 Found 319 hits with Last Name = 'khalil' and Initial = 'e'
Found 319 hits with Last Name = 'khalil' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074092

(6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...)Show SMILES NC(=O)C1CCS[C@@H]2C[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-4-8-24-12-9-16(15(23)20(11)12)5-2-7-19(16)14(22)10-3-1-6-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11?,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50060601
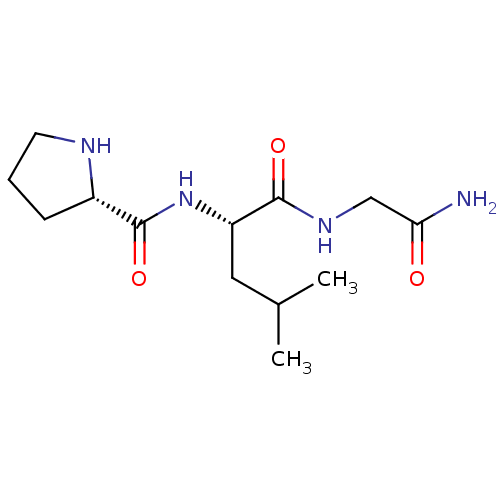
((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)NCC(N)=O Show InChI InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074093
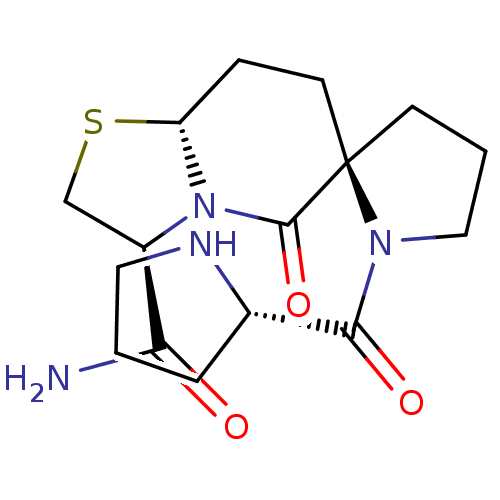
((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...)Show SMILES NC(=O)[C@H]1CS[C@@H]2CC[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 |r| Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-9-24-12-4-6-16(15(23)20(11)12)5-2-8-19(16)14(22)10-3-1-7-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11+,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50060601

((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)NCC(N)=O Show InChI InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074092

(6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...)Show SMILES NC(=O)C1CCS[C@@H]2C[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-4-8-24-12-9-16(15(23)20(11)12)5-2-7-19(16)14(22)10-3-1-6-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11?,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074093

((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...)Show SMILES NC(=O)[C@H]1CS[C@@H]2CC[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 |r| Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-9-24-12-4-6-16(15(23)20(11)12)5-2-8-19(16)14(22)10-3-1-7-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11+,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074092

(6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...)Show SMILES NC(=O)C1CCS[C@@H]2C[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-4-8-24-12-9-16(15(23)20(11)12)5-2-7-19(16)14(22)10-3-1-6-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11?,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074093

((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...)Show SMILES NC(=O)[C@H]1CS[C@@H]2CC[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 |r| Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-9-24-12-4-6-16(15(23)20(11)12)5-2-8-19(16)14(22)10-3-1-7-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11+,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50060601

((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)NCC(N)=O Show InChI InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074092

(6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...)Show SMILES NC(=O)C1CCS[C@@H]2C[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-4-8-24-12-9-16(15(23)20(11)12)5-2-7-19(16)14(22)10-3-1-6-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11?,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50060601

((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)NCC(N)=O Show InChI InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074093

