 Found 335 hits with Last Name = 'kerwar' and Initial = 's'
Found 335 hits with Last Name = 'kerwar' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282079
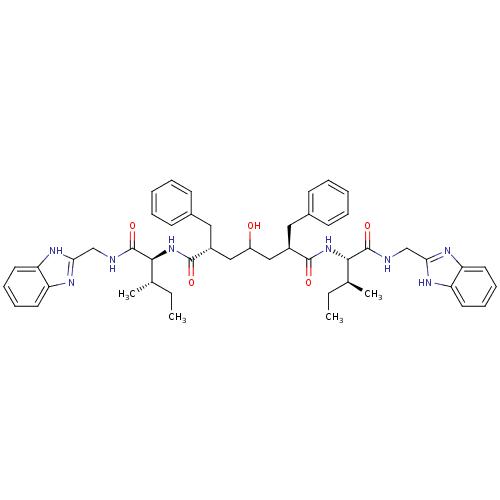
((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCc1nc2ccccc2[nH]1)Cc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C49H60N8O5/c1-5-31(3)44(48(61)50-29-42-52-38-21-13-14-22-39(38)53-42)56-46(59)35(25-33-17-9-7-10-18-33)27-37(58)28-36(26-34-19-11-8-12-20-34)47(60)57-45(32(4)6-2)49(62)51-30-43-54-40-23-15-16-24-41(40)55-43/h7-24,31-32,35-37,44-45,58H,5-6,25-30H2,1-4H3,(H,50,61)(H,51,62)(H,52,53)(H,54,55)(H,56,59)(H,57,60)/t31-,32-,35+,36+,44-,45-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against HIV-1 protease using [125I]-SPA (scintillation proximity assay) |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282079

((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCc1nc2ccccc2[nH]1)Cc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C49H60N8O5/c1-5-31(3)44(48(61)50-29-42-52-38-21-13-14-22-39(38)53-42)56-46(59)35(25-33-17-9-7-10-18-33)27-37(58)28-36(26-34-19-11-8-12-20-34)47(60)57-45(32(4)6-2)49(62)51-30-43-54-40-23-15-16-24-41(40)55-43/h7-24,31-32,35-37,44-45,58H,5-6,25-30H2,1-4H3,(H,50,61)(H,51,62)(H,52,53)(H,54,55)(H,56,59)(H,57,60)/t31-,32-,35+,36+,44-,45-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282083
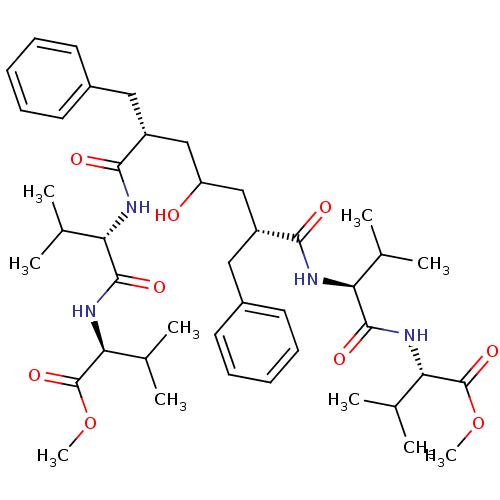
((S)-2-((S)-2-{(2R,6R)-2-Benzyl-4-hydroxy-6-[(S)-1-...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)Cc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C43H64N4O9/c1-25(2)34(40(51)46-36(27(5)6)42(53)55-9)44-38(49)31(21-29-17-13-11-14-18-29)23-33(48)24-32(22-30-19-15-12-16-20-30)39(50)45-35(26(3)4)41(52)47-37(28(7)8)43(54)56-10/h11-20,25-28,31-37,48H,21-24H2,1-10H3,(H,44,49)(H,45,50)(H,46,51)(H,47,52)/t31-,32-,34+,35+,36+,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282077
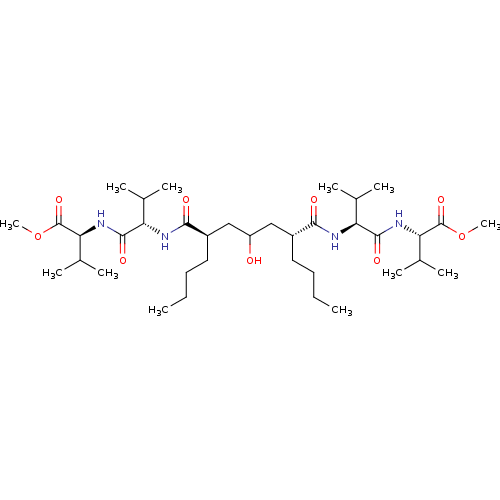
((S)-2-((S)-2-{(2R,6R)-2-Butyl-4-hydroxy-6-[(S)-1-(...)Show SMILES CCCC[C@H](CC(O)C[C@@H](CCCC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC Show InChI InChI=1S/C37H68N4O9/c1-13-15-17-25(32(43)38-28(21(3)4)34(45)40-30(23(7)8)36(47)49-11)19-27(42)20-26(18-16-14-2)33(44)39-29(22(5)6)35(46)41-31(24(9)10)37(48)50-12/h21-31,42H,13-20H2,1-12H3,(H,38,43)(H,39,44)(H,40,45)(H,41,46)/t25-,26-,28+,29+,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282076
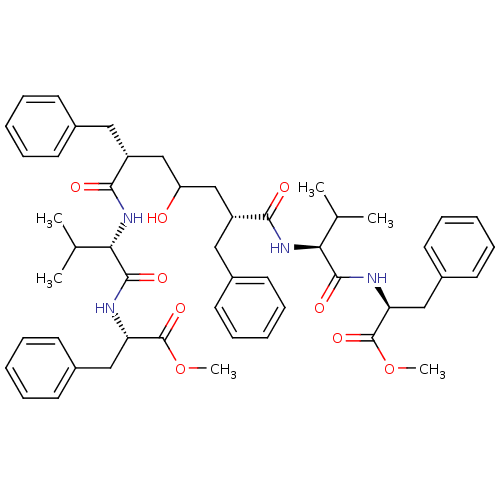
