

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289804 (Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289851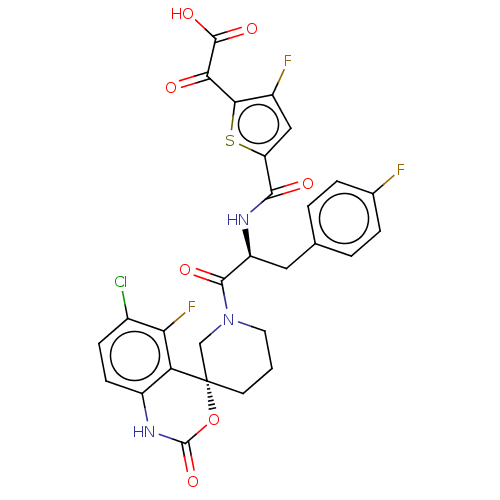 (2-(5-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807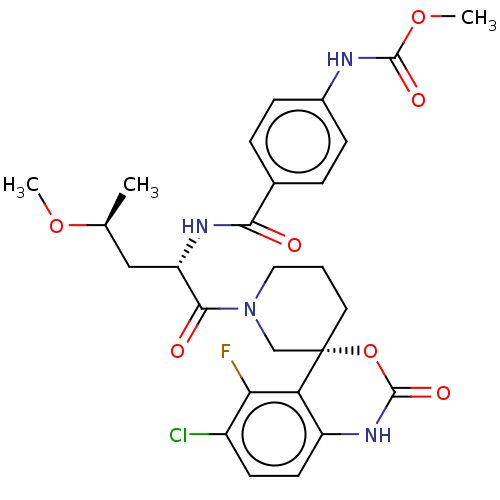 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50174298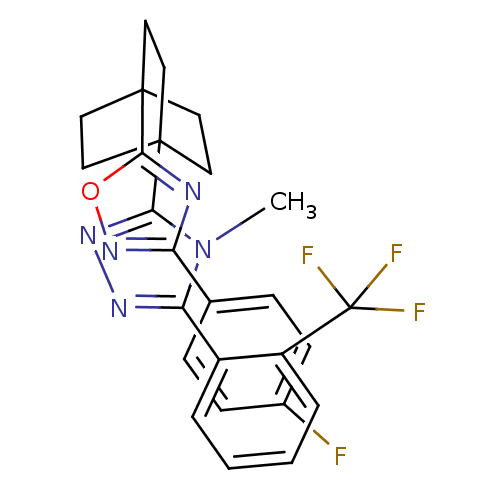 (3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289844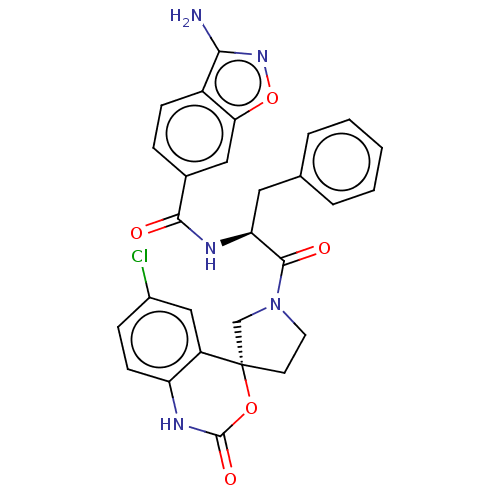 (3-amino-N—((S)-1-(R)-6-chloro-2-oxo-1,2-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.52 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289795 (Methyl 2-amino-3-(5-fluoropyridin-2-yl)propanoate ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.82 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289853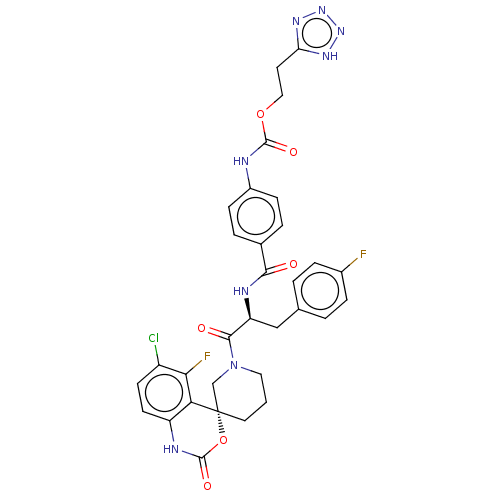 (2-(1H-tetrazol-5-yl)ethyl (4-(((S)-1-((R)-6-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.22 | -48.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50340378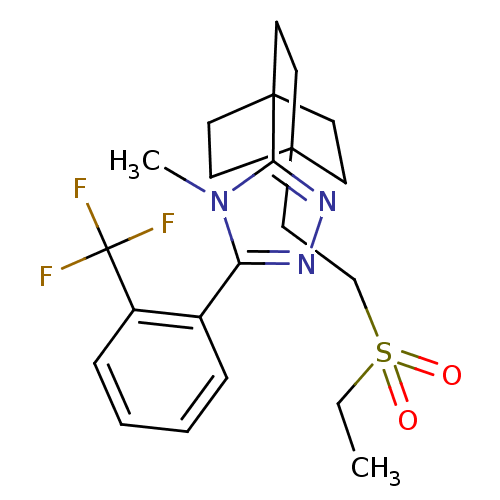 (3-(4-(2-(ethylsulfonyl)ethyl)bicyclo[2.2.2]octan-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289805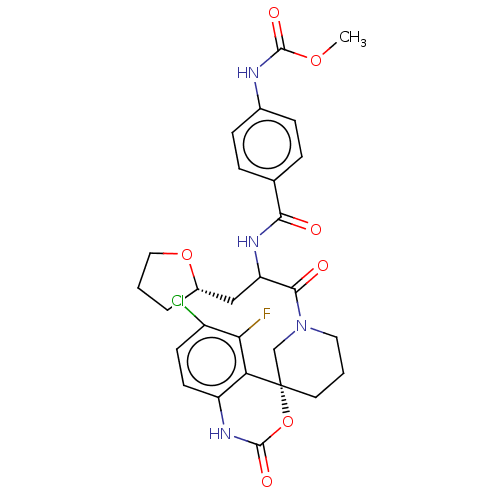 (US10093683, Example 116A | US10093683, Example 116...