

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324036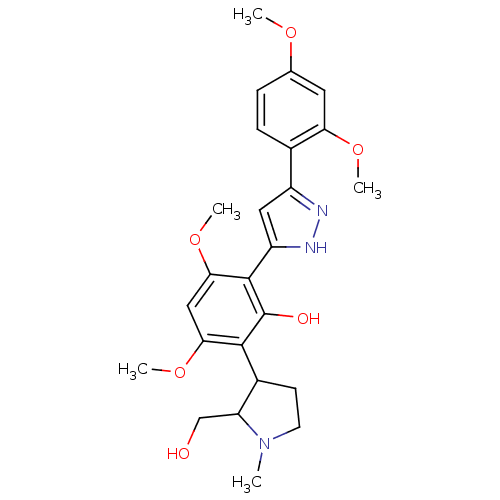 (2-[5-(2,4-Dimethoxy-phenyl)-1H-pyrazol-3-yl]-6-(2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324036 (2-[5-(2,4-Dimethoxy-phenyl)-1H-pyrazol-3-yl]-6-(2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase assessed as oxidation of L-DOPA | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491384 (CHEMBL2380377) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase diphenolase activity assessed as L-DOPA conversion to dopachrome by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491381 (CHEMBL2380379) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase diphenolase activity assessed as L-DOPA conversion to dopachrome by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491382 (CHEMBL2380378) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase diphenolase activity assessed as L-DOPA conversion to dopachrome by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324035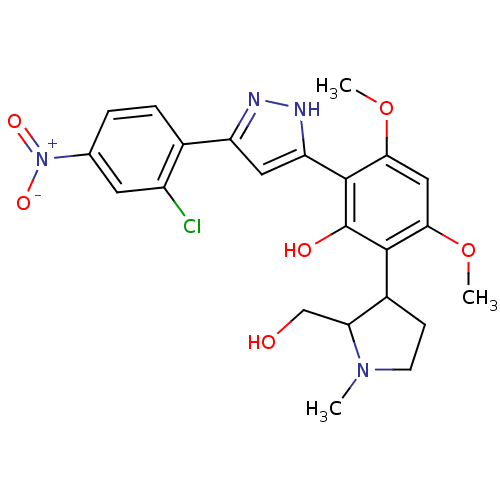 (2-[5-(2-Chloro-4-nitro-phenyl)-1H-pyrazol-3-yl]-6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324034 (2-[5-(2-Chloro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324037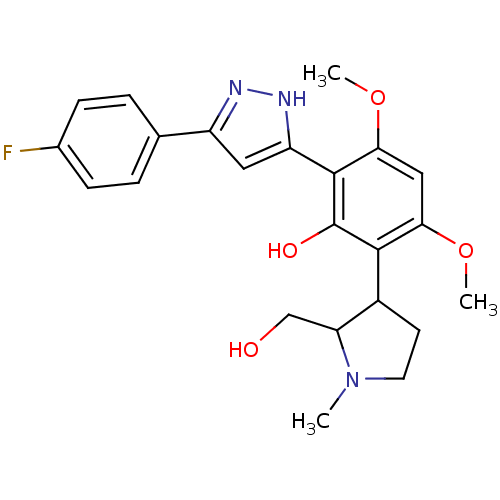 (2-[5-(4-Fluoro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324034 (2-[5-(2-Chloro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase assessed as oxidation of L-DOPA | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324037 (2-[5-(4-Fluoro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase assessed as oxidation of L-DOPA | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324035 (2-[5-(2-Chloro-4-nitro-phenyl)-1H-pyrazol-3-yl]-6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase assessed as oxidation of L-DOPA | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase diphenolase activity assessed as L-DOPA conversion to dopachrome by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491386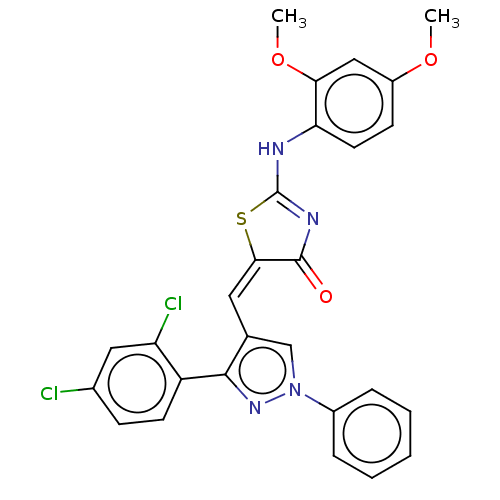 (CHEMBL2380491) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase diphenolase activity assessed as L-DOPA conversion to dopachrome by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491380 (CHEMBL2380380) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase diphenolase activity assessed as L-DOPA conversion to dopachrome by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491383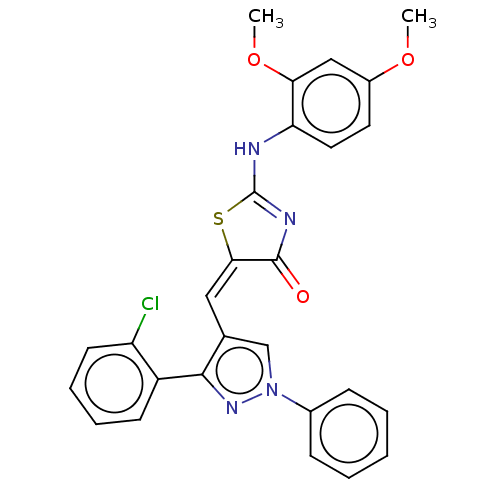 (CHEMBL2380490) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase diphenolase activity assessed as L-DOPA conversion to dopachrome by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324036 (2-[5-(2,4-Dimethoxy-phenyl)-1H-pyrazol-3-yl]-6-(2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324036 (2-[5-(2,4-Dimethoxy-phenyl)-1H-pyrazol-3-yl]-6-(2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase assessed as oxidation of L-DOPA | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM35440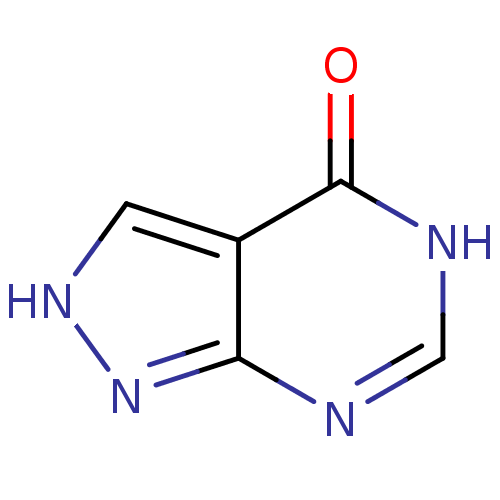 (ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423923 (CHEMBL2313040) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423922 (CHEMBL2313041) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423924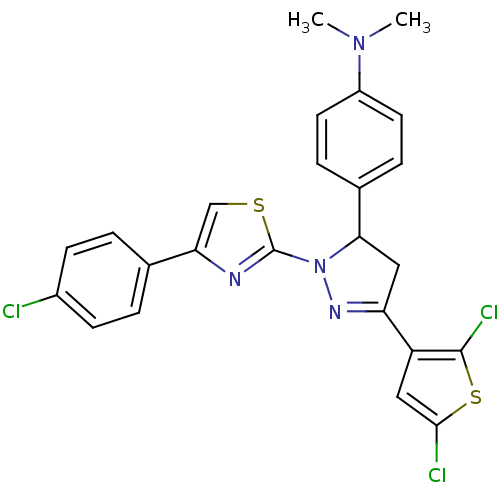 (CHEMBL2313039) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324037 (2-[5-(4-Fluoro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324035 (2-[5-(2-Chloro-4-nitro-phenyl)-1H-pyrazol-3-yl]-6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324037 (2-[5-(4-Fluoro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase assessed as oxidation of L-DOPA | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423932 (CHEMBL2313031) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324035 (2-[5-(2-Chloro-4-nitro-phenyl)-1H-pyrazol-3-yl]-6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase assessed as oxidation of L-DOPA | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324034 (2-[5-(2-Chloro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423931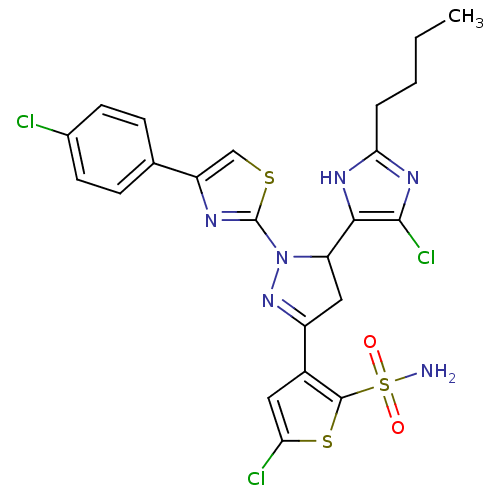 (CHEMBL2313032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423933 (CHEMBL2313030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50324034 (2-[5-(2-Chloro-phenyl)-1H-pyrazol-3-yl]-6-(2-hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Solapur University Curated by ChEMBL | Assay Description Inhibition of mashroom tyrosinase assessed as oxidation of L-DOPA | Bioorg Med Chem 18: 6149-55 (2010) Article DOI: 10.1016/j.bmc.2010.06.046 BindingDB Entry DOI: 10.7270/Q2GQ6XXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423929 (CHEMBL2313034) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491383 (CHEMBL2380490) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423928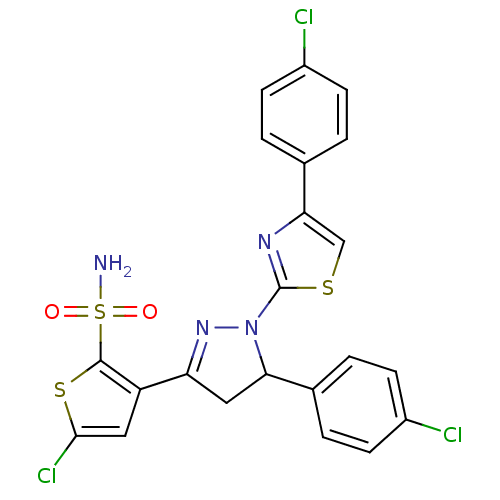 (CHEMBL2313035) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423930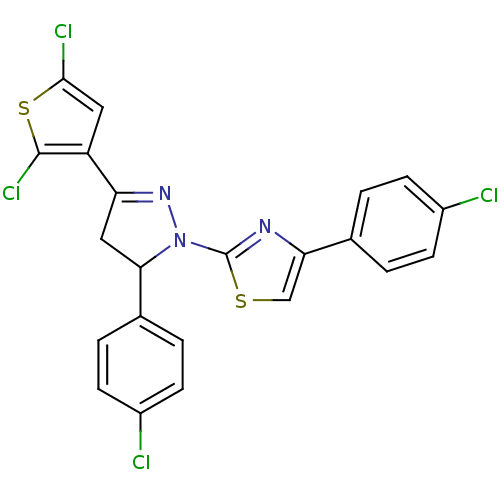 (CHEMBL2313033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423926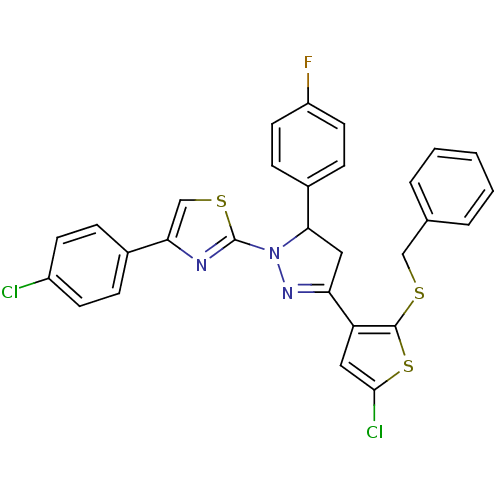 (CHEMBL2313037) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423925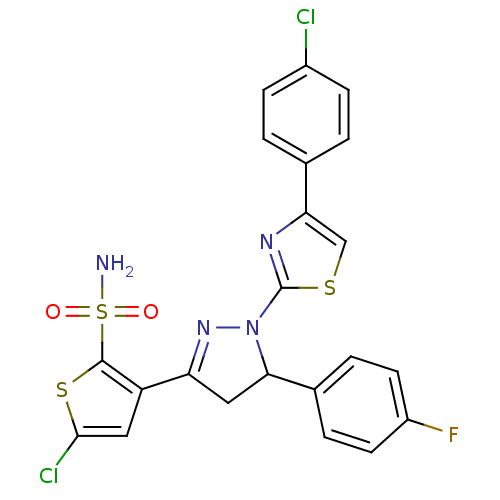 (CHEMBL2313038) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM50423927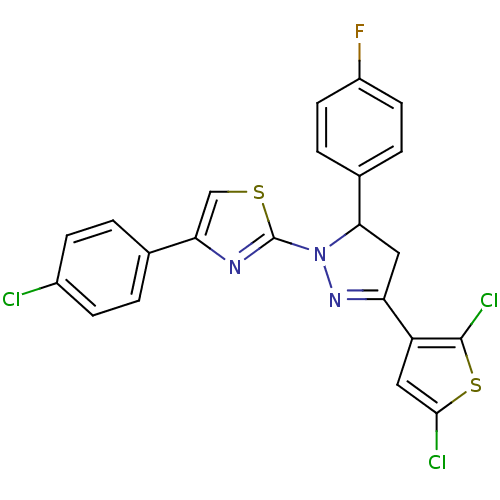 (CHEMBL2313036) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swami Ramanand Teerth Marathwada University Curated by ChEMBL | Assay Description Inhibition of Wistar albino rat xanthine oxidase isolated from liver using xanthine as substrate incubated for 15 mins prior to substrate addition by... | Bioorg Med Chem 21: 365-72 (2012) Article DOI: 10.1016/j.bmc.2012.09.060 BindingDB Entry DOI: 10.7270/Q2RV0Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491380 (CHEMBL2380380) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491384 (CHEMBL2380377) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491386 (CHEMBL2380491) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491381 (CHEMBL2380379) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491379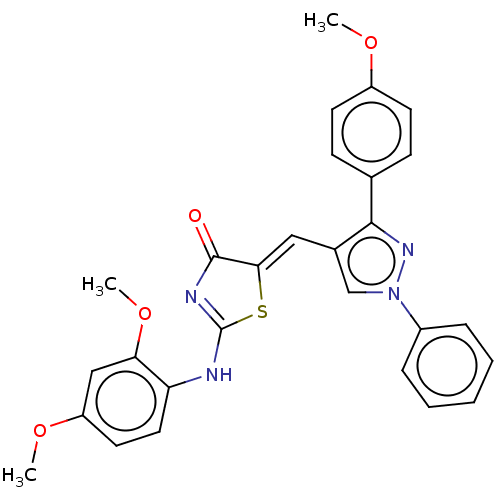 (CHEMBL2380376) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491382 (CHEMBL2380378) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50491385 (CHEMBL2380375) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bahirji Smarak Mahavidyalaya Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins by spectrophotometric analysis | Bioorg Med Chem 21: 2772-7 (2013) Article DOI: 10.1016/j.bmc.2012.12.053 BindingDB Entry DOI: 10.7270/Q2G44T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||