((S)-2-((S)-2-{(2R,6R)-2-Benzyl-4-hydroxy-6-[(S)-1-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)OC)Cc1ccccc1)C(C)C Show InChI InChI=1S/C51H64N4O9/c1-33(2)44(48(59)52-42(50(61)63-5)29-37-23-15-9-16-24-37)54-46(57)39(27-35-19-11-7-12-20-35)31-41(56)32-40(28-36-21-13-8-14-22-36)47(58)55-45(34(3)4)49(60)53-43(51(62)64-6)30-38-25-17-10-18-26-38/h7-26,33-34,39-45,56H,27-32H2,1-6H3,(H,52,59)(H,53,60)(H,54,57)(H,55,58)/t39-,40-,42+,43+,44+,45+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282087
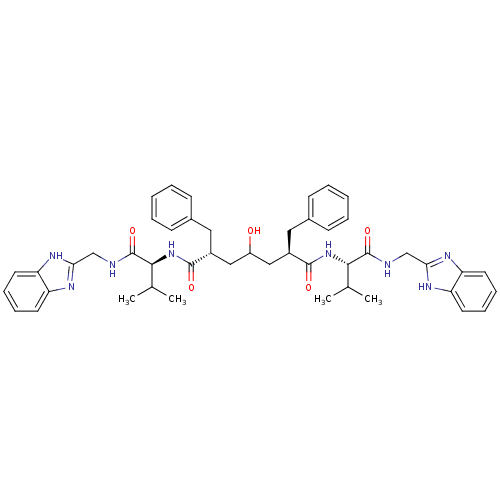
((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)NCc1nc2ccccc2[nH]1)Cc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C47H56N8O5/c1-29(2)42(46(59)48-27-40-50-36-19-11-12-20-37(36)51-40)54-44(57)33(23-31-15-7-5-8-16-31)25-35(56)26-34(24-32-17-9-6-10-18-32)45(58)55-43(30(3)4)47(60)49-28-41-52-38-21-13-14-22-39(38)53-41/h5-22,29-30,33-35,42-43,56H,23-28H2,1-4H3,(H,48,59)(H,49,60)(H,50,51)(H,52,53)(H,54,57)(H,55,58)/t33-,34-,42+,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50005232
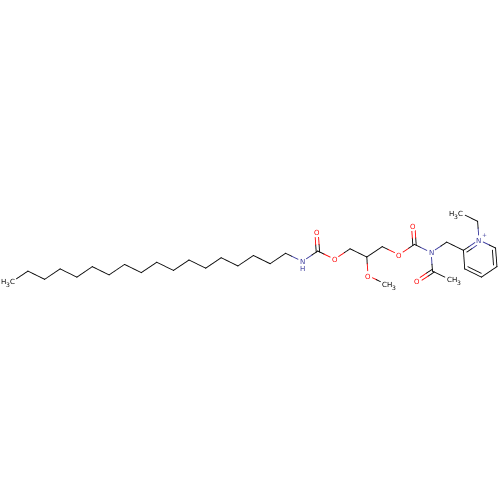
((R)-2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-...)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC(COC(=O)N(Cc1cccc[n+]1CC)C(C)=O)OC Show InChI InChI=1S/C34H59N3O6/c1-5-7-8-9-10-11-12-13-14-15-16-17-18-19-20-22-25-35-33(39)42-28-32(41-4)29-43-34(40)37(30(3)38)27-31-24-21-23-26-36(31)6-2/h21,23-24,26,32H,5-20,22,25,27-29H2,1-4H3/p+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF-induced platelet aggregation in rabbit platelet rich plasma |
J Med Chem 36: 580-90 (1993)
BindingDB Entry DOI: 10.7270/Q2SX6C9X |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50047776
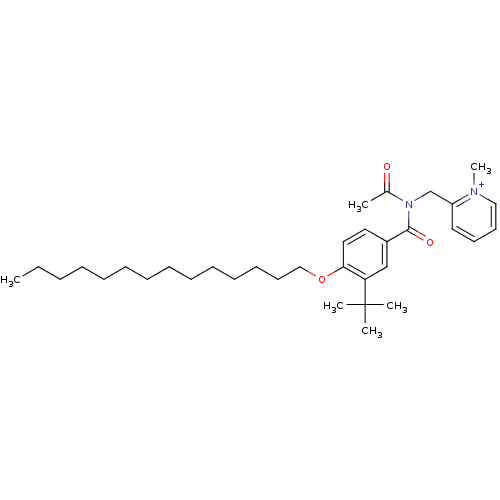
(2-{[Acetyl-(3-tert-butyl-4-tetradecyloxy-benzoyl)-...)Show SMILES CCCCCCCCCCCCCCOc1ccc(cc1C(C)(C)C)C(=O)N(Cc1cccc[n+]1C)C(C)=O Show InChI InChI=1S/C34H53N2O3/c1-7-8-9-10-11-12-13-14-15-16-17-20-25-39-32-23-22-29(26-31(32)34(3,4)5)33(38)36(28(2)37)27-30-21-18-19-24-35(30)6/h18-19,21-24,26H,7-17,20,25,27H2,1-6H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF-induced platelet aggregation in rabbit platelet rich plasma |
J Med Chem 36: 580-90 (1993)
BindingDB Entry DOI: 10.7270/Q2SX6C9X |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282081
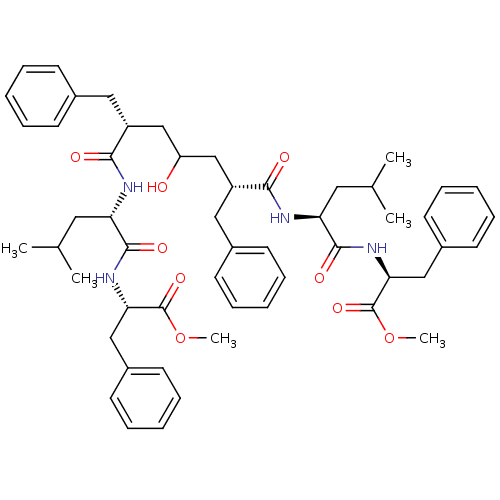
((S)-2-((S)-2-{(2R,6R)-2-Benzyl-4-hydroxy-6-[(S)-1-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)OC)Cc1ccccc1 Show InChI InChI=1S/C53H68N4O9/c1-35(2)27-44(50(61)56-46(52(63)65-5)31-39-23-15-9-16-24-39)54-48(59)41(29-37-19-11-7-12-20-37)33-43(58)34-42(30-38-21-13-8-14-22-38)49(60)55-45(28-36(3)4)51(62)57-47(53(64)66-6)32-40-25-17-10-18-26-40/h7-26,35-36,41-47,58H,27-34H2,1-6H3,(H,54,59)(H,55,60)(H,56,61)(H,57,62)/t41-,42-,44+,45+,46+,47+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282097