((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...)Show SMILES NC(=O)[C@H]1CS[C@@H]2CC[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 |r| Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-9-24-12-4-6-16(15(23)20(11)12)5-2-8-19(16)14(22)10-3-1-7-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11+,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182163
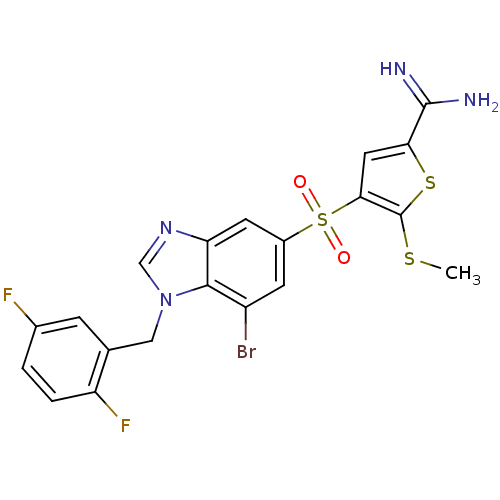
(4-[7-bromo-1-(2,5-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(F)ccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)12-5-13(21)18-15(6-12)26-9-27(18)8-10-4-11(22)2-3-14(10)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233679
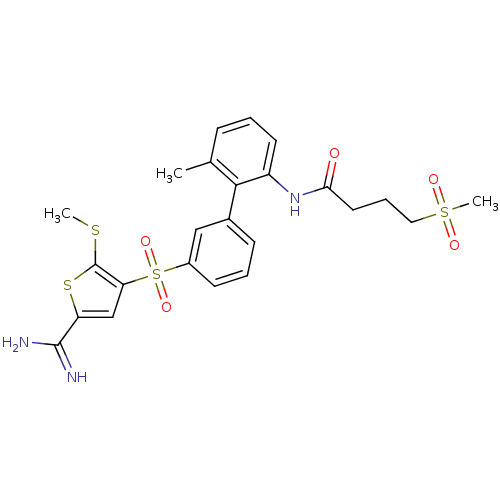
(CHEMBL399284 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C24H27N3O5S4/c1-15-7-4-10-18(27-21(28)11-6-12-35(3,29)30)22(15)16-8-5-9-17(13-16)36(31,32)20-14-19(23(25)26)34-24(20)33-2/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H3,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182160
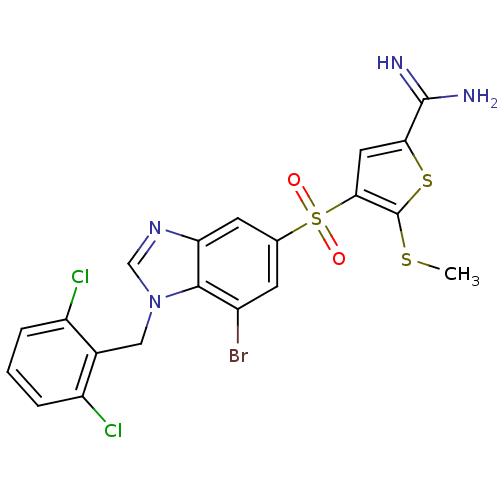
(4-[7-bromo-1-(2,6-dichloro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3c(Cl)cccc3Cl)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrCl2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233691

