 Found 487 hits with Last Name = 'kesicki' and Initial = 'ea'
Found 487 hits with Last Name = 'kesicki' and Initial = 'ea' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27700
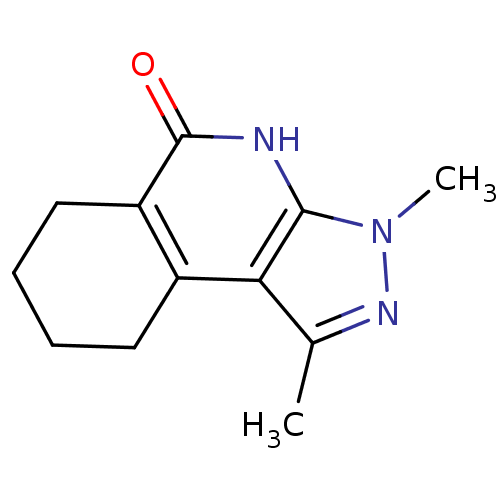
(3,5-dimethyl-4,5,7-triazatricyclo[7.4.0.0^{2,6}]tr...)Show InChI InChI=1S/C12H15N3O/c1-7-10-8-5-3-4-6-9(8)12(16)13-11(10)15(2)14-7/h3-6H2,1-2H3,(H,13,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27685

(2-{4-[(dimethylamino)methyl]phenyl}-3,10-diazatric...)Show SMILES CN(C)Cc1ccc(cc1)-c1[nH]c2cccc3C(=O)NCCc1c23 Show InChI InChI=1S/C20H21N3O/c1-23(2)12-13-6-8-14(9-7-13)19-15-10-11-21-20(24)16-4-3-5-17(22-19)18(15)16/h3-9,22H,10-12H2,1-2H3,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27702

(5-methyl-3-(thiophen-2-yl)-4,5,7-triazatricyclo[7....)Show InChI InChI=1S/C15H15N3OS/c1-18-14-12(13(17-18)11-7-4-8-20-11)9-5-2-3-6-10(9)15(19)16-14/h4,7-8H,2-3,5-6H2,1H3,(H,16,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27701

(5-methyl-3-phenyl-4,5,7-triazatricyclo[7.4.0.0^{2,...)Show InChI InChI=1S/C17H17N3O/c1-20-16-14(15(19-20)11-7-3-2-4-8-11)12-9-5-6-10-13(12)17(21)18-16/h2-4,7-8H,5-6,9-10H2,1H3,(H,18,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150175
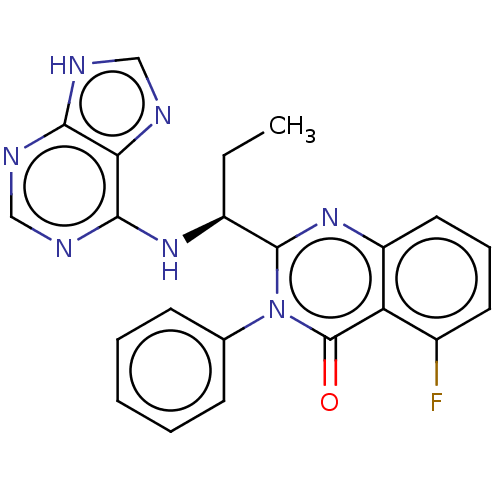
(US8980901, 107 | US9149477, Compound 107)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Using the method described in US9149477, Example 2, compounds of the invention were tested for inhibitory activity and potency against PI3Kδ, an... |
US Patent US9149477 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
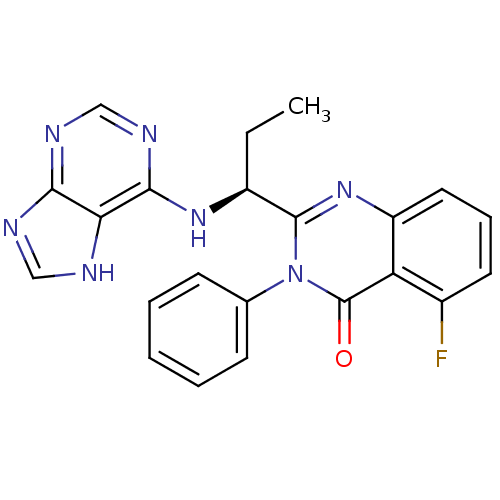
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Biochemical assay using PI3K delta. |
US Patent USRE44599 (2013)
BindingDB Entry DOI: 10.7270/Q2736PJJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068

(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Compounds of the invention were tested for inhibitory activity and potency against PI3Kδ. |
US Patent US8586597 (2013)
BindingDB Entry DOI: 10.7270/Q2RV0M9J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150175