((3S,4aS,8aS)-2-((R)-4-{(1S,2S)-1-[(1H-Benzoimidazo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)CC(O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C37H60N6O4/c1-8-24(4)33(36(47)38-20-32-39-29-15-11-12-16-30(29)40-32)41-34(45)27(17-23(2)3)18-28(44)22-43-21-26-14-10-9-13-25(26)19-31(43)35(46)42-37(5,6)7/h11-12,15-16,23-28,31,33,44H,8-10,13-14,17-22H2,1-7H3,(H,38,47)(H,39,40)(H,41,45)(H,42,46)/t24-,25-,26+,27+,28?,31-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282098

(1-((R)-4-{(1S,2S)-1-[(1H-Benzoimidazol-2-ylmethyl)...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)CN1CCCCC1C(=O)NC(C)(C)C)Cc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C36H52N6O4/c1-6-24(2)32(35(46)37-22-31-38-28-16-10-11-17-29(28)39-31)40-33(44)26(20-25-14-8-7-9-15-25)21-27(43)23-42-19-13-12-18-30(42)34(45)41-36(3,4)5/h7-11,14-17,24,26-27,30,32,43H,6,12-13,18-23H2,1-5H3,(H,37,46)(H,38,39)(H,40,44)(H,41,45)/t24-,26+,27?,30?,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403198
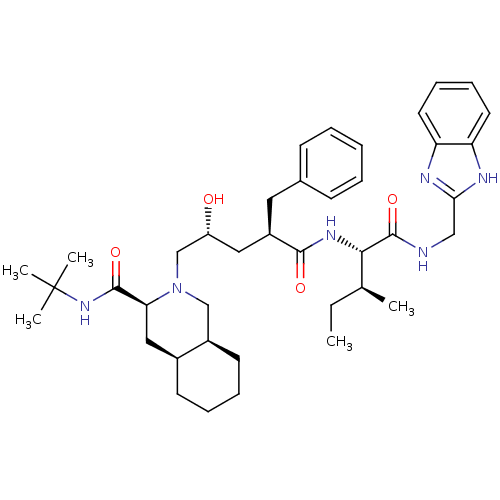
(CHEMBL2114445)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C)Cc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C40H58N6O4/c1-6-26(2)36(39(50)41-23-35-42-32-18-12-13-19-33(32)43-35)44-37(48)30(20-27-14-8-7-9-15-27)21-31(47)25-46-24-29-17-11-10-16-28(29)22-34(46)38(49)45-40(3,4)5/h7-9,12-15,18-19,26,28-31,34,36,47H,6,10-11,16-17,20-25H2,1-5H3,(H,41,50)(H,42,43)(H,44,48)(H,45,49)/t26-,28-,29+,30+,31+,34-,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403197
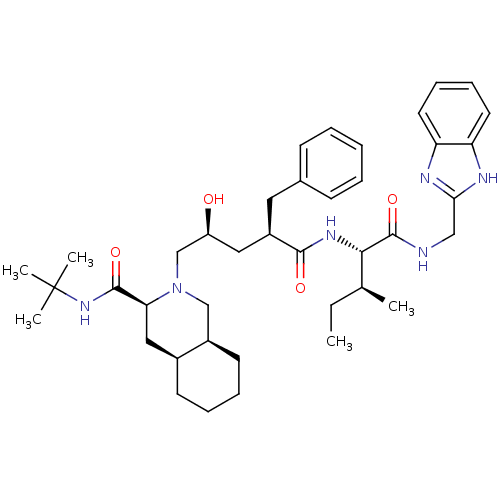
(CHEMBL2115378)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C)Cc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C40H58N6O4/c1-6-26(2)36(39(50)41-23-35-42-32-18-12-13-19-33(32)43-35)44-37(48)30(20-27-14-8-7-9-15-27)21-31(47)25-46-24-29-17-11-10-16-28(29)22-34(46)38(49)45-40(3,4)5/h7-9,12-15,18-19,26,28-31,34,36,47H,6,10-11,16-17,20-25H2,1-5H3,(H,41,50)(H,42,43)(H,44,48)(H,45,49)/t26-,28-,29+,30+,31-,34-,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284036
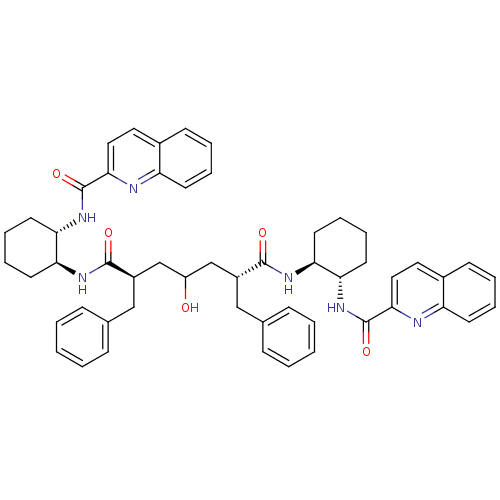
((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...)Show SMILES OC(C[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1ccc2ccccc2n1)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1ccc2ccccc2n1 Show InChI InChI=1S/C53H58N6O5/c60-41(33-39(31-35-15-3-1-4-16-35)50(61)56-44-23-11-13-25-46(44)58-52(63)48-29-27-37-19-7-9-21-42(37)54-48)34-40(32-36-17-5-2-6-18-36)51(62)57-45-24-12-14-26-47(45)59-53(64)49-30-28-38-20-8-10-22-43(38)55-49/h1-10,15-22,27-30,39-41,44-47,60H,11-14,23-26,31-34H2,(H,56,61)(H,57,62)(H,58,63)(H,59,64)/t39-,40-,44+,45+,46+,47+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against recombinant HIV-1 protease using 125 I-SPA (scintillation proximity assay) |
Bioorg Med Chem Lett 4: 583-588 (1994)
Article DOI: 10.1016/S0960-894X(01)80159-1
BindingDB Entry DOI: 10.7270/Q2Q24061 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284034
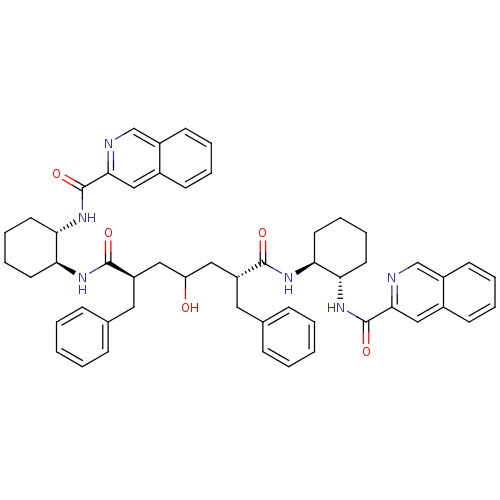
((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...)Show SMILES OC(C[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1cc2ccccc2cn1)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1cc2ccccc2cn1 Show InChI InChI=1S/C53H58N6O5/c60-43(29-41(27-35-15-3-1-4-16-35)50(61)56-44-23-11-13-25-46(44)58-52(63)48-31-37-19-7-9-21-39(37)33-54-48)30-42(28-36-17-5-2-6-18-36)51(62)57-45-24-12-14-26-47(45)59-53(64)49-32-38-20-8-10-22-40(38)34-55-49/h1-10,15-22,31-34,41-47,60H,11-14,23-30H2,(H,56,61)(H,57,62)(H,58,63)(H,59,64)/t41-,42-,44+,45+,46+,47+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against recombinant HIV-1 protease using 125 I-SPA (scintillation proximity assay) |
Bioorg Med Chem Lett 4: 583-588 (1994)
Article DOI: 10.1016/S0960-894X(01)80159-1
BindingDB Entry DOI: 10.7270/Q2Q24061 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50047782

(2-{[Acetyl-(3-methoxy-4-tetradecyloxy-benzoyl)-ami...)Show SMILES CCCCCCCCCCCCCCOc1ccc(cc1OC)C(=O)N(Cc1cccc[n+]1C)C(C)=O Show InChI InChI=1S/C31H47N2O4/c1-5-6-7-8-9-10-11-12-13-14-15-18-23-37-29-21-20-27(24-30(29)36-4)31(35)33(26(2)34)25-28-19-16-17-22-32(28)3/h16-17,19-22,24H,5-15,18,23,25H2,1-4H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF-induced platelet aggregation in rabbit platelet rich plasma |
J Med Chem 36: 580-90 (1993)
BindingDB Entry DOI: 10.7270/Q2SX6C9X |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282103

