

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50341447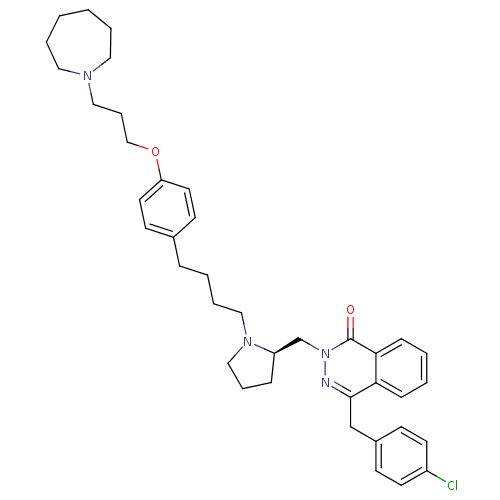 (4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205359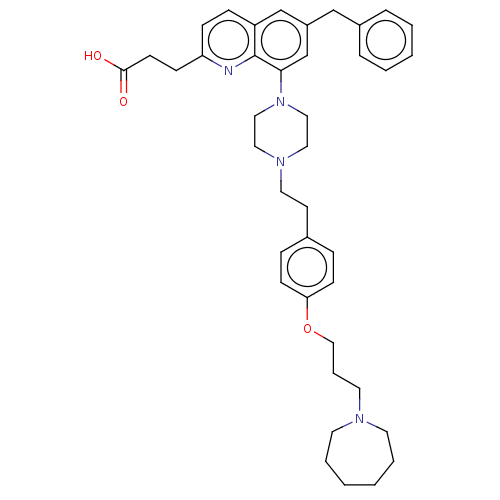 (CHEMBL3917428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205360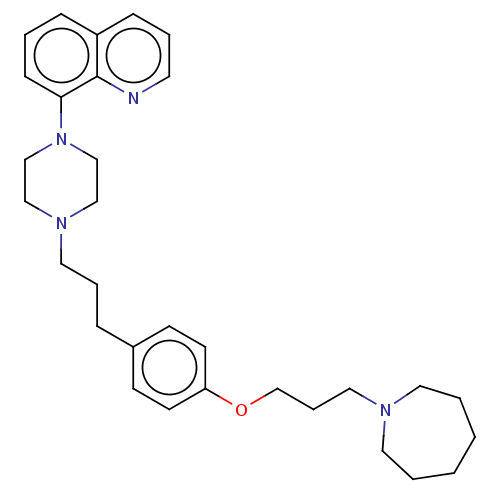 (CHEMBL3921827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205367 (CHEMBL3917794) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205353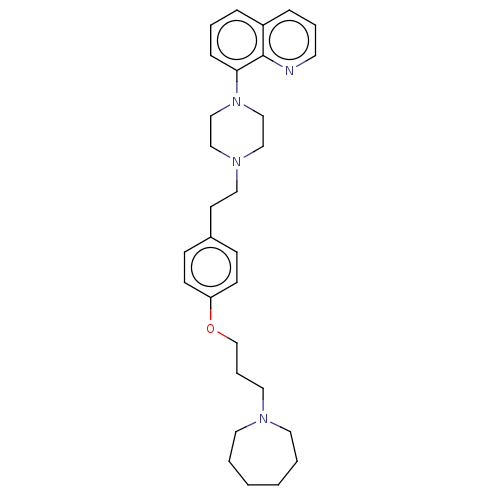 (CHEMBL3947980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205361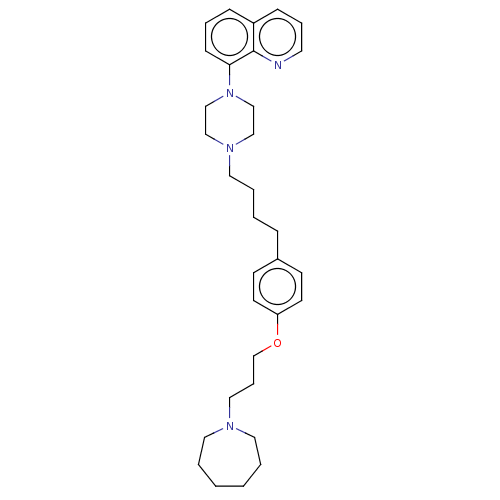 (CHEMBL3893197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205358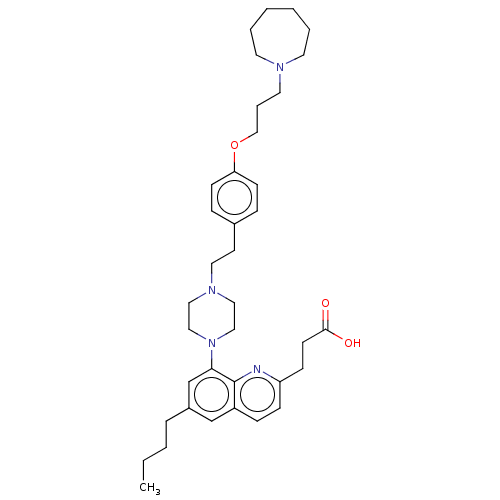 (CHEMBL3898341) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50341448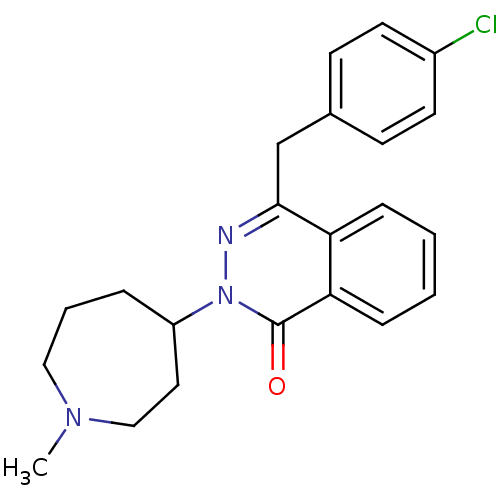 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205369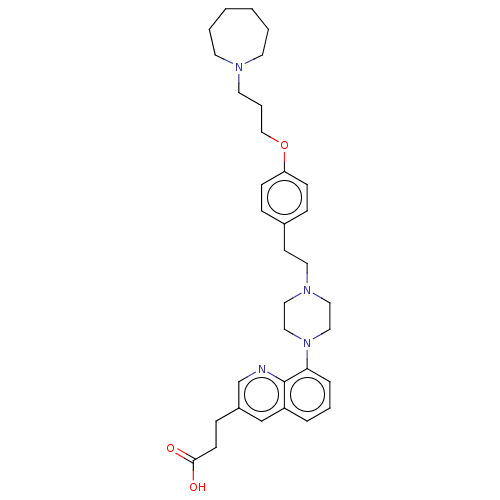 (CHEMBL3907368) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205365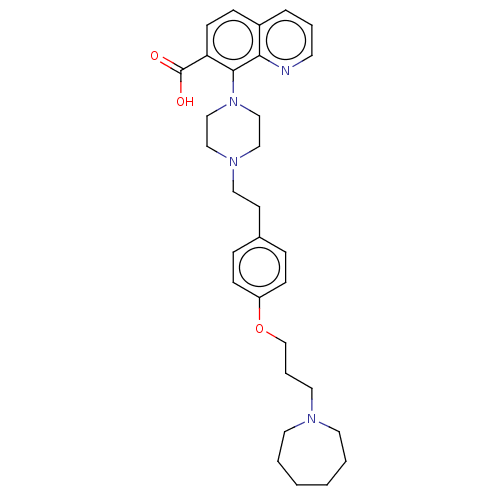 (CHEMBL3925977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205362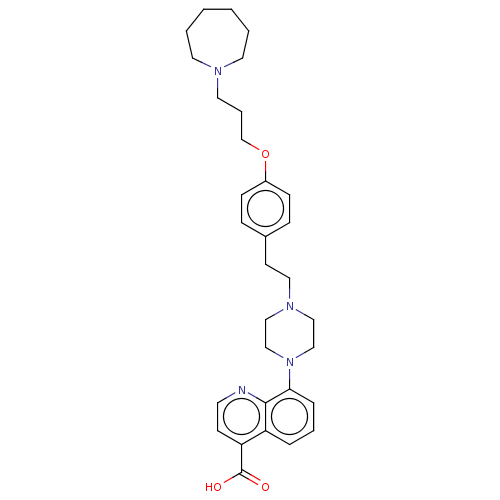 (CHEMBL3896541) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205364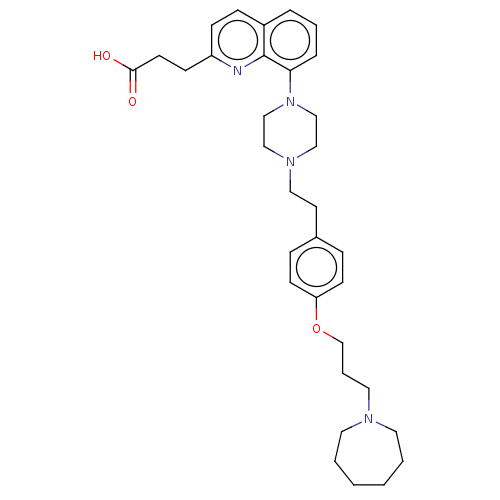 (CHEMBL3935303) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205355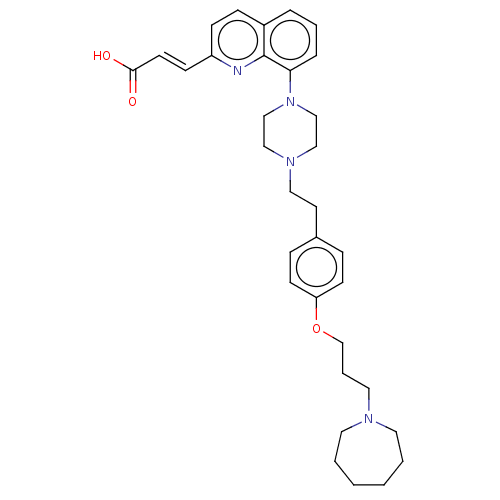 (CHEMBL3952802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205356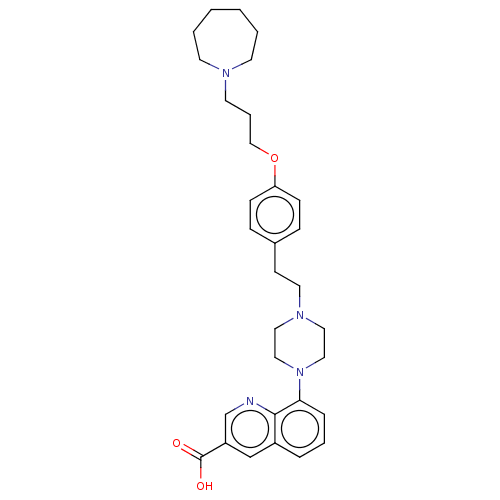 (CHEMBL3967709) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205357 (CHEMBL3925332) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205354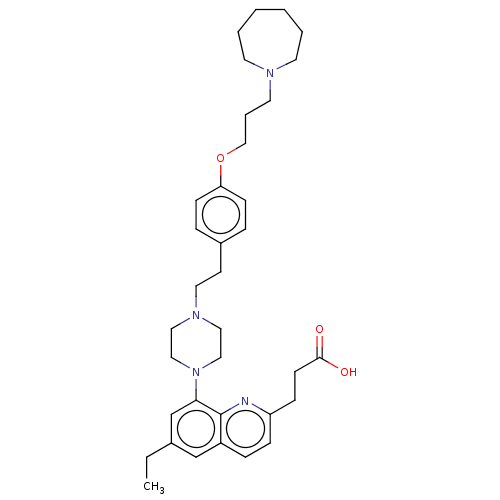 (CHEMBL3926412) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205366 (CHEMBL3953018) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50205353 (CHEMBL3947980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50341447 (4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205364 (CHEMBL3935303) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205358 (CHEMBL3898341) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205354 (CHEMBL3926412) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205361 (CHEMBL3893197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50205353 (CHEMBL3947980) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205353 (CHEMBL3947980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205359 (CHEMBL3917428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50341447 (4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50205360 (CHEMBL3921827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205369 (CHEMBL3907368) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50341447 (4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50341448 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50205361 (CHEMBL3893197) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50205359 (CHEMBL3917428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50341448 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50205355 (CHEMBL3952802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50205361 (CHEMBL3893197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50205355 (CHEMBL3952802) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205360 (CHEMBL3921827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50205360 (CHEMBL3921827) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50341448 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205355 (CHEMBL3952802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50205364 (CHEMBL3935303) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205367 (CHEMBL3917794) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50205359 (CHEMBL3917428) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50205354 (CHEMBL3926412) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50205358 (CHEMBL3898341) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205356 (CHEMBL3967709) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50205358 (CHEMBL3898341) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50205366 (CHEMBL3953018) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50205354 (CHEMBL3926412) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1B receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 390 total ) | Next | Last >> |