2.18,;-2.93,-3.72,;-4.26,-4.49,;-4.26,-6.03,;-2.93,-6.8,;-1.6,-6.03,;-.26,-6.8,;-5.6,-3.72,;-5.6,-2.18,;-4.26,-1.41,;-4.26,.13,;-.26,2.44,;1.07,3.21,)| Show InChI InChI=1S/C27H33N7O/c1-18-3-6-20(7-4-18)34-24-16-28-11-9-21(24)22-15-29-27(32-26(22)34)31-25-8-5-19-17-33(12-2-14-35)13-10-23(19)30-25/h5,8-9,11,15-16,18,20,35H,2-4,6-7,10,12-14,17H2,1H3,(H,29,30,31,32)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Amgen Inc.
US Patent
| Assay Description
The FLT3 inhibitory activity of the CDK4/6-FLT3 inhibitors was determined with a HTRF kinase assay. The FLT3 enzyme (GST-FLT3 fusion) was purchased f... |
US Patent US8623885 (2014)
BindingDB Entry DOI: 10.7270/Q2DZ06Z1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6309
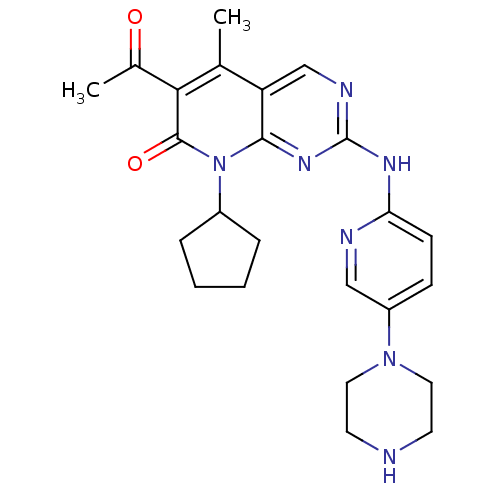
(6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001538

(CHEMBL3237703)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 |r,wU:4.7,wD:1.0,(47.68,-57.06,;47.21,-55.59,;48.24,-54.45,;47.78,-52.99,;46.29,-52.68,;45.25,-53.81,;45.71,-55.26,;45.82,-51.22,;46.72,-49.97,;48.26,-49.81,;48.89,-48.4,;47.98,-47.15,;46.45,-47.31,;45.82,-48.71,;44.35,-49.19,;43,-48.42,;41.68,-49.2,;41.69,-50.73,;40.36,-51.51,;40.36,-53.05,;41.7,-53.81,;41.7,-55.37,;40.36,-56.14,;39.03,-55.37,;39.03,-53.82,;40.36,-57.68,;39.02,-58.45,;39.02,-59.98,;40.35,-60.76,;41.69,-59.99,;41.7,-58.45,;43.02,-51.51,;44.34,-50.74,)| Show InChI InChI=1S/C25H30N8/c1-17-2-4-18(5-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-23-7-6-19(14-28-23)32-12-10-26-11-13-32/h6-9,14-18,26H,2-5,10-13H2,1H3,(H,28,29,30,31)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001544
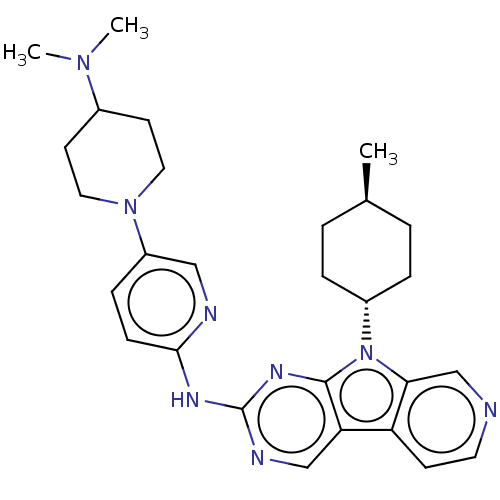
(CHEMBL3237709)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCC(CC3)N(C)C)nc12 |r,wU:4.7,wD:1.0,(23.53,-50.28,;23.06,-48.82,;24.09,-47.68,;23.62,-46.2,;22.12,-45.89,;21.08,-47.03,;21.55,-48.49,;21.65,-44.42,;22.55,-43.17,;24.08,-43.02,;24.72,-41.61,;23.8,-40.36,;22.28,-40.52,;21.65,-41.92,;20.18,-42.4,;18.83,-41.63,;17.51,-42.41,;17.52,-43.94,;16.19,-44.71,;16.17,-46.25,;17.49,-47.02,;17.48,-48.55,;16.14,-49.31,;14.82,-48.53,;14.84,-47,;16.13,-50.84,;14.78,-51.59,;14.77,-53.13,;16.09,-53.92,;17.43,-53.16,;17.45,-51.62,;16.07,-55.46,;14.73,-56.21,;17.4,-56.24,;18.85,-44.71,;20.18,-43.94,)| Show InChI InChI=1S/C28H36N8/c1-19-4-6-21(7-5-19)36-25-18-29-13-10-23(25)24-17-31-28(33-27(24)36)32-26-9-8-22(16-30-26)35-14-11-20(12-15-35)34(2)3/h8-10,13,16-21H,4-7,11-12,14-15H2,1-3H3,(H,30,31,32,33)/t19-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data