((3S,4aS,8aS)-2-((R)-4-{(1S,2S)-1-[(1H-Benzoimidazo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)CC(O)(CO)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C41H60N6O5/c1-6-27(2)36(39(51)42-23-35-43-32-18-12-13-19-33(32)44-35)45-37(49)31(20-28-14-8-7-9-15-28)22-41(52,26-48)25-47-24-30-17-11-10-16-29(30)21-34(47)38(50)46-40(3,4)5/h7-9,12-15,18-19,27,29-31,34,36,48,52H,6,10-11,16-17,20-26H2,1-5H3,(H,42,51)(H,43,44)(H,45,49)(H,46,50)/t27-,29-,30+,31+,34-,36-,41?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282092
(CHEMBL412878 | Isoquinoline-3-carboxylic acid {(1S...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1cc2ccccc2cn1 Show InChI InChI=1S/C42H57N5O4/c1-42(2,3)46-41(51)38-24-30-16-8-10-18-32(30)26-47(38)27-34(48)22-33(21-28-13-5-4-6-14-28)39(49)44-35-19-11-12-20-36(35)45-40(50)37-23-29-15-7-9-17-31(29)25-43-37/h4-7,9,13-15,17,23,25,30,32-36,38,48H,8,10-12,16,18-22,24,26-27H2,1-3H3,(H,44,49)(H,45,50)(H,46,51)/t30-,32+,33+,34?,35-,36-,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282095
(CHEMBL291155 | Quinoline-2-carboxylic acid {(1S,2S...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1ccc2ccccc2n1 Show InChI InChI=1S/C42H57N5O4/c1-42(2,3)46-41(51)38-25-30-16-7-8-17-31(30)26-47(38)27-33(48)24-32(23-28-13-5-4-6-14-28)39(49)44-35-19-11-12-20-36(35)45-40(50)37-22-21-29-15-9-10-18-34(29)43-37/h4-6,9-10,13-15,18,21-22,30-33,35-36,38,48H,7-8,11-12,16-17,19-20,23-27H2,1-3H3,(H,44,49)(H,45,50)(H,46,51)/t30-,31+,32+,33?,35-,36-,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282078
((S)-2-((S)-2-{(2R,6R)-2-Butyl-4-hydroxy-6-[(S)-1-(...)Show SMILES CCCC[C@H](CC(O)C[C@@H](CCCC)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)OC)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)OC Show InChI InChI=1S/C29H52N4O9/c1-9-11-13-21(26(37)30-17(3)24(35)32-19(5)28(39)41-7)15-23(34)16-22(14-12-10-2)27(38)31-18(4)25(36)33-20(6)29(40)42-8/h17-23,34H,9-16H2,1-8H3,(H,30,37)(H,31,38)(H,32,35)(H,33,36)/t17-,18-,19-,20-,21+,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50138607
(2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(CC(=O)N[C@H]4CCCc5ccccc45)CC3)ccc2s1 Show InChI InChI=1S/C27H34N4O3S/c1-19-28-25-15-22(9-10-26(25)35-19)34-18-21(32)16-30-11-13-31(14-12-30)17-27(33)29-24-8-4-6-20-5-2-3-7-23(20)24/h2-3,5,7,9-10,15,21,24,32H,4,6,8,11-14,16-18H2,1H3,(H,29,33)/t21-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. |
Bioorg Med Chem Lett 14: 549-52 (2003)
BindingDB Entry DOI: 10.7270/Q2GH9HD4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282091
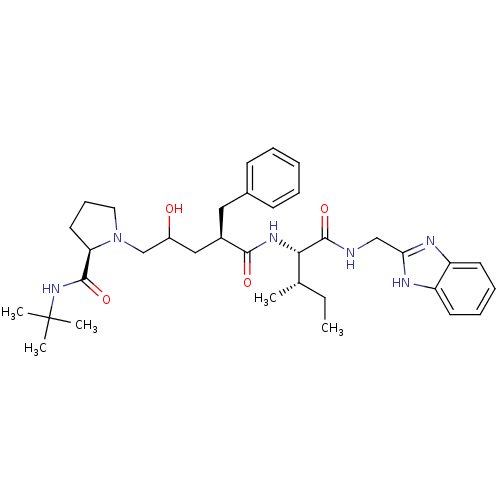
(1-((R)-4-{(1S,2S)-1-[(1H-Benzoimidazol-2-ylmethyl)...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)CN1CCC[C@@H]1C(=O)NC(C)(C)C)Cc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C35H50N6O4/c1-6-23(2)31(34(45)36-21-30-37-27-15-10-11-16-28(27)38-30)39-32(43)25(19-24-13-8-7-9-14-24)20-26(42)22-41-18-12-17-29(41)33(44)40-35(3,4)5/h7-11,13-16,23,25-26,29,31,42H,6,12,17-22H2,1-5H3,(H,36,45)(H,37,38)(H,39,43)(H,40,44)/t23-,25+,26?,29+,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50006082

(3-{3-[(2,5-Di-tert-butyl-4-dodecyloxy-phenoxy)-hyd...)Show SMILES CCCCCCCCCCCCOc1cc(c(OP([O-])(=O)Oc2cccc(C[n+]3csc(C)c3)c2)cc1C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C37H56NO5PS/c1-9-10-11-12-13-14-15-16-17-18-22-41-34-24-33(37(6,7)8)35(25-32(34)36(3,4)5)43-44(39,40)42-31-21-19-20-30(23-31)27-38-26-29(2)45-28-38/h19-21,23-26,28H,9-18,22,27H2,1-8H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF induced platelet aggregation in rabbit |
J Med Chem 35: 1650-62 (1992)
BindingDB Entry DOI: 10.7270/Q2BC4058 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282085

((S)-2-((S)-2-{(2R,6R)-2-Butyl-4-hydroxy-6-[(S)-1-(...)Show SMILES CCCC[C@H](CC(O)C[C@@H](CCCC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)OC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)OC Show InChI InChI=1S/C47H72N4O9/c1-9-11-23-35(42(53)48-38(25-31(3)4)44(55)50-40(46(57)59-7)27-33-19-15-13-16-20-33)29-37(52)30-36(24-12-10-2)43(54)49-39(26-32(5)6)45(56)51-41(47(58)60-8)28-34-21-17-14-18-22-34/h13-22,31-32,35-41,52H,9-12,23-30H2,1-8H3,(H,48,53)(H,49,54)(H,50,55)(H,51,56)/t35-,36-,38+,39+,40+,41+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50047772
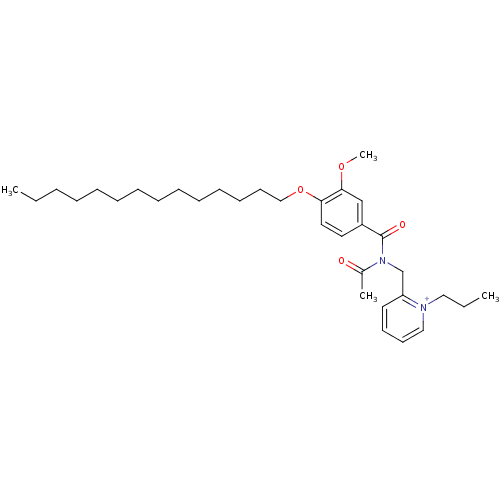
(CHEMBL349327 | Trifluoro-methanesulfonate2-{[acety...)Show SMILES CCCCCCCCCCCCCCOc1ccc(cc1OC)C(=O)N(Cc1cccc[n+]1CCC)C(C)=O Show InChI InChI=1S/C33H51N2O4/c1-5-7-8-9-10-11-12-13-14-15-16-19-25-39-31-22-21-29(26-32(31)38-4)33(37)35(28(3)36)27-30-20-17-18-24-34(30)23-6-2/h17-18,20-22,24,26H,5-16,19,23,25,27H2,1-4H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF-induced platelet aggregation in rabbit platelet rich plasma |
J Med Chem 36: 580-90 (1993)
BindingDB Entry DOI: 10.7270/Q2SX6C9X |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50138628
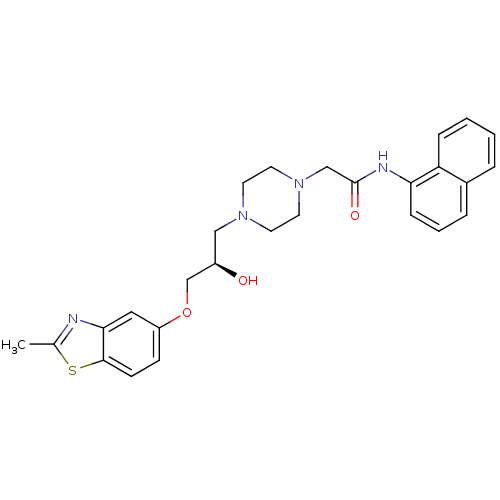
(2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(CC(=O)Nc4cccc5ccccc45)CC3)ccc2s1 Show InChI InChI=1S/C27H30N4O3S/c1-19-28-25-15-22(9-10-26(25)35-19)34-18-21(32)16-30-11-13-31(14-12-30)17-27(33)29-24-8-4-6-20-5-2-3-7-23(20)24/h2-10,15,21,32H,11-14,16-18H2,1H3,(H,29,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. |
Bioorg Med Chem Lett 14: 549-52 (2003)
BindingDB Entry DOI: 10.7270/Q2GH9HD4 |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50156430
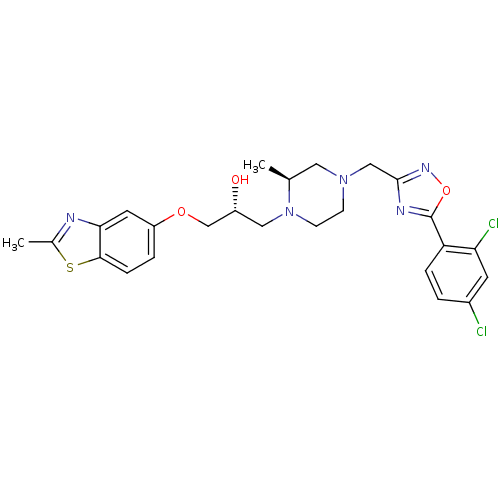
((R)-1-{(S)-4-[5-(2,4-Dichloro-phenyl)-[1,2,4]oxadi...)Show SMILES C[C@H]1CN(Cc2noc(n2)-c2ccc(Cl)cc2Cl)CCN1C[C@@H](O)COc1ccc2sc(C)nc2c1 Show InChI InChI=1S/C25H27Cl2N5O3S/c1-15-11-31(13-24-29-25(35-30-24)20-5-3-17(26)9-21(20)27)7-8-32(15)12-18(33)14-34-19-4-6-23-22(10-19)28-16(2)36-23/h3-6,9-10,15,18,33H,7-8,11-14H2,1-2H3/t15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria |
Bioorg Med Chem Lett 14: 6017-21 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.077
BindingDB Entry DOI: 10.7270/Q2ZS2W0R |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50156430