(US8980901, 107 | US9149477, Compound 107)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Icos Corporation
US Patent
| Assay Description
Using the method described in Example 2, compounds of the invention were
tested for inhibitory activity and potency against PI3Kdelta, and for
sele... |
US Patent US8980901 (2015)
BindingDB Entry DOI: 10.7270/Q28C9V0N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104486

(US8586597, 101 | USRE44599, 117)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(Cl)c2c(=O)n1-c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C22H16ClF2N7O/c1-2-15(30-20-18-19(27-9-26-18)28-10-29-20)21-31-16-5-3-4-14(23)17(16)22(33)32(21)13-7-11(24)6-12(25)8-13/h3-10,15H,2H2,1H3,(H2,26,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Compounds of the invention were tested for inhibitory activity and potency against PI3Kδ. |
US Patent US8586597 (2013)
BindingDB Entry DOI: 10.7270/Q2RV0M9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150172

(US8980901, 117)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2ccccc2c(=O)n1-c1cccc(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Icos Corporation
US Patent
| Assay Description
Using the method described in Example 2, compounds of the invention were
tested for inhibitory activity and potency against PI3Kdelta, and for
sele... |
US Patent US8980901 (2015)
BindingDB Entry DOI: 10.7270/Q28C9V0N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM182844

(US9149477, Compound 117)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1cc(F)cc(F)c1 Show InChI InChI=1S/C22H16ClF2N7O/c1-2-15(30-20-18-19(27-9-26-18)28-10-29-20)21-31-16-5-3-4-14(23)17(16)22(33)32(21)13-7-11(24)6-12(25)8-13/h3-10,15H,2H2,1H3,(H2,26,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Using the method described in US9149477, Example 2, compounds of the invention were tested for inhibitory activity and potency against PI3Kδ, an... |
US Patent US9149477 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104486

(US8586597, 101 | USRE44599, 117)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(Cl)c2c(=O)n1-c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C22H16ClF2N7O/c1-2-15(30-20-18-19(27-9-26-18)28-10-29-20)21-31-16-5-3-4-14(23)17(16)22(33)32(21)13-7-11(24)6-12(25)8-13/h3-10,15H,2H2,1H3,(H2,26,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Biochemical assay using PI3K delta. |
US Patent USRE44599 (2013)
BindingDB Entry DOI: 10.7270/Q2736PJJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104485

(US8586597, 99 | USRE44599, 98)Show SMILES CCC(Nc1ncnc2nc[nH]c12)c1nc2ccccc2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H19N7O/c1-2-16(27-20-18-19(24-12-23-18)25-13-26-20)21-28-17-11-7-6-10-15(17)22(30)29(21)14-8-4-3-5-9-14/h3-13,16H,2H2,1H3,(H2,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Compounds of the invention were tested for inhibitory activity and potency against PI3Kδ. |
US Patent US8586597 (2013)
BindingDB Entry DOI: 10.7270/Q2RV0M9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150171

(US8980901, 98)Show SMILES CC(Nc1ncnc2[nH]cc(C)c12)c1nc2cccc(C)c2c(=O)n1-c1cccc(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Icos Corporation
US Patent
| Assay Description
Using the method described in Example 2, compounds of the invention were
tested for inhibitory activity and potency against PI3Kdelta, and for
sele... |
US Patent US8980901 (2015)
BindingDB Entry DOI: 10.7270/Q28C9V0N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104485

(US8586597, 99 | USRE44599, 98)Show SMILES CCC(Nc1ncnc2nc[nH]c12)c1nc2ccccc2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H19N7O/c1-2-16(27-20-18-19(24-12-23-18)25-13-26-20)21-28-17-11-7-6-10-15(17)22(30)29(21)14-8-4-3-5-9-14/h3-13,16H,2H2,1H3,(H2,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Biochemical assay using PI3K delta. |
US Patent USRE44599 (2013)
BindingDB Entry DOI: 10.7270/Q2736PJJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM182845

(US9149477, Compound 98)Show SMILES CCC(Nc1ncnc2[nH]cnc12)c1nc2ccccc2c(=O)n1-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Using the method described in US9149477, Example 2, compounds of the invention were tested for inhibitory activity and potency against PI3Kδ, an... |
US Patent US9149477 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X11 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27497
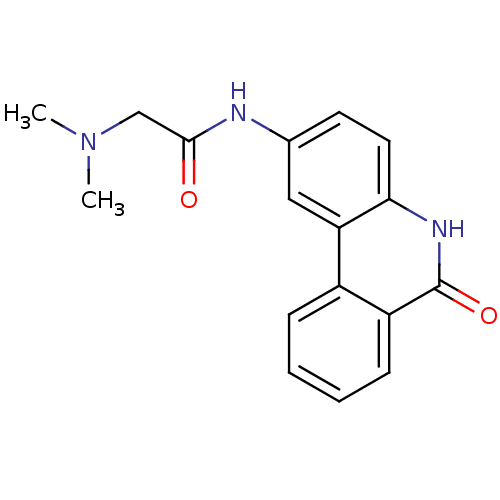
(2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...)Show InChI InChI=1S/C17H17N3O2/c1-20(2)10-16(21)18-11-7-8-15-14(9-11)12-5-3-4-6-13(12)17(22)19-15/h3-9H,10H2,1-2H3,(H,18,21)(H,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286461