(4-(2'-amino-6'-methyl-biphenyl-3-sulfonyl)-5-methy...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1N)C(N)=N Show InChI InChI=1S/C19H19N3O2S3/c1-11-5-3-8-14(20)17(11)12-6-4-7-13(9-12)27(23,24)16-10-15(18(21)22)26-19(16)25-2/h3-10H,20H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233674
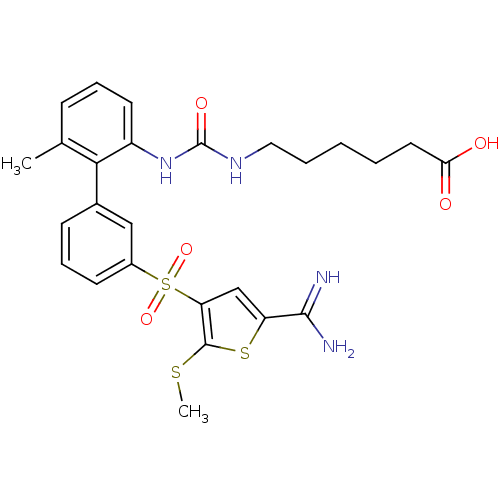
(6-{3-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophe...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCCCCC(O)=O)C(N)=N Show InChI InChI=1S/C26H30N4O5S3/c1-16-8-6-11-19(30-26(33)29-13-5-3-4-12-22(31)32)23(16)17-9-7-10-18(14-17)38(34,35)21-15-20(24(27)28)37-25(21)36-2/h6-11,14-15H,3-5,12-13H2,1-2H3,(H3,27,28)(H,31,32)(H2,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182171
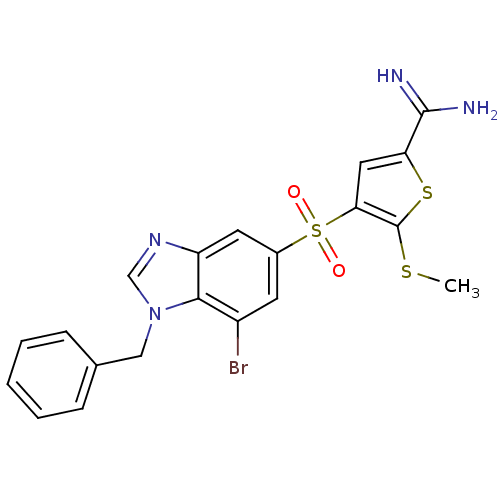
(4-(1-benzyl-7-bromo-1H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3ccccc3)cnc2c1)C(N)=N Show InChI InChI=1S/C20H17BrN4O2S3/c1-28-20-17(9-16(29-20)19(22)23)30(26,27)13-7-14(21)18-15(8-13)24-11-25(18)10-12-5-3-2-4-6-12/h2-9,11H,10H2,1H3,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182170
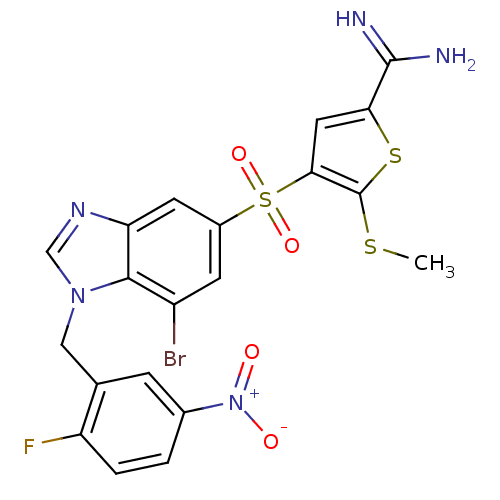
(4-[7-bromo-1-(2-fluoro-5-nitro-benzyl)-1H-benzoimi...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(ccc3F)[N+]([O-])=O)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrFN5O4S3/c1-32-20-17(7-16(33-20)19(23)24)34(30,31)12-5-13(21)18-15(6-12)25-9-26(18)8-10-4-11(27(28)29)2-3-14(10)22/h2-7,9H,8H2,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233686

(4-(2'-methyl-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C)C(N)=N Show InChI InChI=1S/C19H18N2O2S3/c1-12-6-3-4-9-15(12)13-7-5-8-14(10-13)26(22,23)17-11-16(18(20)21)25-19(17)24-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233692

(4-[3-(6-methyl-pyridin-2-yl)-benzenesulfonyl]-5-me...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(C)n1)C(N)=N Show InChI InChI=1S/C18H17N3O2S3/c1-11-5-3-8-14(21-11)12-6-4-7-13(9-12)26(22,23)16-10-15(17(19)20)25-18(16)24-2/h3-10H,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182159
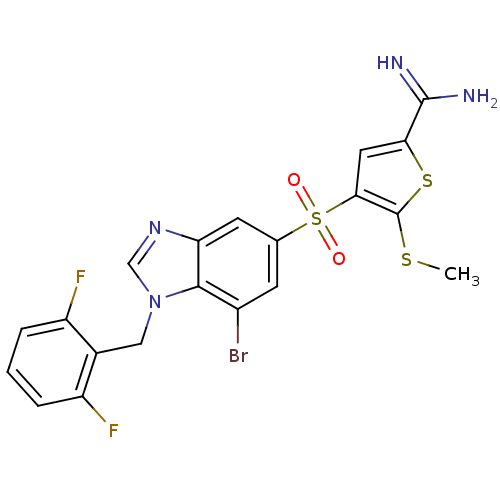
(4-[7-bromo-1-(2,6-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3c(F)cccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233688