((R)-1-{(S)-4-[5-(2,4-Dichloro-phenyl)-[1,2,4]oxadi...)Show SMILES C[C@H]1CN(Cc2noc(n2)-c2ccc(Cl)cc2Cl)CCN1C[C@@H](O)COc1ccc2sc(C)nc2c1 Show InChI InChI=1S/C25H27Cl2N5O3S/c1-15-11-31(13-24-29-25(35-30-24)20-5-3-17(26)9-21(20)27)7-8-32(15)12-18(33)14-34-19-4-6-23-22(10-19)28-16(2)36-23/h3-6,9-10,15,18,33H,7-8,11-14H2,1-2H3/t15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria |
Bioorg Med Chem Lett 14: 6017-21 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.077
BindingDB Entry DOI: 10.7270/Q2ZS2W0R |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50156421
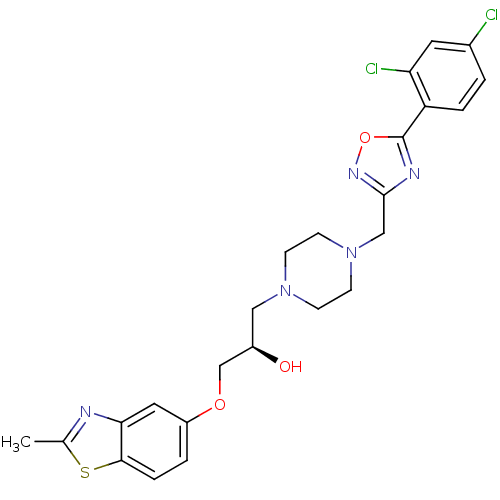
((R)-1-{4-[5-(2,4-Dichloro-phenyl)-[1,2,4]oxadiazol...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(Cc4noc(n4)-c4ccc(Cl)cc4Cl)CC3)ccc2s1 Show InChI InChI=1S/C24H25Cl2N5O3S/c1-15-27-21-11-18(3-5-22(21)35-15)33-14-17(32)12-30-6-8-31(9-7-30)13-23-28-24(34-29-23)19-4-2-16(25)10-20(19)26/h2-5,10-11,17,32H,6-9,12-14H2,1H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria |
Bioorg Med Chem Lett 14: 6017-21 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.077
BindingDB Entry DOI: 10.7270/Q2ZS2W0R |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282099
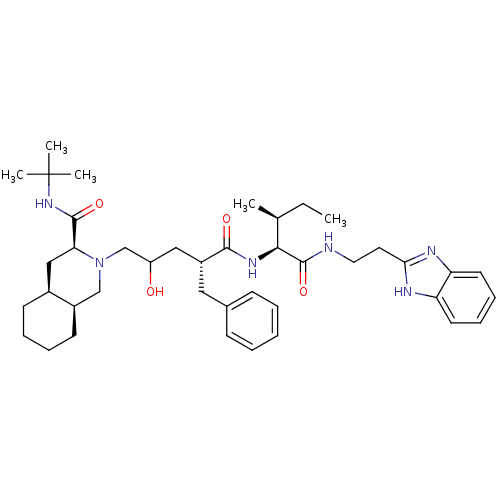
((4S,8aS)-2-((S)-4-{1-[(S,S)-(R)-2-(1H-Benzoimidazo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C)Cc1ccccc1)C(=O)NCCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C41H60N6O4/c1-6-27(2)37(40(51)42-21-20-36-43-33-18-12-13-19-34(33)44-36)45-38(49)31(22-28-14-8-7-9-15-28)23-32(48)26-47-25-30-17-11-10-16-29(30)24-35(47)39(50)46-41(3,4)5/h7-9,12-15,18-19,27,29-32,35,37,48H,6,10-11,16-17,20-26H2,1-5H3,(H,42,51)(H,43,44)(H,45,49)(H,46,50)/t27-,29-,30+,31+,32?,35-,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50006092