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.21 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50435691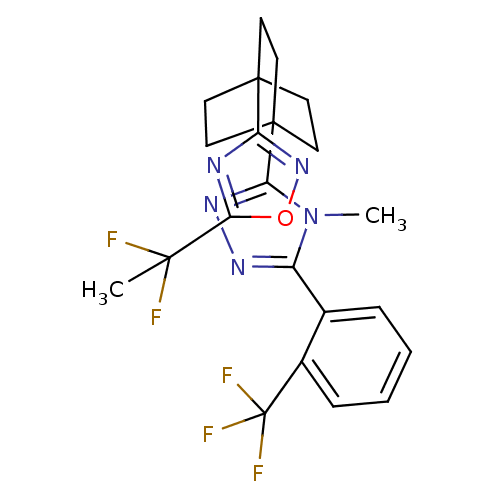 (CHEMBL2391968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289805 (US10093683, Example 116A | US10093683, Example 116...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.51 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289849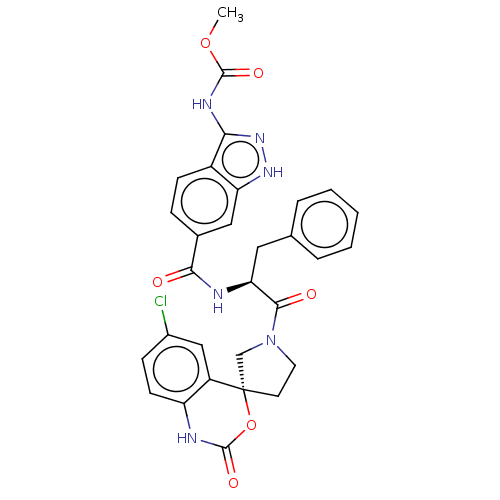 (Methyl 3-amino-6-(((S)-1-((R)-6-chloro-2-oxo-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289787 (US10093683, Example 14b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.5 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289811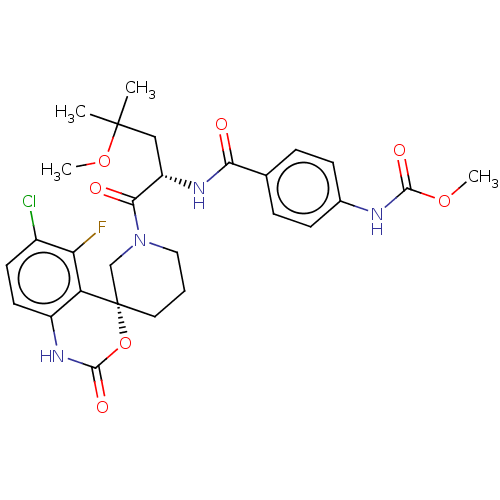 (Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.2 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289801 (Methyl (4-(((S)-1-((R)-6-chloro-2-oxo-1,2-dihydros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50340386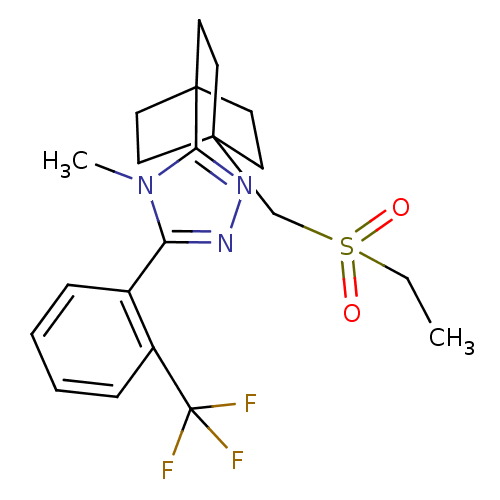 (3-(4-(ethylsulfonylmethyl)bicyclo[2.2.2]octan-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289803 (N—((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.6 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289848 (Methyl 3-amino-6-(((S)-1-((R)-6-chloro-2-oxo-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.1 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289796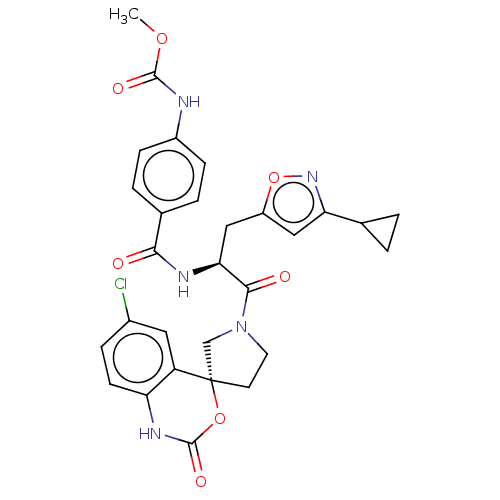 (Methyl(4-(((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28.1 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289794 (US10093683, Example 21b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 31.2 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289797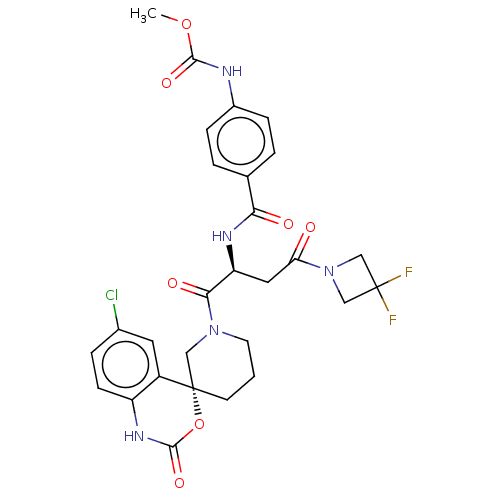 (Methyl (4-(((S)-1-((R)-6-chloro-2-oxo-1,2-dihydros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33.3 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289816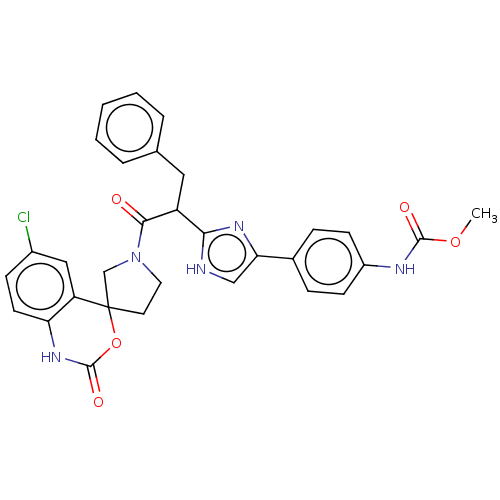 (US10093683, Example 122a | US10093683, Example 122...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33.6 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289799 (Methyl (4-(((S)-1-((R)-6-chloro-2-oxo-1,2-dihydros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 50.7 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289798 (Methyl (4-(((S)-1-((R)-6-chloro-2-oxo-1,2-dihydros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 52 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289800 (Methyl (4-(((2S)-1-((R)-6-chloro-2-oxo-1,2-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 52 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289843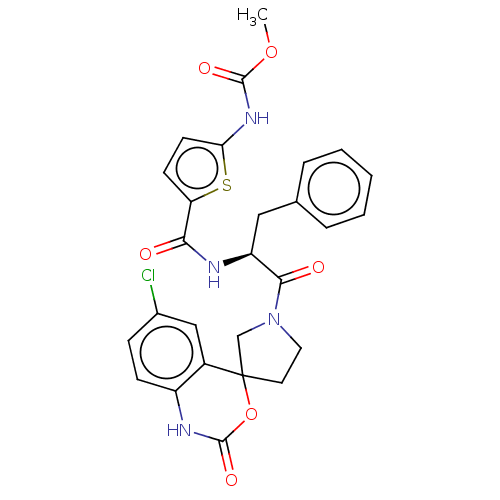 (Methyl (5-(((2S)-1-(6-chloro-2-oxo-1,2-dihydrospir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 63.4 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289840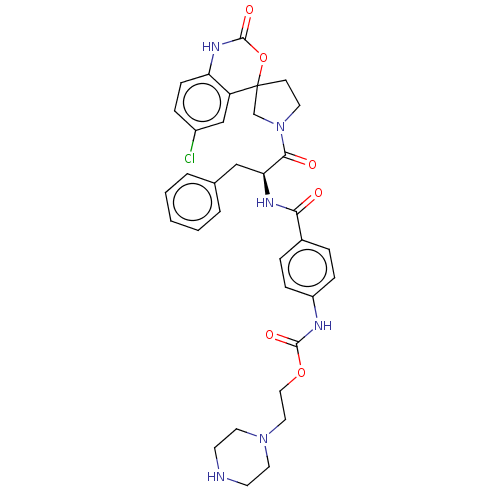 (2-(Piperazin-1-yl)ethyl (4-(((2S)-1-(6-chloro-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 76.7 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289826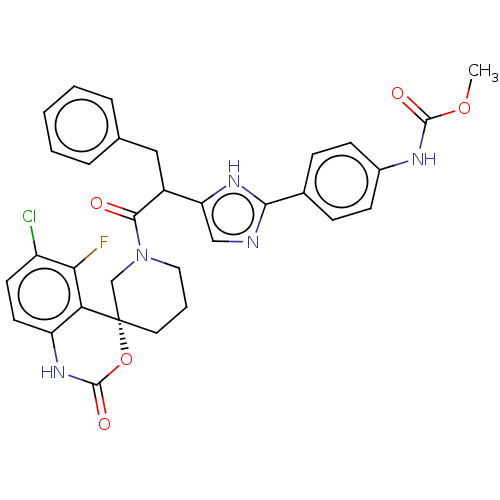 (US10093683, Example 130 | US10093683, Example 130A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 91.9 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 95.4 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289841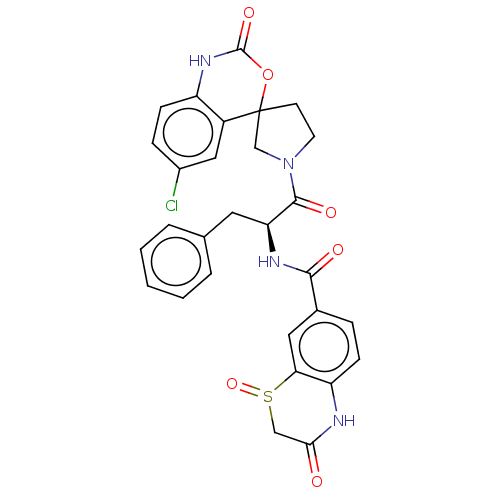 (N-((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro[benzo[d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 99.7 