

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50343725 ((S)-5-fluoro-2-(1-(4-fluorophenyl)ethylamino)-6-(5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech Curated by ChEMBL | Assay Description Inhibition of JAK2 V617F mutant (unknown origin) using L-Ahx-IPTSPITTTYFFFKKK-COOH as substrate in presence of ATP | Bioorg Med Chem 24: 4647-4651 (2016) Article DOI: 10.1016/j.bmc.2016.07.069 BindingDB Entry DOI: 10.7270/Q2D79FW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11694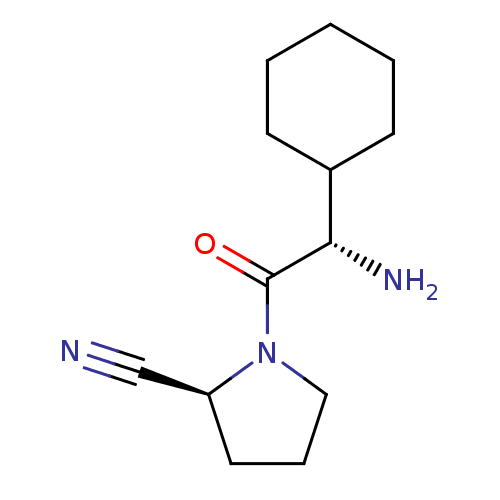 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards Dipeptidyl peptidase IV (DPP-IV) | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM404301 (US10017501, Compound 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM404301 (US10017501, Compound 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249309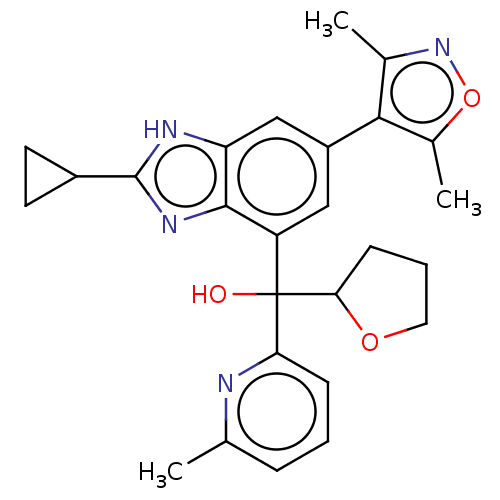 (US10017501, Compound 1020-114 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249314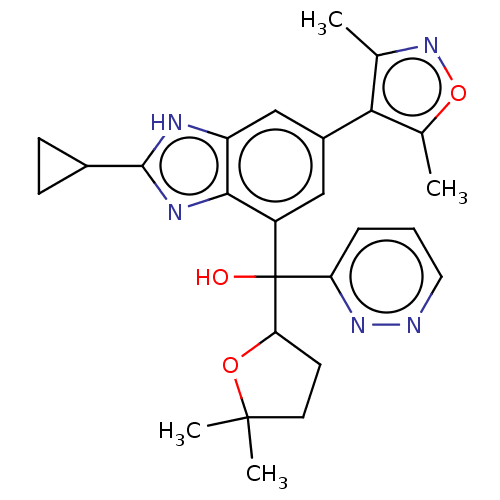 (US10017501, Compound 1020-298 | US9458145, 1020-29...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249309 (US10017501, Compound 1020-114 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277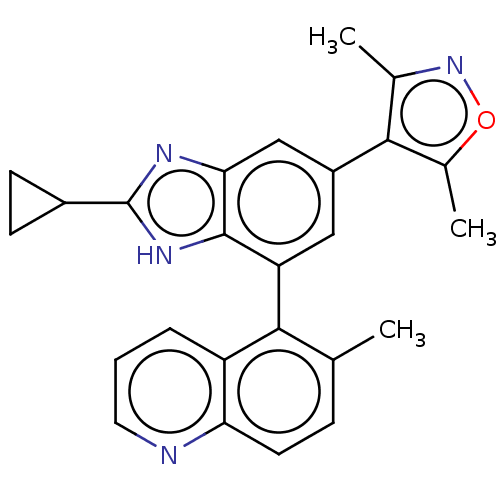 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249305 (US10017501, Compound 1020-103 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249312 (US10017501, Compound 1020-257 | US9458145, 1020-25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249314 (US10017501, Compound 1020-298 | US9458145, 1020-29...