(4-(2'-chloro-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1Cl)C(N)=N Show InChI InChI=1S/C18H15ClN2O2S3/c1-24-18-16(10-15(25-18)17(20)21)26(22,23)12-6-4-5-11(9-12)13-7-2-3-8-14(13)19/h2-10H,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233694

(5-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCCC(O)=O)C(N)=N Show InChI InChI=1S/C25H27N3O5S3/c1-15-7-5-10-18(28-21(29)11-3-4-12-22(30)31)23(15)16-8-6-9-17(13-16)36(32,33)20-14-19(24(26)27)35-25(20)34-2/h5-10,13-14H,3-4,11-12H2,1-2H3,(H3,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182176
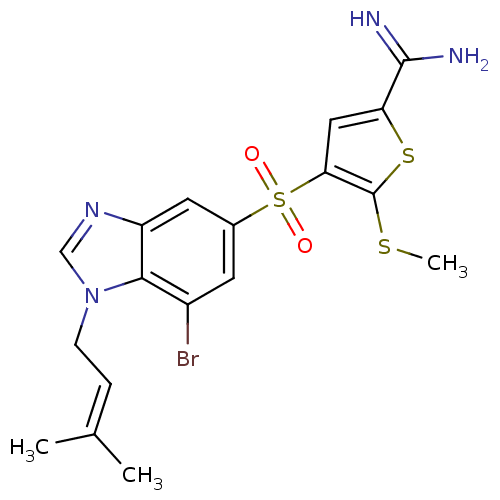
(4-[7-bromo-1-(3-methyl-but-2-enyl)-1H-benzoimidazo...)Show SMILES [#6]-[#16]-c1sc(cc1S(=O)(=O)c1cc(Br)c2n(-[#6]\[#6]=[#6](/[#6])-[#6])cnc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C18H19BrN4O2S3/c1-10(2)4-5-23-9-22-13-7-11(6-12(19)16(13)23)28(24,25)15-8-14(17(20)21)27-18(15)26-3/h4,6-9H,5H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233677

(4-(2'-hydroxymethyl-6'-methyl-biphenyl-3-sulfonyl)...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1CO)C(N)=N Show InChI InChI=1S/C20H20N2O3S3/c1-12-5-3-7-14(11-23)18(12)13-6-4-8-15(9-13)28(24,25)17-10-16(19(21)22)27-20(17)26-2/h3-10,23H,11H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147047
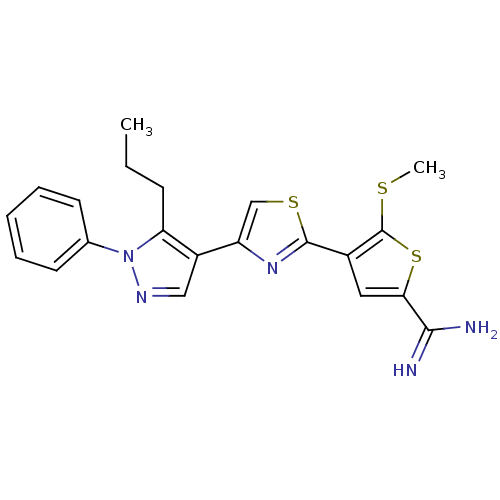
(5-Methylsulfanyl-4-[4-(1-phenyl-5-propyl-1H-pyrazo...)Show SMILES CCCc1c(cnn1-c1ccccc1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C21H21N5S3/c1-3-7-17-15(11-24-26(17)13-8-5-4-6-9-13)16-12-28-20(25-16)14-10-18(19(22)23)29-21(14)27-2/h4-6,8-12H,3,7H2,1-2H3,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50060601