(CHEMBL4166626)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccccc3)[C@@H](C)C3CC3)C2=O)c1 |r| Show InChI InChI=1S/C27H30N4O5/c1-17(19-8-9-19)30(15-18-6-4-3-5-7-18)23(32)16-31-24(33)27(36-26(31)35)13-12-20-14-21(10-11-22(20)27)29-25(34)28-2/h3-7,10-11,14,17,19H,8-9,12-13,15-16H2,1-2H3,(H2,28,29,34)/t17-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP (unknown origin) using biotinylated synthetic Histone H4 Peptide as substrate pretreated for 30 mins followed by substrate add... |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150174

(US8980901, 93 | US9149477, Compound 93)Show SMILES CCC(Nc1ncnc2[nH]cnc12)c1nc2cccc(C)c2c(=O)n1-c1cc(F)cc(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Icos Corporation
US Patent
| Assay Description
Using the method described in Example 2, compounds of the invention were
tested for inhibitory activity and potency against PI3Kdelta, and for
sele... |
US Patent US8980901 (2015)
BindingDB Entry DOI: 10.7270/Q28C9V0N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104488
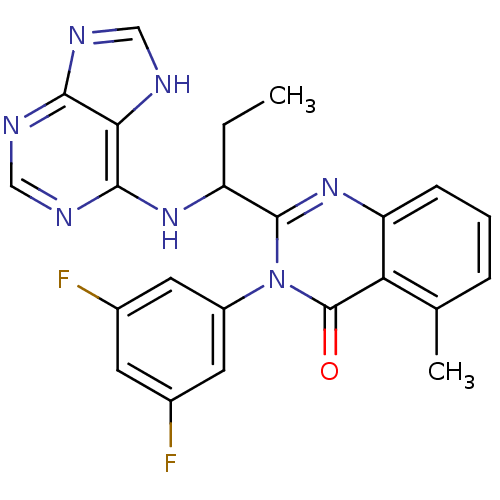
(US8586597, 98 | USRE44599, 93)Show SMILES CCC(Nc1ncnc2nc[nH]c12)c1nc2cccc(C)c2c(=O)n1-c1cc(F)cc(F)c1 Show InChI InChI=1S/C23H19F2N7O/c1-3-16(30-21-19-20(27-10-26-19)28-11-29-21)22-31-17-6-4-5-12(2)18(17)23(33)32(22)15-8-13(24)7-14(25)9-15/h4-11,16H,3H2,1-2H3,(H2,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Compounds of the invention were tested for inhibitory activity and potency against PI3Kδ. |
US Patent US8586597 (2013)
BindingDB Entry DOI: 10.7270/Q2RV0M9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104488

(US8586597, 98 | USRE44599, 93)Show SMILES CCC(Nc1ncnc2nc[nH]c12)c1nc2cccc(C)c2c(=O)n1-c1cc(F)cc(F)c1 Show InChI InChI=1S/C23H19F2N7O/c1-3-16(30-21-19-20(27-10-26-19)28-11-29-21)22-31-17-6-4-5-12(2)18(17)23(33)32(22)15-8-13(24)7-14(25)9-15/h4-11,16H,3H2,1-2H3,(H2,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Biochemical assay using PI3K delta. |
US Patent USRE44599 (2013)
BindingDB Entry DOI: 10.7270/Q2736PJJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150174

