

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50349203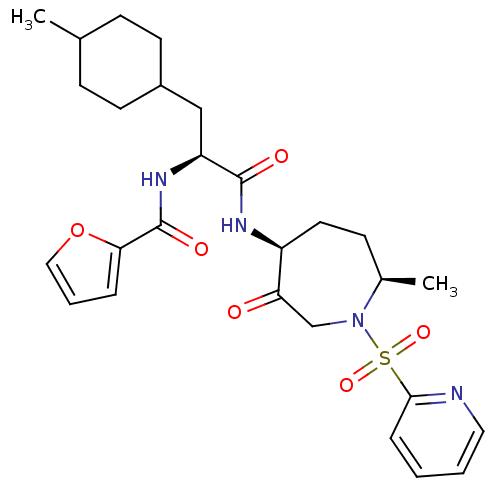 (CHEMBL1807698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349197 (CHEMBL1807647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349198 (CHEMBL1807649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349205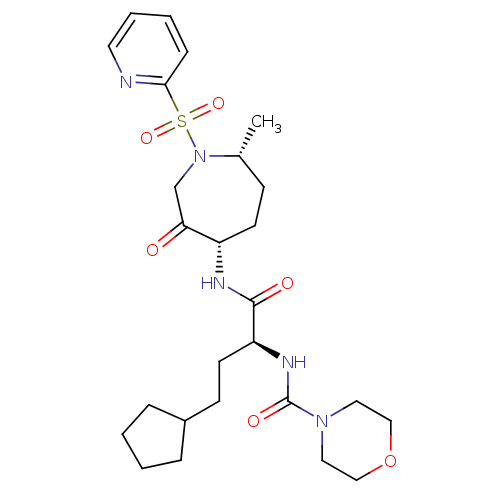 (CHEMBL1807700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349196 (CHEMBL1807646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50349198 (CHEMBL1807649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349203 (CHEMBL1807698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349197 (CHEMBL1807647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349204 (CHEMBL1807699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349199 (CHEMBL1807650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349207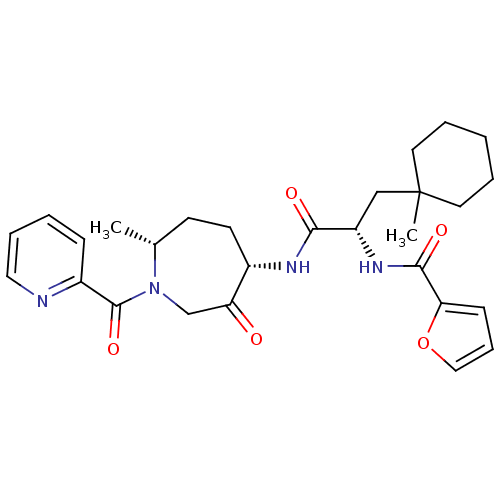 (CHEMBL1807703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349206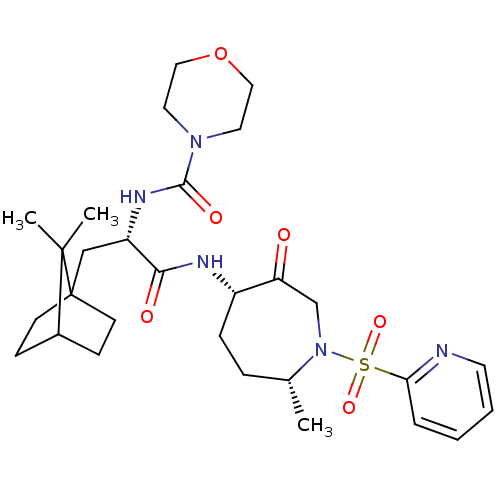 (CHEMBL1807702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349202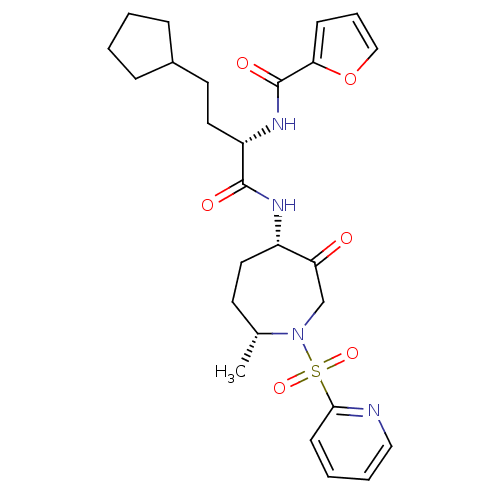 (CHEMBL1807697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349198 (CHEMBL1807649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349195 (CHEMBL1807645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50349199 (CHEMBL1807650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349196 (CHEMBL1807646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50349195 (CHEMBL1807645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507365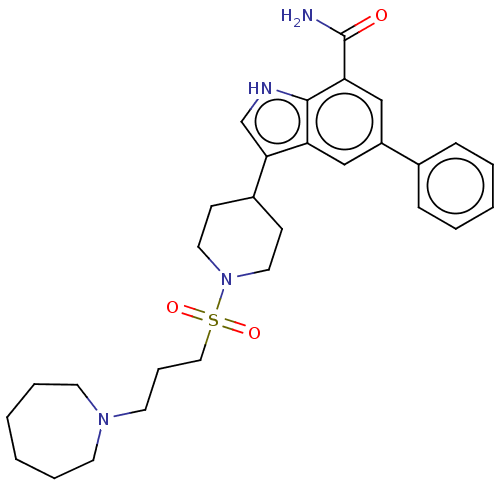 (CHEMBL4522601) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507345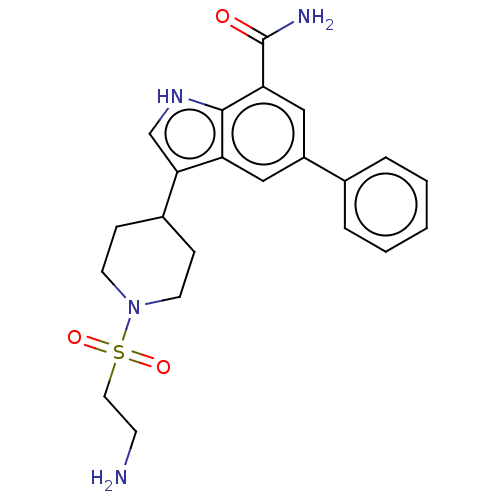 (CHEMBL4457925) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507355 (CHEMBL4565091) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507357 (CHEMBL4462412) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507362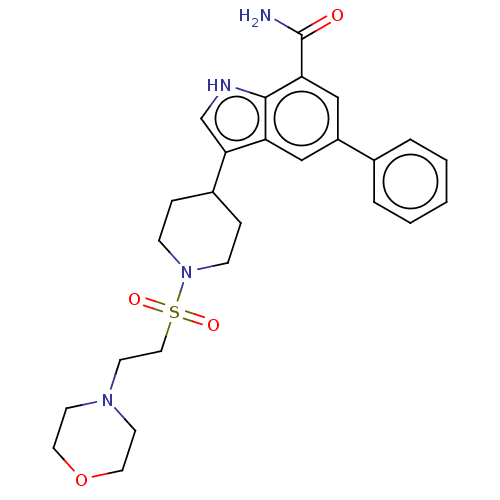 (CHEMBL4473469) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507354 (CHEMBL4577079) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50418169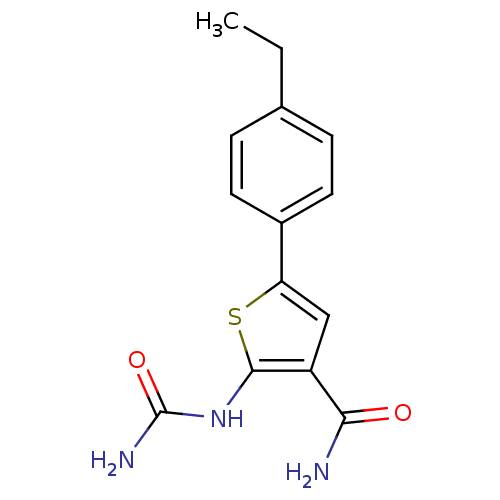 (CHEMBL1761516) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of IKK-beta | Bioorg Med Chem Lett 21: 2255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.107 BindingDB Entry DOI: 10.7270/Q2GF0VS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50098576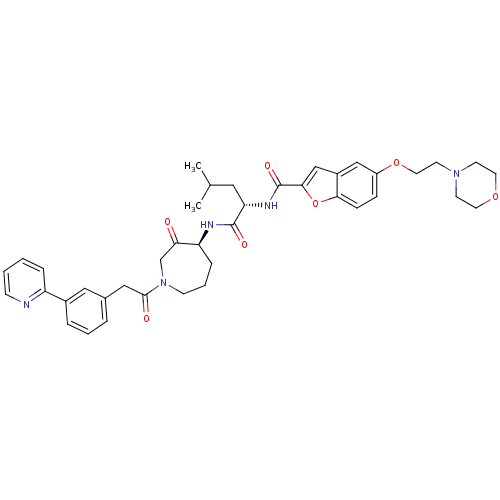 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50349195 (CHEMBL1807645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507356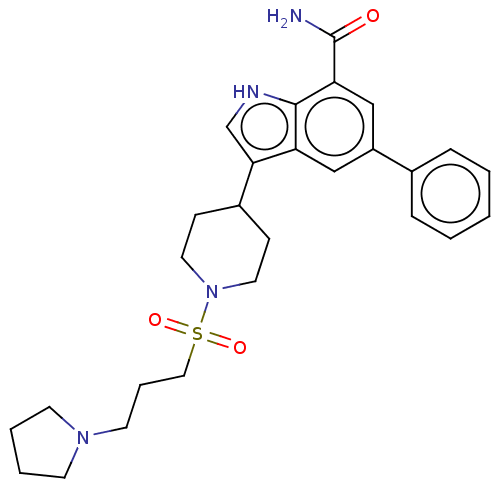 (CHEMBL4436805) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304732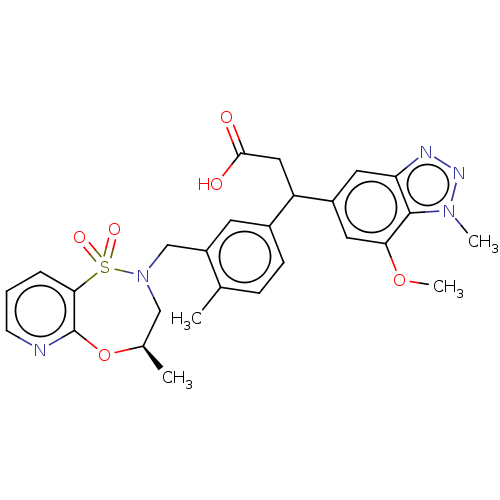 