((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)NCC(N)=O Show InChI InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of ... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50060601

((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)NCC(N)=O Show InChI InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of ... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50060601

((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)NCC(N)=O Show InChI InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of G... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233689
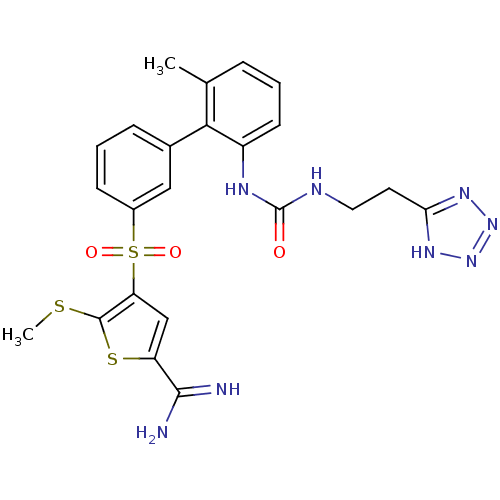
(5-methylsulfanyl-4-(6'-methyl-2'-{3-[2-(2H-tetrazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCc1nnn[nH]1)C(N)=N Show InChI InChI=1S/C23H24N8O3S3/c1-13-5-3-8-16(27-23(32)26-10-9-19-28-30-31-29-19)20(13)14-6-4-7-15(11-14)37(33,34)18-12-17(21(24)25)36-22(18)35-2/h3-8,11-12H,9-10H2,1-2H3,(H3,24,25)(H2,26,27,32)(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147046
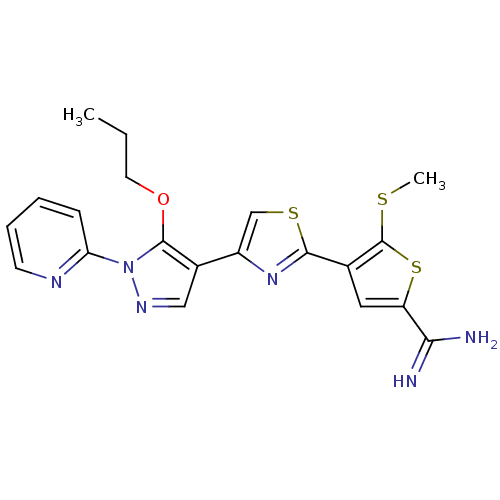
(5-Methylsulfanyl-4-[4-(5-propoxy-1-pyridin-2-yl-1H...)Show SMILES CCCOc1c(cnn1-c1ccccn1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C20H20N6OS3/c1-3-8-27-19-13(10-24-26(19)16-6-4-5-7-23-16)14-11-29-18(25-14)12-9-15(17(21)22)30-20(12)28-2/h4-7,9-11H,3,8H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182185
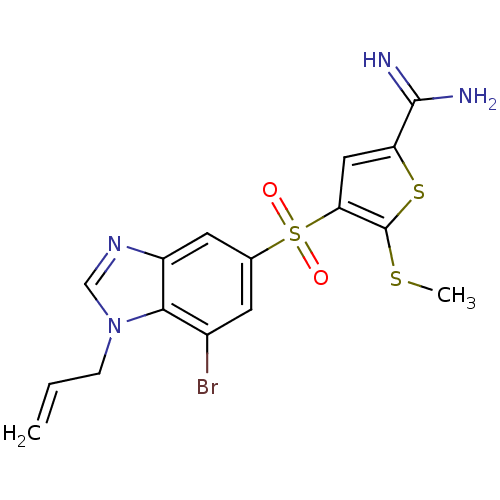
(4-(1-allyl-7-bromo-1H-benzoimidazole-5-sulfonyl)-5...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(CC=C)cnc2c1)C(N)=N Show InChI InChI=1S/C16H15BrN4O2S3/c1-3-4-21-8-20-11-6-9(5-10(17)14(11)21)26(22,23)13-7-12(15(18)19)25-16(13)24-2/h3,5-8H,1,4H2,2H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50060601