(US8980901, 93 | US9149477, Compound 93)Show SMILES CCC(Nc1ncnc2[nH]cnc12)c1nc2cccc(C)c2c(=O)n1-c1cc(F)cc(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Using the method described in US9149477, Example 2, compounds of the invention were tested for inhibitory activity and potency against PI3Kδ, an... |
US Patent US9149477 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X11 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27694
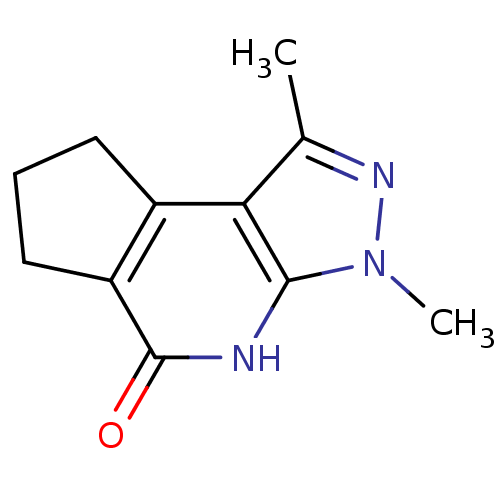
(3,5-dimethyl-4,5,7-triazatricyclo[7.3.0.0^{2,6}]do...)Show InChI InChI=1S/C11H13N3O/c1-6-9-7-4-3-5-8(7)11(15)12-10(9)14(2)13-6/h3-5H2,1-2H3,(H,12,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29.8 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286424
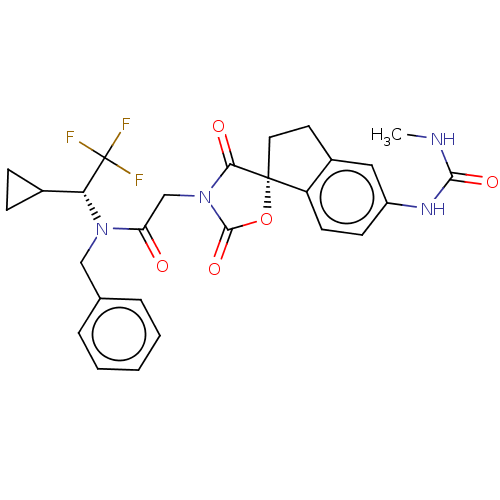
(CHEMBL4170186)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccccc3)[C@H](C3CC3)C(F)(F)F)C2=O)c1 |r| Show InChI InChI=1S/C27H27F3N4O5/c1-31-24(37)32-19-9-10-20-18(13-19)11-12-26(20)23(36)34(25(38)39-26)15-21(35)33(14-16-5-3-2-4-6-16)22(17-7-8-17)27(28,29)30/h2-6,9-10,13,17,22H,7-8,11-12,14-15H2,1H3,(H2,31,32,37)/t22-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP (unknown origin) using biotinylated synthetic Histone H4 Peptide as substrate pretreated for 30 mins followed by substrate add... |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM182846

(US9149477, Compound 174)Show SMILES CCC(Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1c(F)cccc1F |(-6.81,-.53,;-5.47,.24,;-4.14,-.53,;-2.8,.24,;-2.8,1.78,;-1.47,2.55,;-1.47,4.09,;-2.8,4.86,;-4.14,4.09,;-5.6,4.56,;-6.51,3.32,;-5.6,2.07,;-4.14,2.55,;-4.14,-2.07,;-5.47,-2.84,;-5.47,-4.38,;-6.81,-5.15,;-6.81,-6.69,;-5.47,-7.46,;-4.14,-6.69,;-2.8,-7.46,;-4.14,-5.15,;-2.8,-4.38,;-1.47,-5.15,;-2.8,-2.84,;-1.47,-2.07,;-1.47,-.53,;-2.1,.87,;-.14,.24,;1.2,-.53,;1.2,-2.07,;-.14,-2.84,;-.14,-4.38,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Using the method described in US9149477, Example 2, compounds of the invention were tested for inhibitory activity and potency against PI3Kδ, an... |
US Patent US9149477 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104487

(US8586597, 102 | USRE44599, 174)Show SMILES CCC(Nc1ncnc2nc[nH]c12)c1nc2cccc(Cl)c2c(=O)n1-c1c(F)cccc1F |(4.1,.06,;2.77,.83,;1.44,.06,;1.44,-1.48,;2.77,-2.25,;4.1,-1.48,;5.44,-2.25,;5.44,-3.79,;4.1,-4.56,;3.78,-6.06,;2.25,-6.22,;1.63,-4.82,;2.77,-3.79,;.1,.83,;-1.23,.06,;-2.56,.83,;-3.9,.06,;-5.23,.83,;-5.23,2.37,;-3.9,3.14,;-3.9,4.68,;-2.56,2.37,;-1.23,3.14,;-1.23,4.68,;.1,2.37,;1.44,3.14,;1.44,4.68,;-.05,5.08,;2.77,5.45,;4.1,4.68,;4.1,3.14,;2.77,2.37,;4.1,1.6,)| Show InChI InChI=1S/C22H16ClF2N7O/c1-2-14(30-20-17-19(27-9-26-17)28-10-29-20)21-31-15-8-3-5-11(23)16(15)22(33)32(21)18-12(24)6-4-7-13(18)25/h3-10,14H,2H2,1H3,(H2,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Compounds of the invention were tested for inhibitory activity and potency against PI3Kδ. |
US Patent US8586597 (2013)
BindingDB Entry DOI: 10.7270/Q2RV0M9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150173

(US8980901, 174)Show SMILES CC(Nc1ncnc2[nH]cnc12)c1nc2ccc(F)cc2c(=O)n1-c1cc(F)cc(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Icos Corporation
US Patent
| Assay Description
Using the method described in Example 2, compounds of the invention were
tested for inhibitory activity and potency against PI3Kdelta, and for
sele... |
US Patent US8980901 (2015)
BindingDB Entry DOI: 10.7270/Q28C9V0N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104487