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289842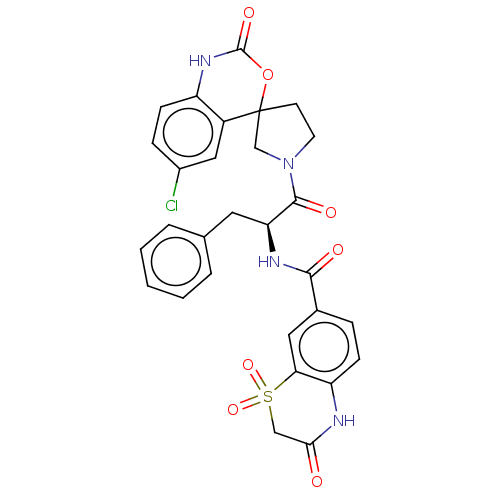 (N-((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro[benzo[d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 145 | -39.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289823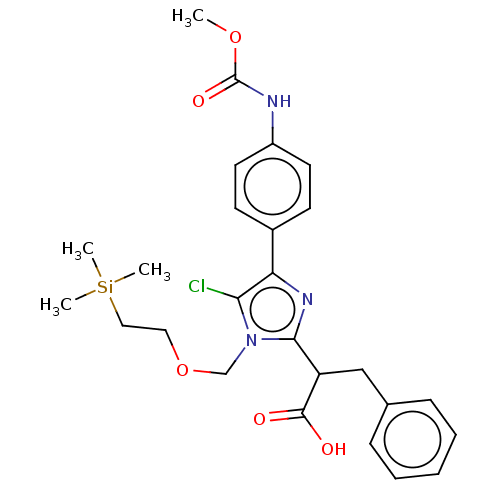 (US10093683, Example 126B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 154 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289820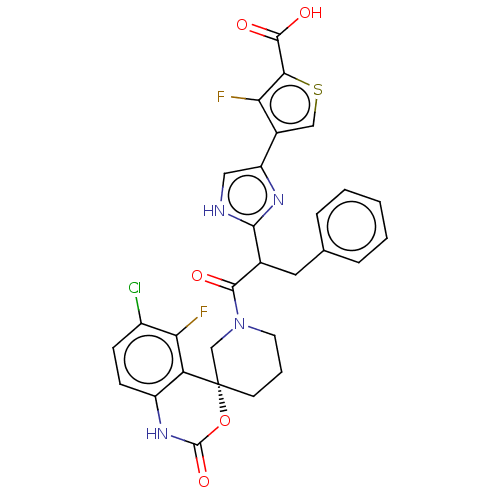 (Methyl 4-(5-(1-(tert-butoxy)-1-oxo-3-phenylpropan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 209 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289802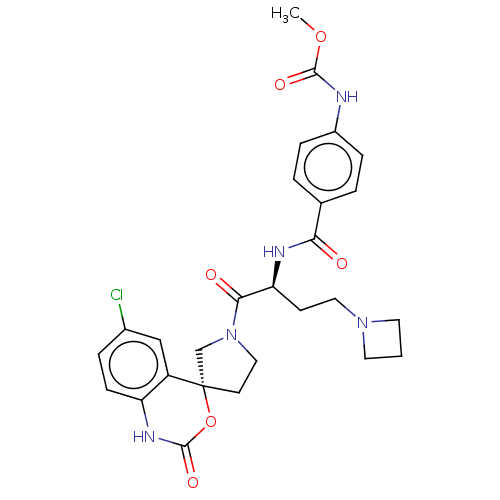 (Methyl (4-(((S)-4-(azetidin-1-yl)-1-((R)-6-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 219 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 246 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289771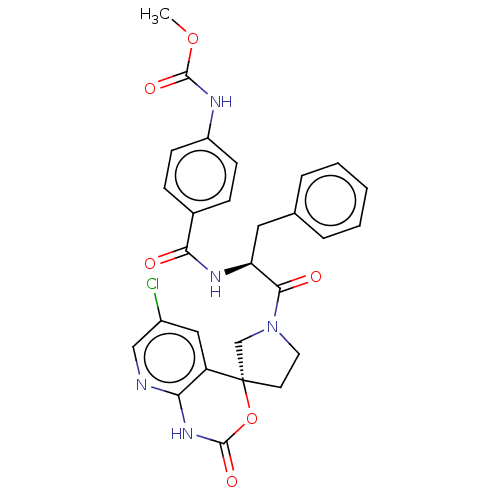 (US10093683, Example 1b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 246 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289824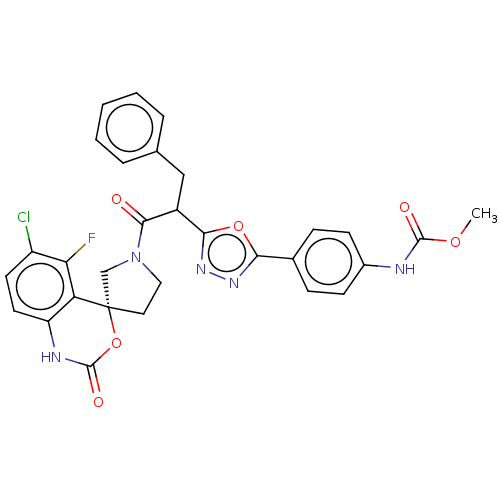 (US10093683, Example 129A | US10093683, Example 129...