(3-{3-[(2,6-Di-tert-butyl-4-dodecyloxy-phenoxy)-hyd...)Show SMILES CCCCCCCCCCCCOc1cc(c(OP([O-])(=O)Oc2cccc(C[n+]3ccsc3)c2)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C36H54NO5PS/c1-8-9-10-11-12-13-14-15-16-17-22-40-31-25-32(35(2,3)4)34(33(26-31)36(5,6)7)42-43(38,39)41-30-20-18-19-29(24-30)27-37-21-23-44-28-37/h18-21,23-26,28H,8-17,22,27H2,1-7H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF induced platelet aggregation in rabbit |
J Med Chem 35: 1650-62 (1992)
BindingDB Entry DOI: 10.7270/Q2BC4058 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282100
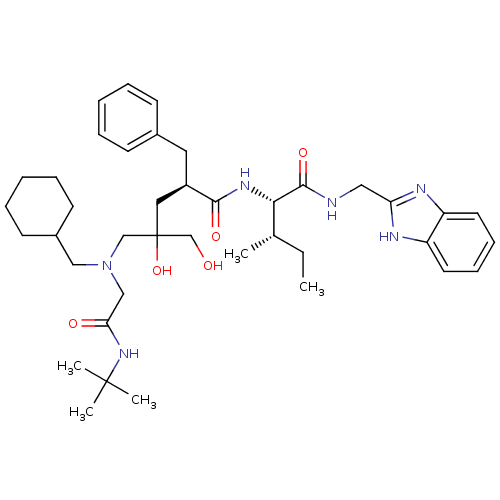
((R)-2-Benzyl-5-[(tert-butylcarbamoyl-methyl)-cyclo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)CC(O)(CO)CN(CC1CCCCC1)CC(=O)NC(C)(C)C)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C40H60N6O5/c1-6-28(2)36(38(50)41-23-34-42-32-19-13-14-20-33(32)43-34)44-37(49)31(21-29-15-9-7-10-16-29)22-40(51,27-47)26-46(24-30-17-11-8-12-18-30)25-35(48)45-39(3,4)5/h7,9-10,13-16,19-20,28,30-31,36,47,51H,6,8,11-12,17-18,21-27H2,1-5H3,(H,41,50)(H,42,43)(H,44,49)(H,45,48)/t28-,31+,36-,40?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50138619
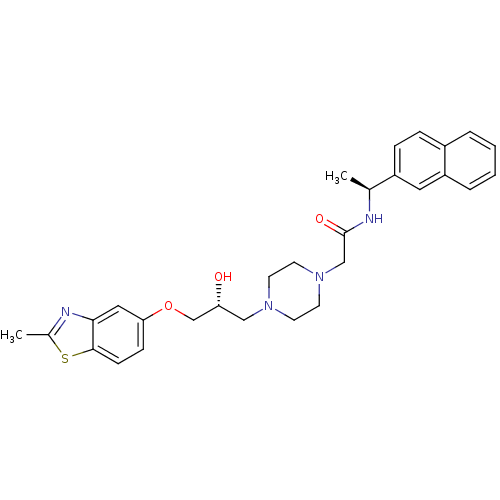
(2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...)Show SMILES C[C@H](NC(=O)CN1CCN(C[C@@H](O)COc2ccc3sc(C)nc3c2)CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C29H34N4O3S/c1-20(23-8-7-22-5-3-4-6-24(22)15-23)30-29(35)18-33-13-11-32(12-14-33)17-25(34)19-36-26-9-10-28-27(16-26)31-21(2)37-28/h3-10,15-16,20,25,34H,11-14,17-19H2,1-2H3,(H,30,35)/t20-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. |
Bioorg Med Chem Lett 14: 549-52 (2003)
BindingDB Entry DOI: 10.7270/Q2GH9HD4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282101
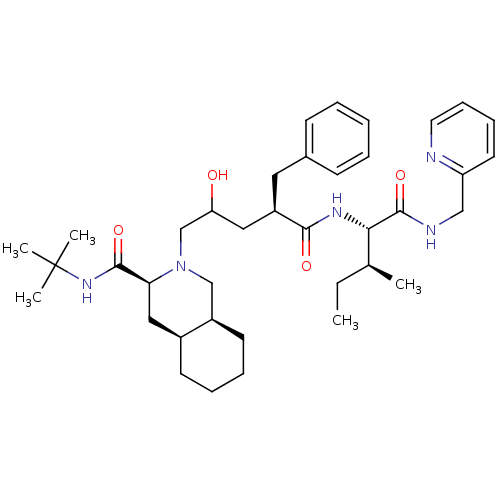
((3S,4aS,8aS)-2-((R)-2-Hydroxy-4-{(1S,2S)-2-methyl-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C)Cc1ccccc1)C(=O)NCc1ccccn1 Show InChI InChI=1S/C38H57N5O4/c1-6-26(2)34(37(47)40-23-31-18-12-13-19-39-31)41-35(45)30(20-27-14-8-7-9-15-27)21-32(44)25-43-24-29-17-11-10-16-28(29)22-33(43)36(46)42-38(3,4)5/h7-9,12-15,18-19,26,28-30,32-34,44H,6,10-11,16-17,20-25H2,1-5H3,(H,40,47)(H,41,45)(H,42,46)/t26-,28-,29+,30+,32?,33-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50047761
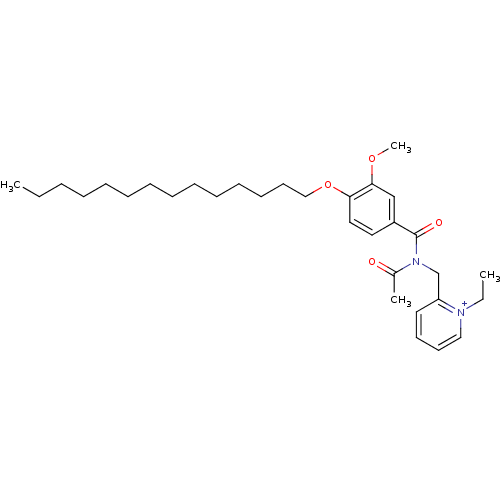
(2-{[Acetyl-(3-methoxy-4-tetradecyloxy-benzoyl)-ami...)Show SMILES CCCCCCCCCCCCCCOc1ccc(cc1OC)C(=O)N(Cc1cccc[n+]1CC)C(C)=O Show InChI InChI=1S/C32H49N2O4/c1-5-7-8-9-10-11-12-13-14-15-16-19-24-38-30-22-21-28(25-31(30)37-4)32(36)34(27(3)35)26-29-20-17-18-23-33(29)6-2/h17-18,20-23,25H,5-16,19,24,26H2,1-4H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF-induced platelet aggregation in rabbit platelet rich plasma |
J Med Chem 36: 580-90 (1993)
BindingDB Entry DOI: 10.7270/Q2SX6C9X |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50006071