(US8586597, 102 | USRE44599, 174)Show SMILES CCC(Nc1ncnc2nc[nH]c12)c1nc2cccc(Cl)c2c(=O)n1-c1c(F)cccc1F |(4.1,.06,;2.77,.83,;1.44,.06,;1.44,-1.48,;2.77,-2.25,;4.1,-1.48,;5.44,-2.25,;5.44,-3.79,;4.1,-4.56,;3.78,-6.06,;2.25,-6.22,;1.63,-4.82,;2.77,-3.79,;.1,.83,;-1.23,.06,;-2.56,.83,;-3.9,.06,;-5.23,.83,;-5.23,2.37,;-3.9,3.14,;-3.9,4.68,;-2.56,2.37,;-1.23,3.14,;-1.23,4.68,;.1,2.37,;1.44,3.14,;1.44,4.68,;-.05,5.08,;2.77,5.45,;4.1,4.68,;4.1,3.14,;2.77,2.37,;4.1,1.6,)| Show InChI InChI=1S/C22H16ClF2N7O/c1-2-14(30-20-17-19(27-9-26-17)28-10-29-20)21-31-15-8-3-5-11(23)16(15)22(33)32(21)18-12(24)6-4-7-13(18)25/h3-10,14H,2H2,1H3,(H2,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Biochemical assay using PI3K delta. |
US Patent USRE44599 (2013)
BindingDB Entry DOI: 10.7270/Q2736PJJ |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286424

(CHEMBL4170186)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccccc3)[C@H](C3CC3)C(F)(F)F)C2=O)c1 |r| Show InChI InChI=1S/C27H27F3N4O5/c1-31-24(37)32-19-9-10-20-18(13-19)11-12-26(20)23(36)34(25(38)39-26)15-21(35)33(14-16-5-3-2-4-6-16)22(17-7-8-17)27(28,29)30/h2-6,9-10,13,17,22H,7-8,11-12,14-15H2,1H3,(H2,31,32,37)/t22-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP in human PC3 cells assessed as reduction in acetylated H3K27 levels after 3 hrs by fluorescence assay |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286461

(CHEMBL4166626)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccccc3)[C@@H](C)C3CC3)C2=O)c1 |r| Show InChI InChI=1S/C27H30N4O5/c1-17(19-8-9-19)30(15-18-6-4-3-5-7-18)23(32)16-31-24(33)27(36-26(31)35)13-12-20-14-21(10-11-22(20)27)29-25(34)28-2/h3-7,10-11,14,17,19H,8-9,12-13,15-16H2,1-2H3,(H2,28,29,34)/t17-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP in human PC3 cells assessed as reduction in acetylated H3K27 levels after 3 hrs by fluorescence assay |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27684
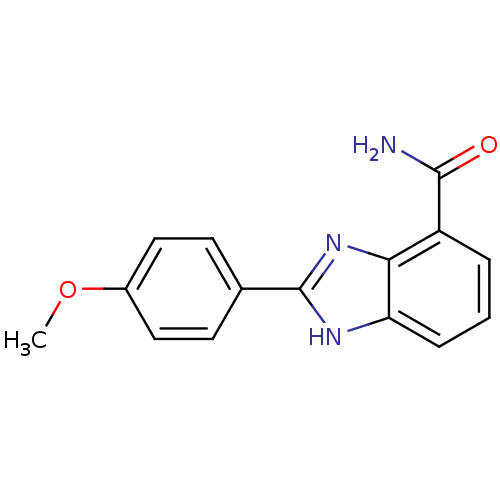
(2-(4-methoxyphenyl)-1H-1,3-benzodiazole-4-carboxam...)Show InChI InChI=1S/C15H13N3O2/c1-20-10-7-5-9(6-8-10)15-17-12-4-2-3-11(14(16)19)13(12)18-15/h2-8H,1H3,(H2,16,19)(H,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286423