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249312 (US10017501, Compound 1020-257 | US9458145, 1020-25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249311 (US10017501, Compound 1020-239 | US9458145, 1020-23...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249311 (US10017501, Compound 1020-239 | US9458145, 1020-23...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249306 (US10017501, Compound 1020-104 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249307 (US10017501, Compound 1020-112 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249308 (US10017501, Compound 1020-113 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249310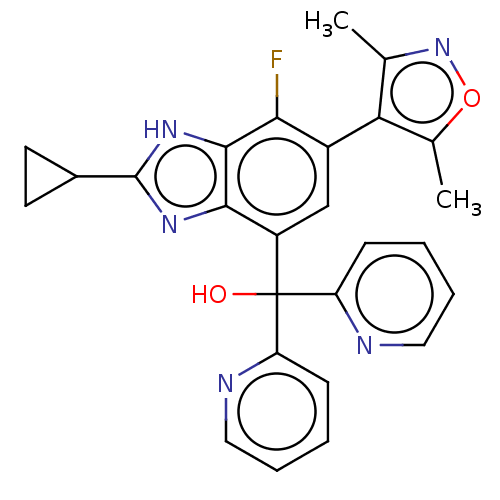 (US10017501, Compound 1020-224 | US9458145, 1020-22...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249306 (US10017501, Compound 1020-104 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249305 (US10017501, Compound 1020-103 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249308 (US10017501, Compound 1020-113 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249307 (US10017501, Compound 1020-112 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249310 (US10017501, Compound 1020-224 | US9458145, 1020-22...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50380682 (CHEMBL2017291 | I-BET151 (16)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50380682 (CHEMBL2017291 | I-BET151 (16)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50299583 ((S)-N-(3-bromophenyl)-N'-cyano-2-methyl-4-(5-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of LIMK1 | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299619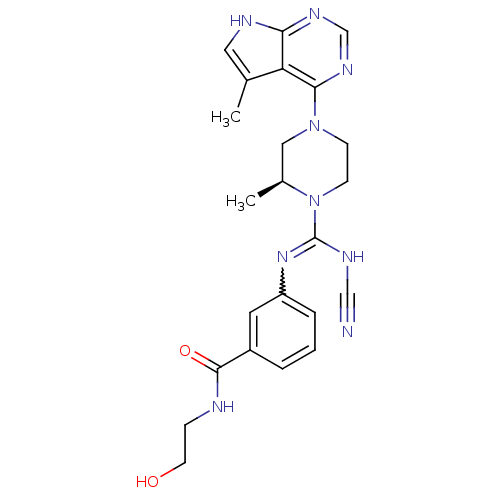 ((S)-3-(N'-cyano-2-methyl-4-(5-methyl-7H-pyrrolo[2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299583 ((S)-N-(3-bromophenyl)-N'-cyano-2-methyl-4-(5-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50134209 (CHEMBL3342974) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299616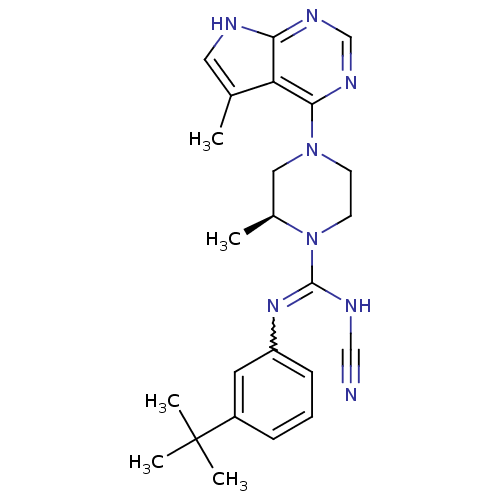 ((S)-N-(3-tert-butylphenyl)-N'-cyano-2-methyl-4-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299615 ((S)-N'-cyano-N-(3-cyanophenyl)-2-methyl-4-(5-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299586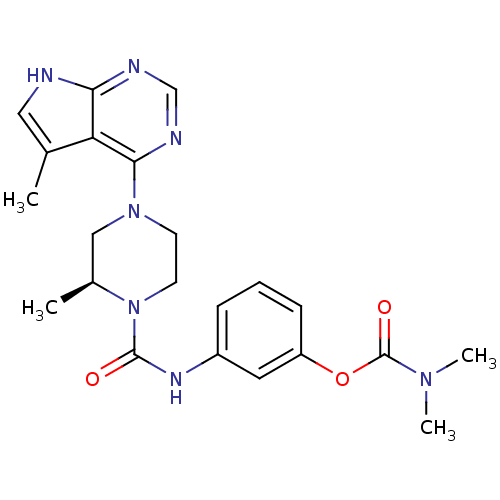 ((S)-3-(2-methyl-4-(5-methyl-7H-pyrrolo[2,3-d]pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299607 ((S)-N-(3-bromophenyl)-4-(5-chloro-7H-pyrrolo[2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299584 ((S)-N-(3-bromo-4-fluorophenyl)-N'-cyano-2-methyl-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299611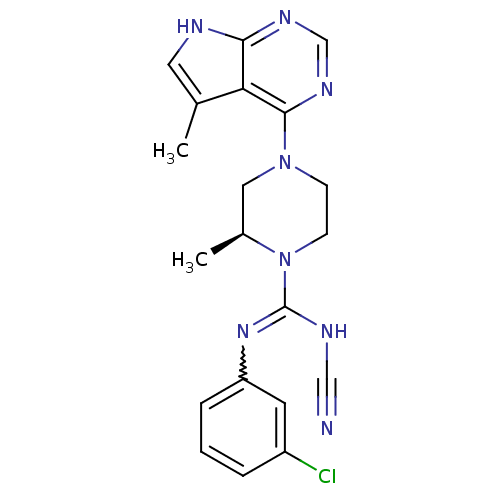 ((S)-N-(3-chlorophenyl)-N'-cyano-2-methyl-4-(5-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299623 ((S)-N'-cyano-N-(3-(N-isopropylsulfamoyl)phenyl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299613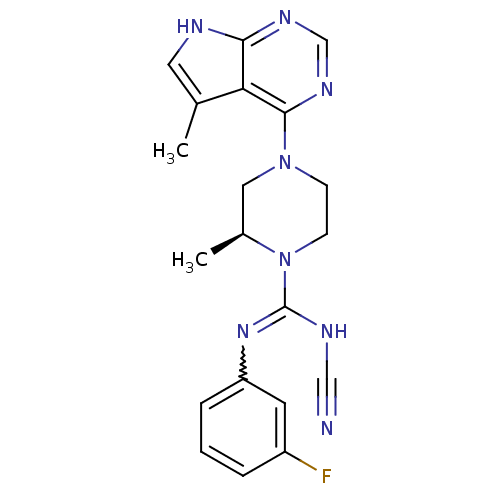 ((S)-N'-cyano-N-(3-fluorophenyl)-2-methyl-4-(5-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299614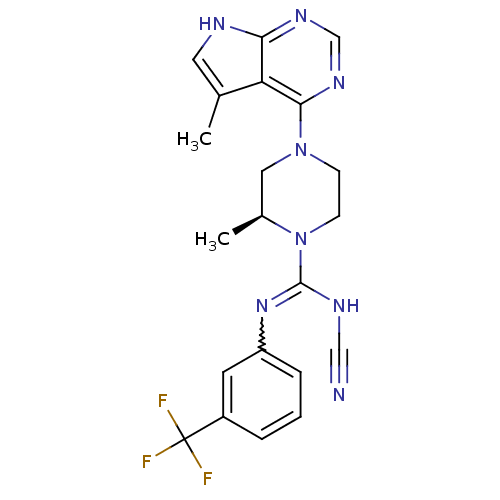 ((S)-N'-cyano-2-methyl-4-(5-methyl-7H-pyrrolo[2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249313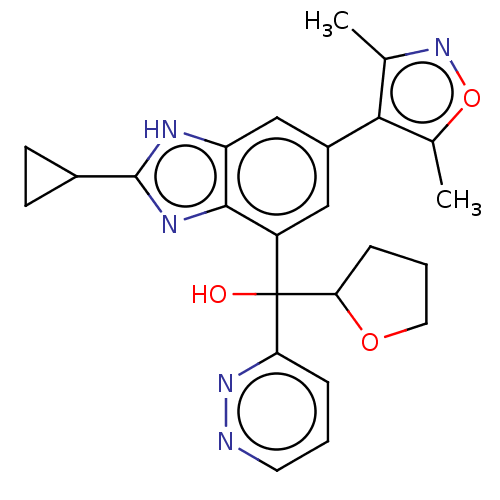 (US9458145, 