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507359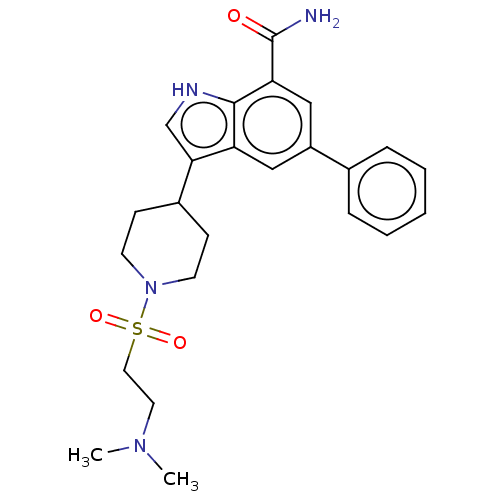 (CHEMBL4529539) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM304727 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... | J Med Chem 62: 4683-4702 (2019) Article DOI: 10.1021/acs.jmedchem.9b00279 BindingDB Entry DOI: 10.7270/Q2PK0KKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304755 (3-(3-(((R)-4-ethyl-1,1-dioxido-3,4-dihydro-2H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304887 (3-(3-(((R)-4-ethyl-1,1-dioxido-3,4-dihydro-2H-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304734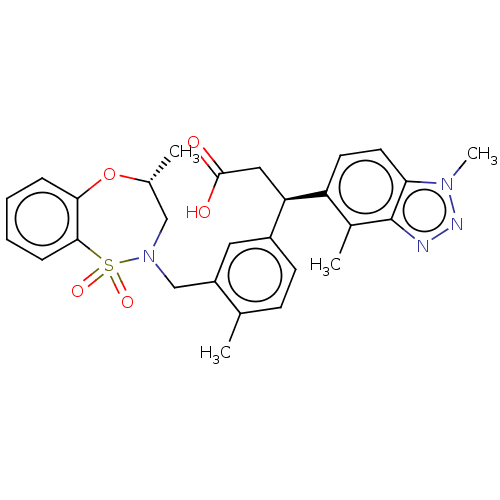 ((R)-3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304736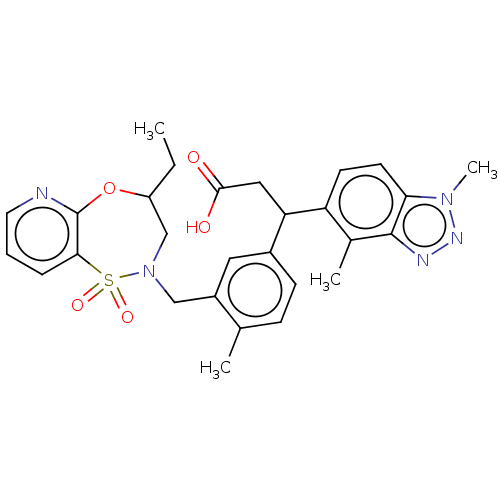 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304737 (3-(3-((4-ethyl-1,1-dioxido-3,4-dihydro-2H-benzo[b]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304738 (3-(3-((4-ethyl-1,1-dioxido-3,4-dihydro-2H-pyrido[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304740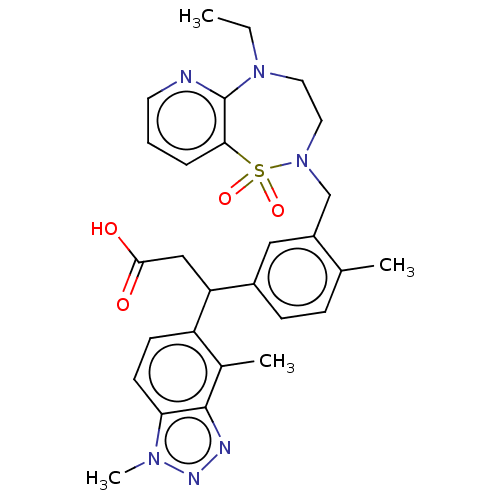 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50349208 (CHEMBL1807704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507364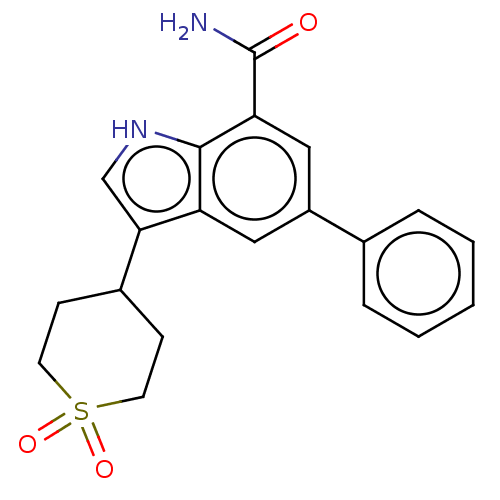 (CHEMBL4590548) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304730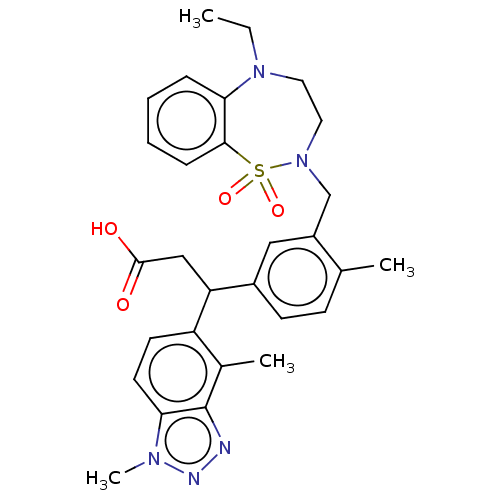 