(CHEMBL4174931)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccccc3)[C@@H](C)C(F)(F)F)C2=O)c1 |r| Show InChI InChI=1S/C25H25F3N4O5/c1-15(25(26,27)28)31(13-16-6-4-3-5-7-16)20(33)14-32-21(34)24(37-23(32)36)11-10-17-12-18(8-9-19(17)24)30-22(35)29-2/h3-9,12,15H,10-11,13-14H2,1-2H3,(H2,29,30,35)/t15-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP (unknown origin) using biotinylated synthetic Histone H4 Peptide as substrate pretreated for 30 mins followed by substrate add... |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27689
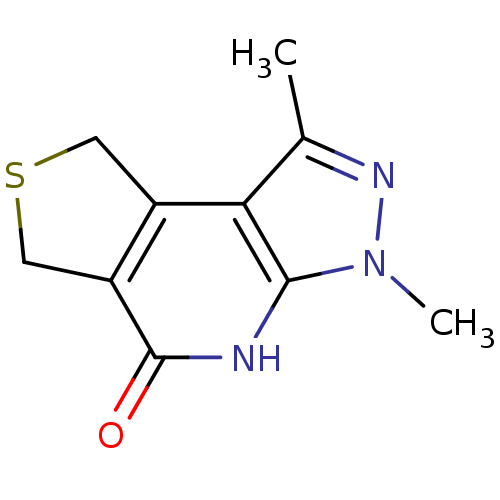
(3,5-dimethyl-11-thia-4,5,7-triazatricyclo[7.3.0.0^...)Show InChI InChI=1S/C10H11N3OS/c1-5-8-6-3-15-4-7(6)10(14)11-9(8)13(2)12-5/h3-4H2,1-2H3,(H,11,14) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92.7 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286423

(CHEMBL4174931)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccccc3)[C@@H](C)C(F)(F)F)C2=O)c1 |r| Show InChI InChI=1S/C25H25F3N4O5/c1-15(25(26,27)28)31(13-16-6-4-3-5-7-16)20(33)14-32-21(34)24(37-23(32)36)11-10-17-12-18(8-9-19(17)24)30-22(35)29-2/h3-9,12,15H,10-11,13-14H2,1-2H3,(H2,29,30,35)/t15-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP in human PC3 cells assessed as reduction in acetylated H3K27 levels after 3 hrs by fluorescence assay |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27695
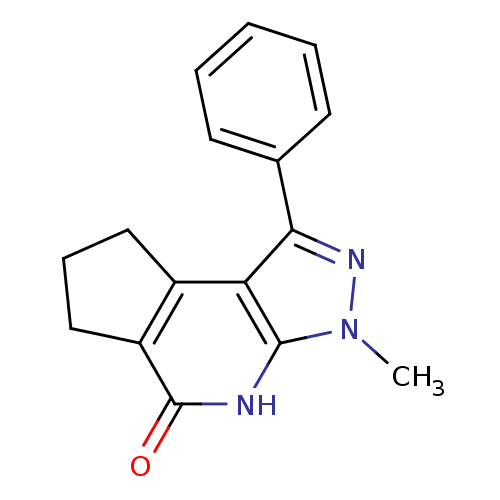
(5-methyl-3-phenyl-4,5,7-triazatricyclo[7.3.0.0^{2,...)Show InChI InChI=1S/C16H15N3O/c1-19-15-13(11-8-5-9-12(11)16(20)17-15)14(18-19)10-6-3-2-4-7-10/h2-4,6-7H,5,8-9H2,1H3,(H,17,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM602728

(2-[1-(2-Isoindolin-2-yl-6-methyl-4-oxo-chromen-8-y...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27690
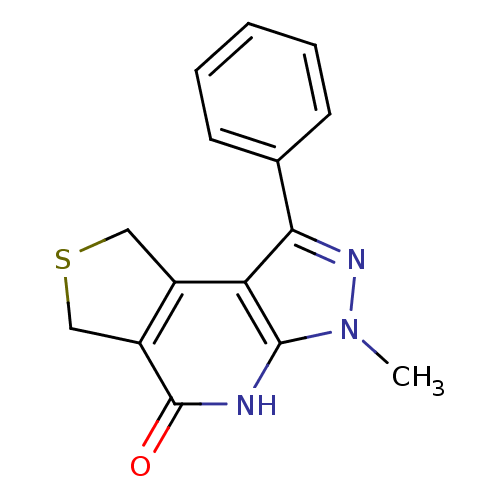
(5-methyl-3-phenyl-11-thia-4,5,7-triazatricyclo[7.3...)Show InChI InChI=1S/C15H13N3OS/c1-18-14-12(10-7-20-8-11(10)15(19)16-14)13(17-18)9-5-3-2-4-6-9/h2-6H,7-8H2,1H3,(H,16,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286465