((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)NCC(N)=O Show InChI InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 73.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of G... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147059
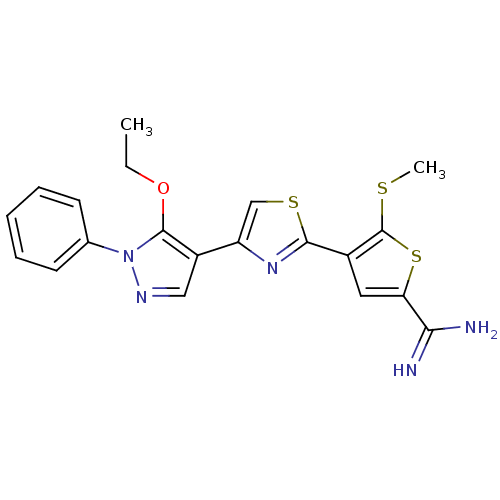
(4-[4-(5-Ethoxy-1-phenyl-1H-pyrazol-4-yl)-thiazol-2...)Show SMILES CCOc1c(cnn1-c1ccccc1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C20H19N5OS3/c1-3-26-19-14(10-23-25(19)12-7-5-4-6-8-12)15-11-28-18(24-15)13-9-16(17(21)22)29-20(13)27-2/h4-11H,3H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182183

(4-[7-chloro-3-(2,6-difluoro-benzyl)-3H-benzoimidaz...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Cl)c2ncn(Cc3c(F)cccc3F)c2c1)C(N)=N Show InChI InChI=1S/C20H15ClF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182173
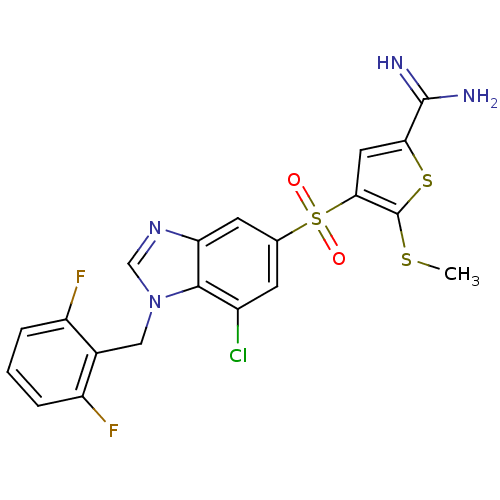
(4-[7-chloro-1-(2,6-difluoro-benzyl)-1H-benzoimidaz...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Cl)c2n(Cc3c(F)cccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15ClF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233693

(CHEMBL252619 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C22H23N3O5S4/c1-13-6-4-9-16(25-19(26)12-33(3,27)28)20(13)14-7-5-8-15(10-14)34(29,30)18-11-17(21(23)24)32-22(18)31-2/h4-11H,12H2,1-3H3,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233680

(4-(2'-hydroxymethyl-biphenyl-3-sulfonyl)-5-methyls...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1CO)C(N)=N Show InChI InChI=1S/C19H18N2O3S3/c1-25-19-17(10-16(26-19)18(20)21)27(23,24)14-7-4-6-12(9-14)15-8-3-2-5-13(15)11-22/h2-10,22H,11H2,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233681
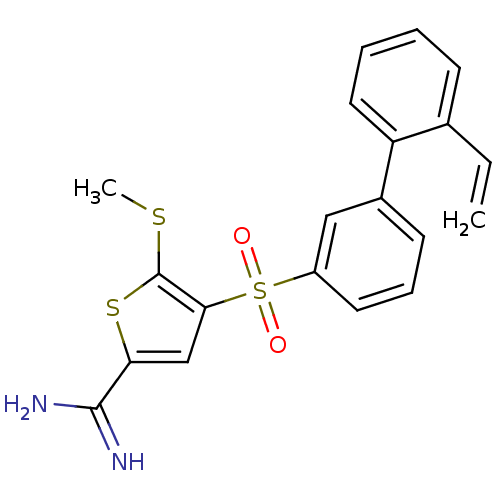
(5-methylsulfanyl-4-(2'-vinyl-biphenyl-3-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C=C)C(N)=N Show InChI InChI=1S/C20H18N2O2S3/c1-3-13-7-4-5-10-16(13)14-8-6-9-15(11-14)27(23,24)18-12-17(19(21)22)26-20(18)25-2/h3-12H,1H2,2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233676