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349208 (CHEMBL1807704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304725 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304727 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349202 (CHEMBL1807697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349205 (CHEMBL1807700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50349210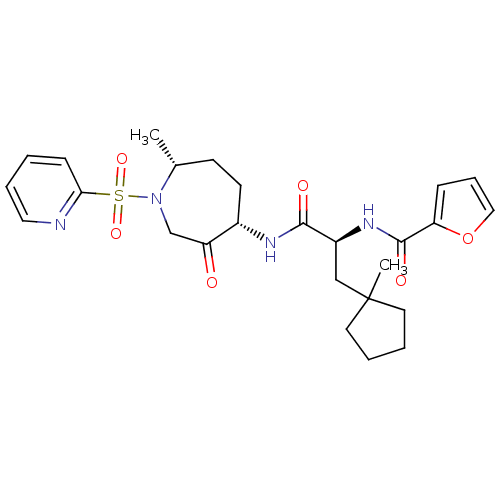 (CHEMBL1807651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50349199 (CHEMBL1807650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 21: 4409-15 (2011) Article DOI: 10.1016/j.bmcl.2011.06.045 BindingDB Entry DOI: 10.7270/Q2TT4R90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50418165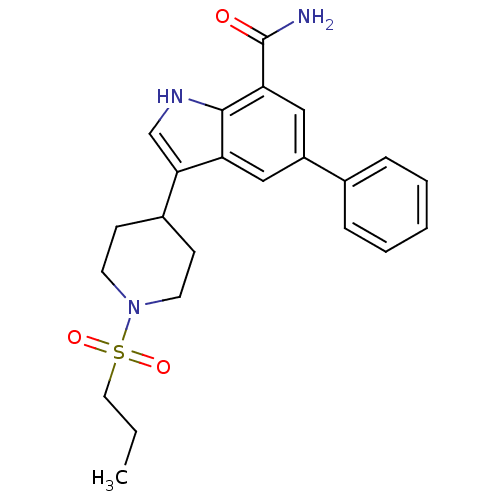 (CHEMBL1761512) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of IKK-beta | Bioorg Med Chem Lett 21: 2255-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.107 BindingDB Entry DOI: 10.7270/Q2GF0VS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50507353 (CHEMBL4564744) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged IKKbeta (1 to 737 residues) expressed in baculovirus expression system using GST-IkBalpha subst... | ACS Med Chem Lett 9: 1164-1169 (2018) Article DOI: 10.1021/acsmedchemlett.8b00291 BindingDB Entry DOI: 10.7270/Q24F1V1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1083 total ) | Next | Last >> |