(CHEMBL4177251)Show SMILES CNC(=O)Nc1ccc2c(CC[C@]22OC(=O)N(CC(=O)N(Cc3ccccc3)[C@@H](C)C3CC3)C2=O)c1 |r| Show InChI InChI=1S/C27H30N4O5/c1-17(19-8-9-19)30(15-18-6-4-3-5-7-18)23(32)16-31-24(33)27(36-26(31)35)13-12-20-14-21(10-11-22(20)27)29-25(34)28-2/h3-7,10-11,14,17,19H,8-9,12-13,15-16H2,1-2H3,(H2,28,29,34)/t17-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP (unknown origin) using biotinylated synthetic Histone H4 Peptide as substrate pretreated for 30 mins followed by substrate add... |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27696
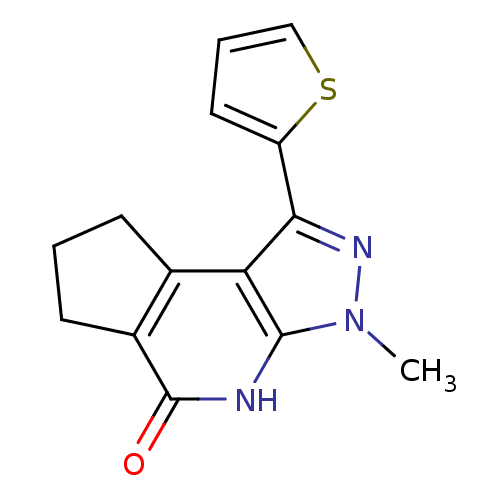
(5-methyl-3-(thiophen-2-yl)-4,5,7-triazatricyclo[7....)Show InChI InChI=1S/C14H13N3OS/c1-17-13-11(12(16-17)10-6-3-7-19-10)8-4-2-5-9(8)14(18)15-13/h3,6-7H,2,4-5H2,1H3,(H,15,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27682
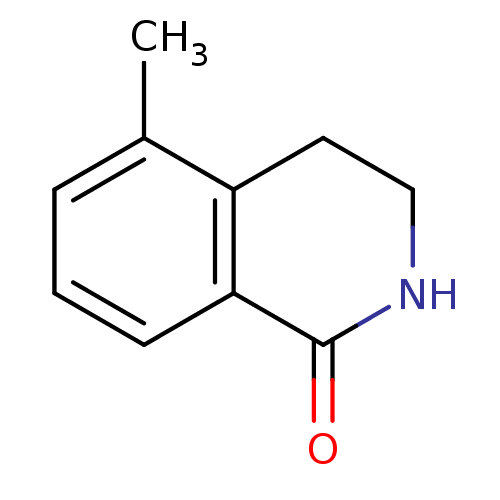
(5-methyl-1,2,3,4-tetrahydroisoquinolin-1-one | CHE...)Show InChI InChI=1S/C10H11NO/c1-7-3-2-4-9-8(7)5-6-11-10(9)12/h2-4H,5-6H2,1H3,(H,11,12) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286459

(CHEMBL4163183)Show SMILES CNC(=O)Nc1ccc2c(CC[C@]22NC(=O)N(CC(=O)N(Cc3ccccc3)[C@@H](C)C3CC3)C2=O)c1 |r| Show InChI InChI=1S/C27H31N5O4/c1-17(19-8-9-19)31(15-18-6-4-3-5-7-18)23(33)16-32-24(34)27(30-26(32)36)13-12-20-14-21(10-11-22(20)27)29-25(35)28-2/h3-7,10-11,14,17,19H,8-9,12-13,15-16H2,1-2H3,(H,30,36)(H2,28,29,35)/t17-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP (unknown origin) using biotinylated synthetic Histone H4 Peptide as substrate pretreated for 30 mins followed by substrate add... |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286469

(CHEMBL4173894)Show SMILES CNC(=O)Nc1ccc2c(CCC22NC(=O)N(CC(=O)N(Cc3ccccc3)[C@@H](C)C3CC3)C2=O)c1 |r| Show InChI InChI=1S/C27H31N5O4/c1-17(19-8-9-19)31(15-18-6-4-3-5-7-18)23(33)16-32-24(34)27(30-26(32)36)13-12-20-14-21(10-11-22(20)27)29-25(35)28-2/h3-7,10-11,14,17,19H,8-9,12-13,15-16H2,1-2H3,(H,30,36)(H2,28,29,35)/t17-,27?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP (unknown origin) using biotinylated synthetic Histone H4 Peptide as substrate pretreated for 30 mins followed by substrate add... |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27691

(5-methyl-3-(thiophen-2-yl)-11-thia-4,5,7-triazatri...)Show InChI InChI=1S/C13H11N3OS2/c1-16-12-10(11(15-16)9-3-2-4-19-9)7-5-18-6-8(7)13(17)14-12/h2-4H,5-6H2,1H3,(H,14,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 428 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Deltagen Research Laboratories
| Assay Description
To assess the inhibitory activity of novel inhibitors, the PARP-1 enzyme assay was carried out in reaction mixture consisting of Escherichia coli str... |
Bioorg Med Chem Lett 18: 5126-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.091
BindingDB Entry DOI: 10.7270/Q29S1PCX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM602728

(2-[1-(2-Isoindolin-2-yl-6-methyl-4-oxo-chromen-8-y...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50286459