(3-{3-[Hydroxy-(2-methoxy-3-octadecylcarbamoyloxy-p...)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC(COP([O-])(=O)Oc1cccc(C[n+]2csc(C)c2)c1)OC Show InChI InChI=1S/C34H57N2O7PS/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23-35-34(37)41-27-33(40-3)28-42-44(38,39)43-32-22-20-21-31(24-32)26-36-25-30(2)45-29-36/h20-22,24-25,29,33H,4-19,23,26-28H2,1-3H3,(H-,35,37,38,39) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF induced platelet aggregation in rabbit |
J Med Chem 35: 1650-62 (1992)
BindingDB Entry DOI: 10.7270/Q2BC4058 |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50138602
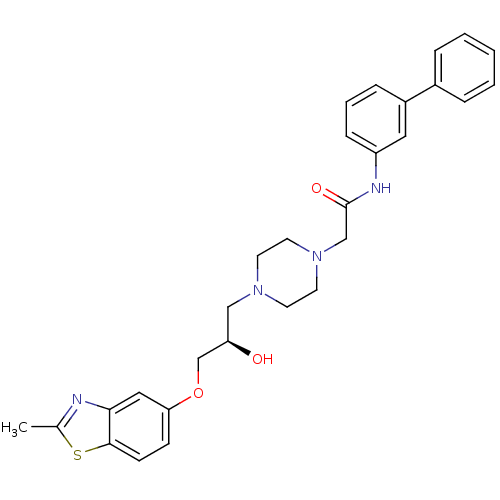
(CHEMBL152968 | N-Biphenyl-3-yl-2-{4-[(R)-2-hydroxy...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(CC(=O)Nc4cccc(c4)-c4ccccc4)CC3)ccc2s1 Show InChI InChI=1S/C29H32N4O3S/c1-21-30-27-17-26(10-11-28(27)37-21)36-20-25(34)18-32-12-14-33(15-13-32)19-29(35)31-24-9-5-8-23(16-24)22-6-3-2-4-7-22/h2-11,16-17,25,34H,12-15,18-20H2,1H3,(H,31,35)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. |
Bioorg Med Chem Lett 14: 549-52 (2003)
BindingDB Entry DOI: 10.7270/Q2GH9HD4 |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50156415
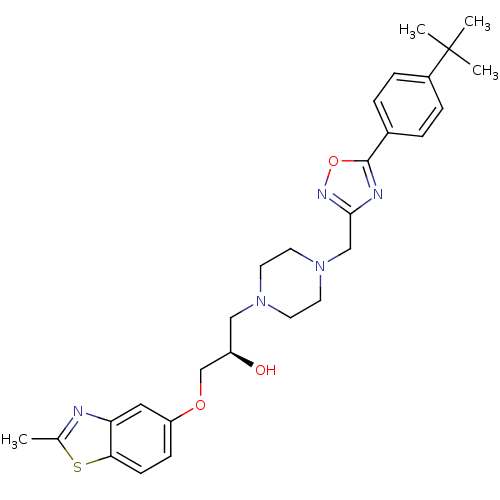
((R)-1-{4-[5-(4-tert-Butyl-phenyl)-[1,2,4]oxadiazol...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(Cc4noc(n4)-c4ccc(cc4)C(C)(C)C)CC3)ccc2s1 Show InChI InChI=1S/C28H35N5O3S/c1-19-29-24-15-23(9-10-25(24)37-19)35-18-22(34)16-32-11-13-33(14-12-32)17-26-30-27(36-31-26)20-5-7-21(8-6-20)28(2,3)4/h5-10,15,22,34H,11-14,16-18H2,1-4H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria |
Bioorg Med Chem Lett 14: 6017-21 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.077
BindingDB Entry DOI: 10.7270/Q2ZS2W0R |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50138617
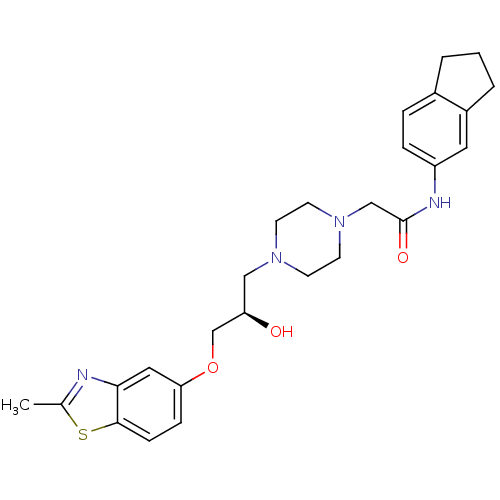
(2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(CC(=O)Nc4ccc5CCCc5c4)CC3)ccc2s1 Show InChI InChI=1S/C26H32N4O3S/c1-18-27-24-14-23(7-8-25(24)34-18)33-17-22(31)15-29-9-11-30(12-10-29)16-26(32)28-21-6-5-19-3-2-4-20(19)13-21/h5-8,13-14,22,31H,2-4,9-12,15-17H2,1H3,(H,28,32)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. |
Bioorg Med Chem Lett 14: 549-52 (2003)
BindingDB Entry DOI: 10.7270/Q2GH9HD4 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50006003

(3-{3-[Hydroxy-(5-tetradecyloxy-biphenyl-2-yloxy)-p...)Show SMILES CCCCCCCCCCCCCCOc1ccc(OP([O-])(=O)Oc2cccc(C[n+]3csc(C)c3)c2)c(c1)-c1ccccc1 Show InChI InChI=1S/C37H48NO5PS/c1-3-4-5-6-7-8-9-10-11-12-13-17-25-41-34-23-24-37(36(27-34)33-20-15-14-16-21-33)43-44(39,40)42-35-22-18-19-32(26-35)29-38-28-31(2)45-30-38/h14-16,18-24,26-28,30H,3-13,17,25,29H2,1-2H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF induced platelet aggregation in rabbit |
J Med Chem 35: 1650-62 (1992)
BindingDB Entry DOI: 10.7270/Q2BC4058 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50006061

(3-{3-[Hydroxy-(2,3,6-trimethyl-4-tetradecyloxy-phe...)Show SMILES CCCCCCCCCCCCCCOc1cc(C)c(OP([O-])(=O)Oc2cccc(C[n+]3csc(C)c3)c2)c(C)c1C Show InChI InChI=1S/C34H50NO5PS/c1-6-7-8-9-10-11-12-13-14-15-16-17-21-38-33-22-27(2)34(30(5)29(33)4)40-41(36,37)39-32-20-18-19-31(23-32)25-35-24-28(3)42-26-35/h18-20,22-24,26H,6-17,21,25H2,1-5H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF induced platelet aggregation in rabbit |
J Med Chem 35: 1650-62 (1992)
BindingDB Entry DOI: 10.7270/Q2BC4058 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50006076

(3-(3-{[3-(3-Dodecyloxy-2-methyl-phenoxy)-2-ethoxy-...)Show SMILES CCCCCCCCCCCCOc1cccc(OCC(COP([O-])(=O)Oc2cccc(C[n+]3csc(C)c3)c2)OCC)c1C Show InChI InChI=1S/C35H52NO7PS/c1-5-7-8-9-10-11-12-13-14-15-22-40-34-20-17-21-35(30(34)4)41-26-33(39-6-2)27-42-44(37,38)43-32-19-16-18-31(23-32)25-36-24-29(3)45-28-36/h16-21,23-24,28,33H,5-15,22,25-27H2,1-4H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF induced platelet aggregation in rabbit |
J Med Chem 35: 1650-62 (1992)
BindingDB Entry DOI: 10.7270/Q2BC4058 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282102