(3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-3-s...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1C(O)=O)C(N)=N Show InChI InChI=1S/C20H18N2O4S3/c1-11-5-3-8-14(19(23)24)17(11)12-6-4-7-13(9-12)29(25,26)16-10-15(18(21)22)28-20(16)27-2/h3-10H,1-2H3,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50147054
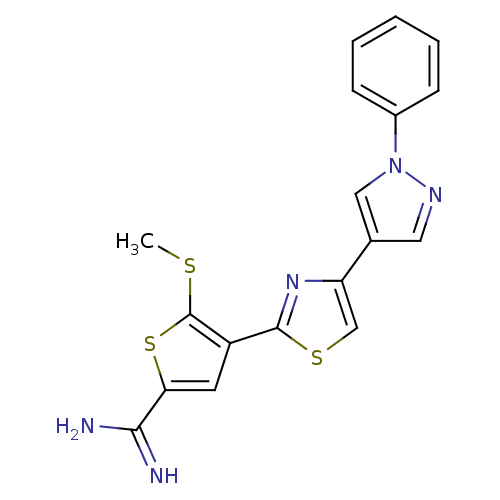
(5-Methylsulfanyl-4-[4-(1-phenyl-1H-pyrazol-4-yl)-t...)Show SMILES CSc1sc(cc1-c1nc(cs1)-c1cnn(c1)-c1ccccc1)C(N)=N Show InChI InChI=1S/C18H15N5S3/c1-24-18-13(7-15(26-18)16(19)20)17-22-14(10-25-17)11-8-21-23(9-11)12-5-3-2-4-6-12/h2-10H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Complement C1s subcomponent |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233683
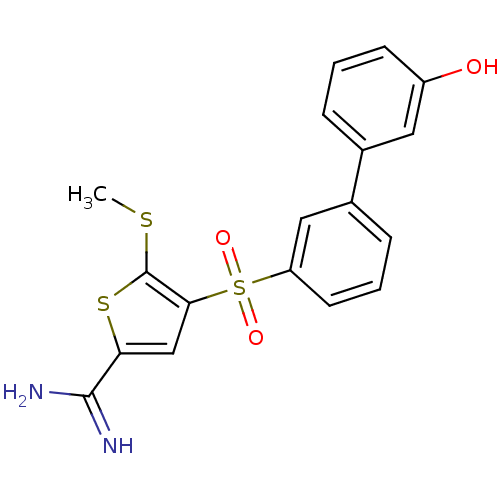
(4-(3'-hydroxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(O)c1)C(N)=N Show InChI InChI=1S/C18H16N2O3S3/c1-24-18-16(10-15(25-18)17(19)20)26(22,23)14-7-3-5-12(9-14)11-4-2-6-13(21)8-11/h2-10,21H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182164

(4-[7-bromo-3-(2,6-dichloro-benzyl)-3H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3c(Cl)cccc3Cl)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrCl2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074092