(CHEMBL4163183)Show SMILES CNC(=O)Nc1ccc2c(CC[C@]22NC(=O)N(CC(=O)N(Cc3ccccc3)[C@@H](C)C3CC3)C2=O)c1 |r| Show InChI InChI=1S/C27H31N5O4/c1-17(19-8-9-19)31(15-18-6-4-3-5-7-18)23(33)16-32-24(34)27(30-26(32)36)13-12-20-14-21(10-11-22(20)27)29-25(35)28-2/h3-7,10-11,14,17,19H,8-9,12-13,15-16H2,1-2H3,(H,30,36)(H2,28,29,35)/t17-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p300/CBP in human PC3 cells assessed as reduction in acetylated H3K27 levels after 3 hrs by fluorescence assay |
ACS Med Chem Lett 9: 28-33 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00395
BindingDB Entry DOI: 10.7270/Q2HQ42FP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602616
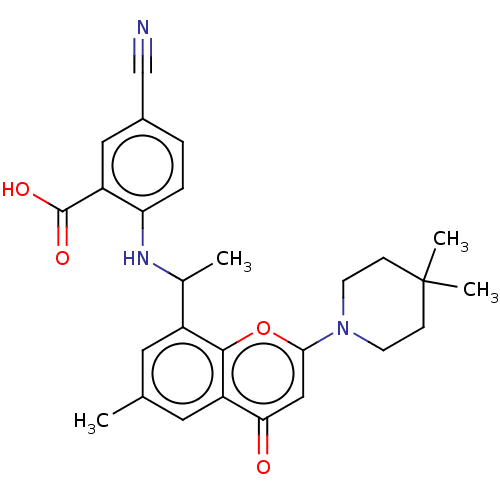
(5-Cyano-2-[1-[2-(4,4-dimethyl-1- piperidyl)-6-meth...)Show SMILES CC(Nc1ccc(cc1C(O)=O)C#N)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602619

(2-[1-[2-(4,4-Dimethyl-1-piperidyl)-6- methyl-4-oxo...)Show SMILES CONC(=O)c1ccccc1NC(C)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602620
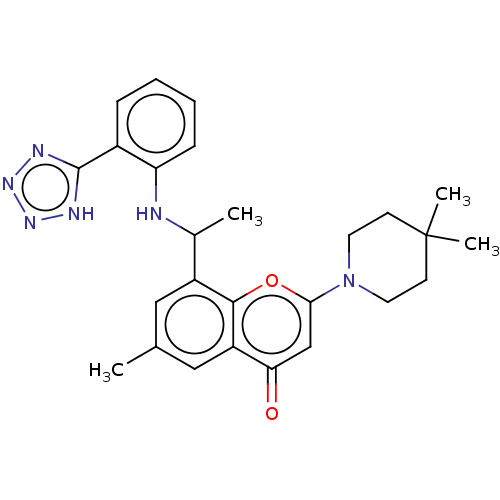
(2-(4,4-Dimethyl-1-piperidyl)-6-methyl- 8-[1-[2-(1H...)Show SMILES CC(Nc1ccccc1-c1nnn[nH]1)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602628
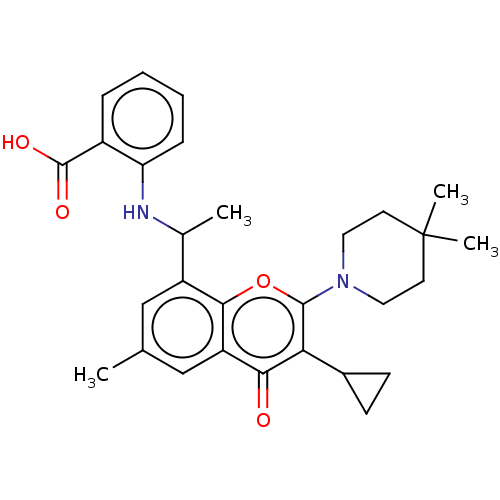
(2-[1-[3-Cyclopropyl-2-(4,4-dimethyl-1- piperidyl)-...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CCC(C)(C)CC1)c(C1CC1)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602629
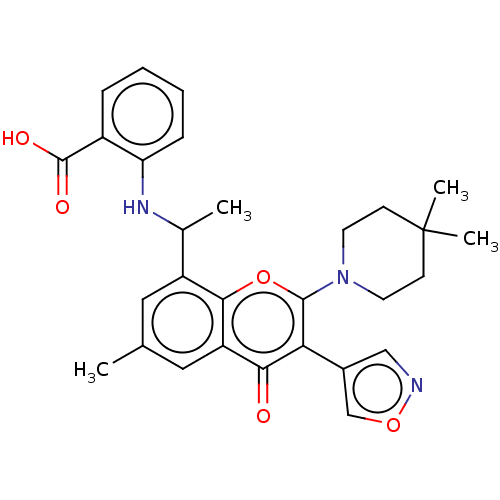
(2-[1-[2-(4,4-Dimethyl-1-piperidyl)-6- isoxazol-4-y...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(N1CCC(C)(C)CC1)c(-c1cnoc1)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data