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 320 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289821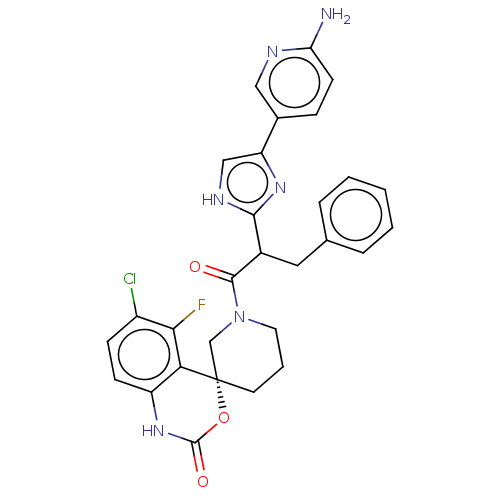 (2-(5-(6-aminopyridin-3-yl)-1H-imidazol-2-yl)-3-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 353 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289829 (US10093683, Example 131a | US10093683, Example 131...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 354 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289826 (US10093683, Example 130 | US10093683, Example 130A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 360 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289816 (US10093683, Example 122a | US10093683, Example 122...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 364 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289852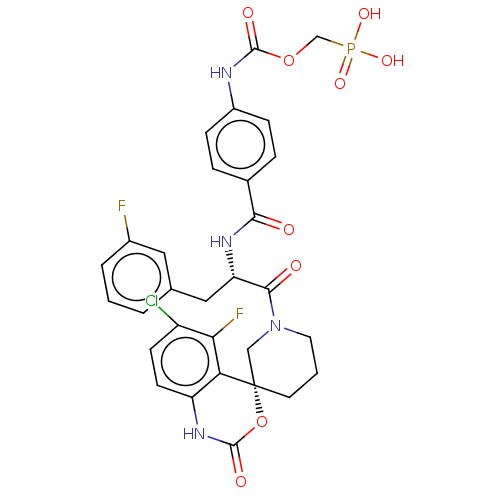 ((2-(4-(((S)-1-((R)-6-Chloro-5-fluoro-2-oxo-1,2-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 367 | -36.