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description The labeled ligand specifically binds BRD4-1 and BRD4-2 and can be displaced by a small molecule inhibitor that shares a similar or overlapping bindi... | US Patent US9458145 (2016) BindingDB Entry DOI: 10.7270/Q26Q1W53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11694 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249313 (US9458145, 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description The labeled ligand specifically binds BRD4-1 and BRD4-2 and can be displaced by a small molecule inhibitor that shares a similar or overlapping bindi... | US Patent US9458145 (2016) BindingDB Entry DOI: 10.7270/Q26Q1W53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129897 (1-(2-Amino-3-methyl-butyryl)-pyrrolidine-2-carboni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50129889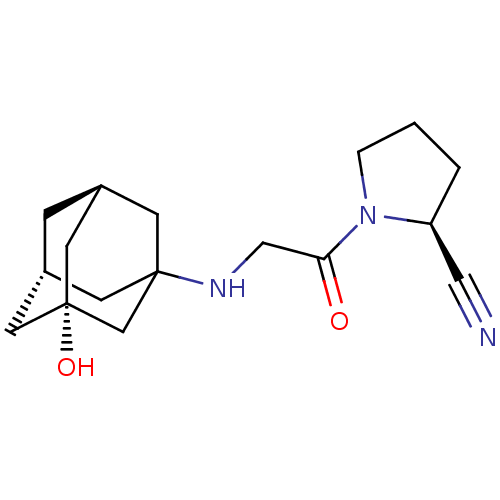 (1-[2-(3-Hydroxy-adamantan-1-ylamino)-acetyl]-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) obtained from rat plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50299612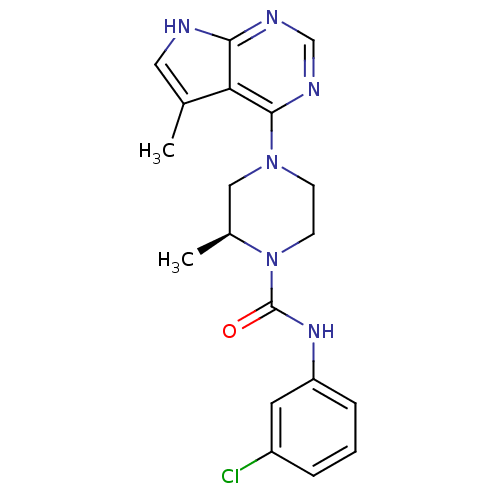 ((S)-N-(3-chlorophenyl)-2-methyl-4-(5-methyl-7H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting | J Med Chem 52: 6515-8 (2009) Article DOI: 10.1021/jm901226j BindingDB Entry DOI: 10.7270/Q2HH6K4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129889 (1-[2-(3-Hydroxy-adamantan-1-ylamino)-acetyl]-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) obtained from human plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 803 total ) | Next | Last >> |