(1-((R)-2-Hydroxy-4-{(1S,2S)-2-methyl-1-[(pyridin-2...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)CN1CCCCC1C(=O)NC(C)(C)C)Cc1ccccc1)C(=O)NCc1ccccn1 Show InChI InChI=1S/C34H51N5O4/c1-6-24(2)30(33(43)36-22-27-16-10-12-18-35-27)37-31(41)26(20-25-14-8-7-9-15-25)21-28(40)23-39-19-13-11-17-29(39)32(42)38-34(3,4)5/h7-10,12,14-16,18,24,26,28-30,40H,6,11,13,17,19-23H2,1-5H3,(H,36,43)(H,37,41)(H,38,42)/t24-,26+,28?,29?,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibitory activity against HIV-1 Protease |
Bioorg Med Chem Lett 3: 1595-1600 (1993)
Article DOI: 10.1016/S0960-894X(00)80024-4
BindingDB Entry DOI: 10.7270/Q2154GZH |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50156413
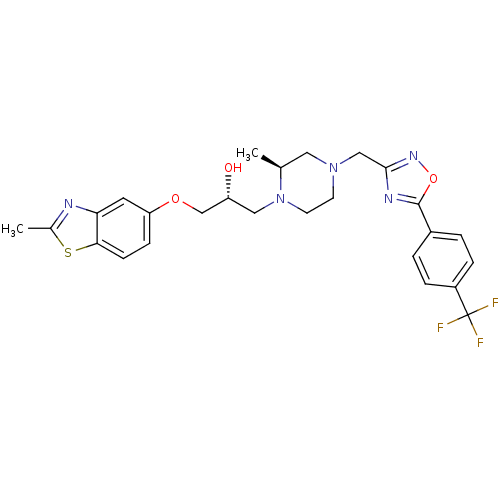
((R)-1-(2-Methyl-benzothiazol-5-yloxy)-3-{(S)-2-met...)Show SMILES C[C@H]1CN(Cc2noc(n2)-c2ccc(cc2)C(F)(F)F)CCN1C[C@@H](O)COc1ccc2sc(C)nc2c1 Show InChI InChI=1S/C26H28F3N5O3S/c1-16-12-33(14-24-31-25(37-32-24)18-3-5-19(6-4-18)26(27,28)29)9-10-34(16)13-20(35)15-36-21-7-8-23-22(11-21)30-17(2)38-23/h3-8,11,16,20,35H,9-10,12-15H2,1-2H3/t16-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria |
Bioorg Med Chem Lett 14: 6017-21 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.077
BindingDB Entry DOI: 10.7270/Q2ZS2W0R |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50006029
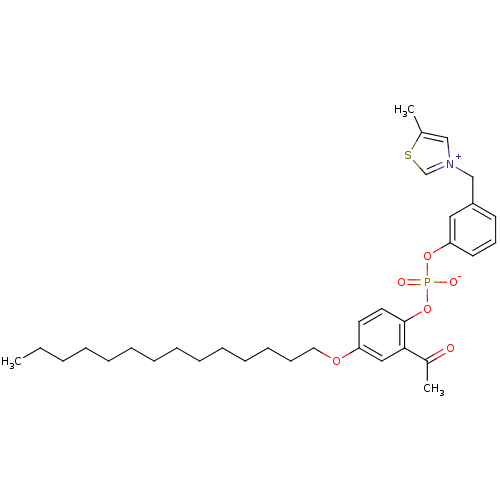
(3-{3-[(2-Acetyl-4-tetradecyloxy-phenoxy)-hydroxy-p...)Show SMILES CCCCCCCCCCCCCCOc1ccc(OP([O-])(=O)Oc2cccc(C[n+]3csc(C)c3)c2)c(c1)C(C)=O Show InChI InChI=1S/C33H46NO6PS/c1-4-5-6-7-8-9-10-11-12-13-14-15-21-38-30-19-20-33(32(23-30)28(3)35)40-41(36,37)39-31-18-16-17-29(22-31)25-34-24-27(2)42-26-34/h16-20,22-24,26H,4-15,21,25H2,1-3H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company
Curated by ChEMBL
| Assay Description
Inhibition of PAF induced platelet aggregation in rabbit |
J Med Chem 35: 1650-62 (1992)
BindingDB Entry DOI: 10.7270/Q2BC4058 |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50138625
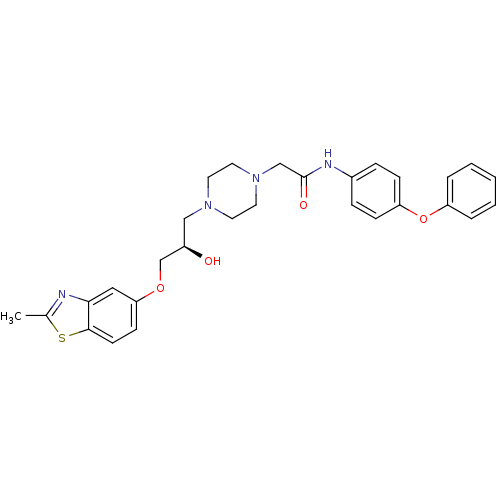
(2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(CC(=O)Nc4ccc(Oc5ccccc5)cc4)CC3)ccc2s1 Show InChI InChI=1S/C29H32N4O4S/c1-21-30-27-17-26(11-12-28(27)38-21)36-20-23(34)18-32-13-15-33(16-14-32)19-29(35)31-22-7-9-25(10-8-22)37-24-5-3-2-4-6-24/h2-12,17,23,34H,13-16,18-20H2,1H3,(H,31,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. |
Bioorg Med Chem Lett 14: 549-52 (2003)
BindingDB Entry DOI: 10.7270/Q2GH9HD4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284031

((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...)Show SMILES OC(C[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1ccncc1)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1ccncc1 Show InChI InChI=1S/C45H54N6O5/c52-37(29-35(27-31-11-3-1-4-12-31)44(55)50-40-17-9-7-15-38(40)48-42(53)33-19-23-46-24-20-33)30-36(28-32-13-5-2-6-14-32)45(56)51-41-18-10-8-16-39(41)49-43(54)34-21-25-47-26-22-34/h1-6,11-14,19-26,35-41,52H,7-10,15-18,27-30H2,(H,48,53)(H,49,54)(H,50,55)(H,51,56)/t35-,36-,38+,39+,40+,41+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against recombinant HIV-1 protease using 125 I-SPA (scintillation proximity assay) |
Bioorg Med Chem Lett 4: 583-588 (1994)
Article DOI: 10.1016/S0960-894X(01)80159-1
BindingDB Entry DOI: 10.7270/Q2Q24061 |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50138627
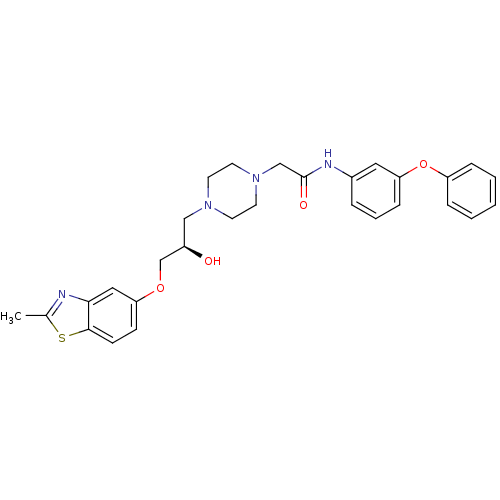
(2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(CC(=O)Nc4cccc(Oc5ccccc5)c4)CC3)ccc2s1 Show InChI InChI=1S/C29H32N4O4S/c1-21-30-27-17-25(10-11-28(27)38-21)36-20-23(34)18-32-12-14-33(15-13-32)19-29(35)31-22-6-5-9-26(16-22)37-24-7-3-2-4-8-24/h2-11,16-17,23,34H,12-15,18-20H2,1H3,(H,31,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. |
Bioorg Med Chem Lett 14: 549-52 (2003)
BindingDB Entry DOI: 10.7270/Q2GH9HD4 |
More data for this
Ligand-Target Pair | |
Peroxisomal acyl-coenzyme A oxidase 1
(Rattus norvegicus) | BDBM50156414
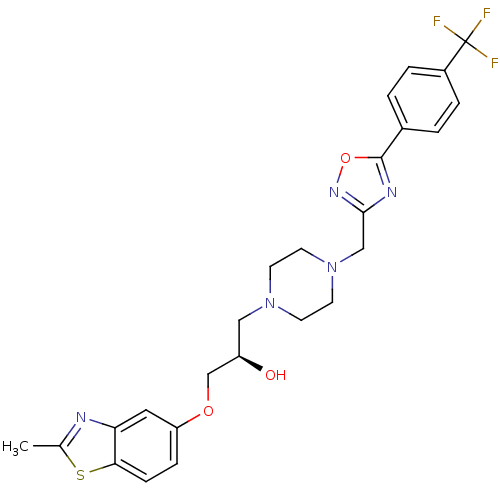
((R)-1-(2-Methyl-benzothiazol-5-yloxy)-3-{4-[5-(4-t...)Show SMILES Cc1nc2cc(OC[C@H](O)CN3CCN(Cc4noc(n4)-c4ccc(cc4)C(F)(F)F)CC3)ccc2s1 Show InChI InChI=1S/C25H26F3N5O3S/c1-16-29-21-12-20(6-7-22(21)37-16)35-15-19(34)13-32-8-10-33(11-9-32)14-23-30-24(36-31-23)17-2-4-18(5-3-17)25(26,27)28/h2-7,12,19,34H,8-11,13-15H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria |
Bioorg Med Chem Lett 14: 6017-21 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.077
BindingDB Entry DOI: 10.7270/Q2ZS2W0R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data