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289835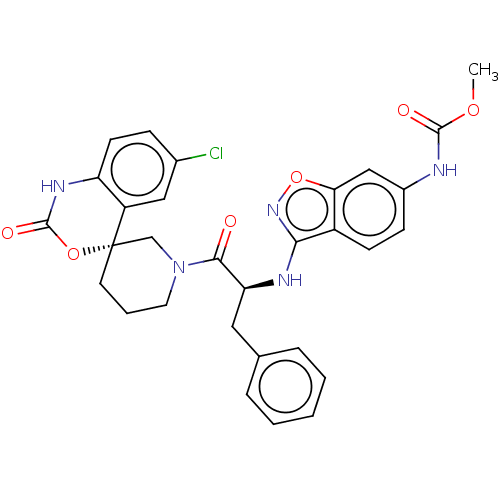 (Methyl (3-(((S)-1-((R)-6-chloro-2-oxo-1,2-dihydros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 523 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289792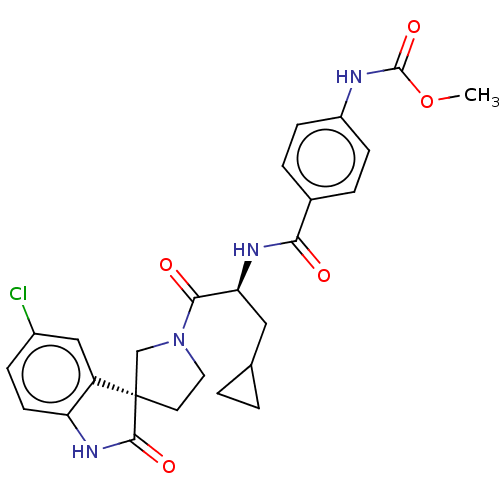 (US10093683, Example 20B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 539 | -35.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289790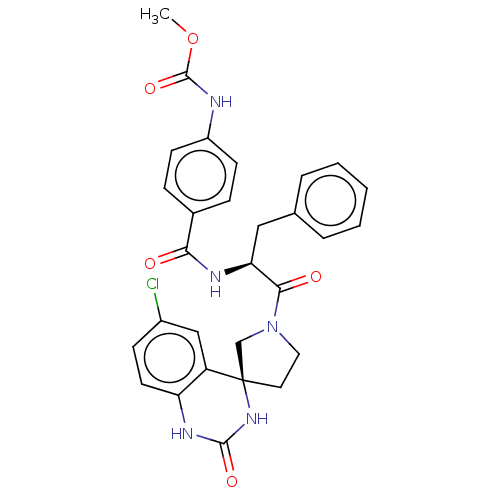 (US10093683, Example 19b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 708 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289793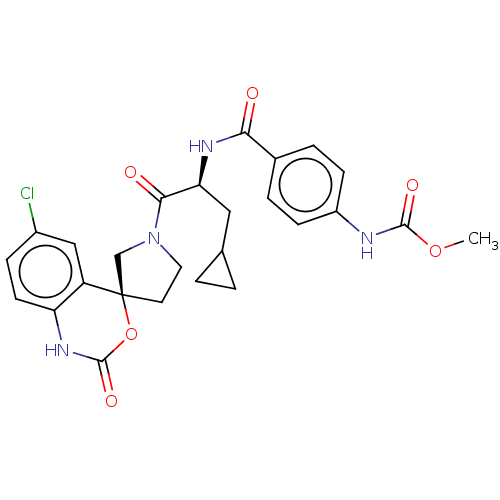 (US10093683, Example 21a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 726 | -35.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289833 (Methyl (2-(1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >875 | >-34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289826 (US10093683, Example 130 | US10093683, Example 130A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >875 | >-34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289824 (US10093683, Example 129A | US10093683, Example 129...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >875 | >-34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 296 total ) | Next | Last >> |