(6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...)Show SMILES NC(=O)C1CCS[C@@H]2C[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-4-8-24-12-9-16(15(23)20(11)12)5-2-7-19(16)14(22)10-3-1-6-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11?,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of G... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182181
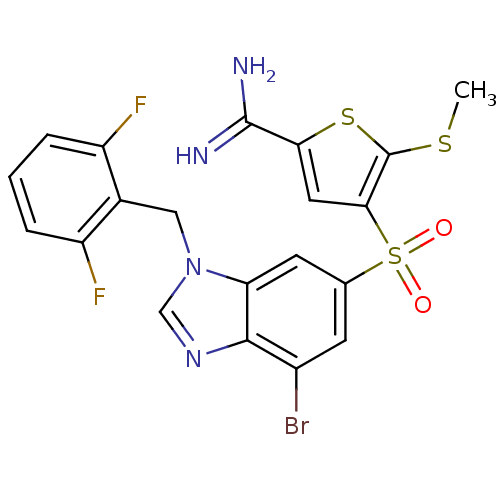
(4-[7-bromo-3-(2,6-difluoro-benzyl)-3H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3c(F)cccc3F)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182174
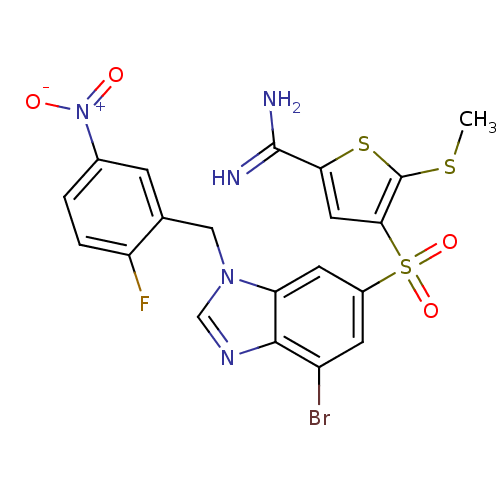
(4-[7-bromo-3-(2-fluoro-5-nitro-benzyl)-3H-benzoimi...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3cc(ccc3F)[N+]([O-])=O)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrFN5O4S3/c1-32-20-17(7-16(33-20)19(23)24)34(30,31)12-5-13(21)18-15(6-12)26(9-25-18)8-10-4-11(27(28)29)2-3-14(10)22/h2-7,9H,8H2,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50147046

(5-Methylsulfanyl-4-[4-(5-propoxy-1-pyridin-2-yl-1H...)Show SMILES CCCOc1c(cnn1-c1ccccn1)-c1csc(n1)-c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C20H20N6OS3/c1-3-8-27-19-13(10-24-26(19)16-6-4-5-7-23-16)14-11-29-18(25-14)12-9-15(17(21)22)30-20(12)28-2/h4-7,9-11H,3,8H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards trypsin was determined |
Bioorg Med Chem Lett 14: 3043-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.034
BindingDB Entry DOI: 10.7270/Q2K35T33 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182180
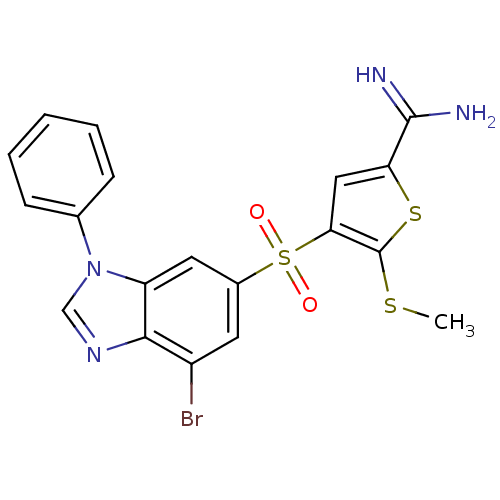
(4-(7-bromo-3-phenyl-3H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(-c3ccccc3)c2c1)C(N)=N Show InChI InChI=1S/C19H15BrN4O2S3/c1-27-19-16(9-15(28-19)18(21)22)29(25,26)12-7-13(20)17-14(8-12)24(10-23-17)11-5-3-2-4-6-11/h2-10H,1H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50074093

((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...)Show SMILES NC(=O)[C@H]1CS[C@@H]2CC[C@]3(CCCN3C(=O)[C@@H]3CCCN3)C(=O)N12 |r| Show InChI InChI=1S/C16H24N4O3S/c17-13(21)11-9-24-12-4-6-16(15(23)20(11)12)5-2-8-19(16)14(22)10-3-1-7-18-10/h10-12,18H,1-9H2,(H2,17,21)/t10-,11+,12+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of G... |
J Med Chem 42: 628-37 (1999)
Article DOI: 10.1021/jm980525q
BindingDB Entry DOI: 10.7270/Q27080MW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data