

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50059414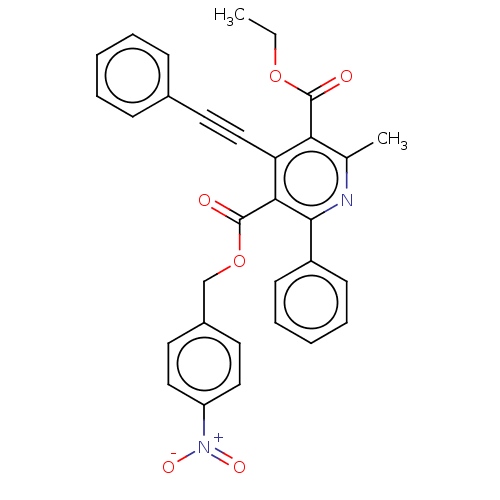 (2-Methyl-6-phenyl-4-phenylethynyl-1,4-dihydro-pyri...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553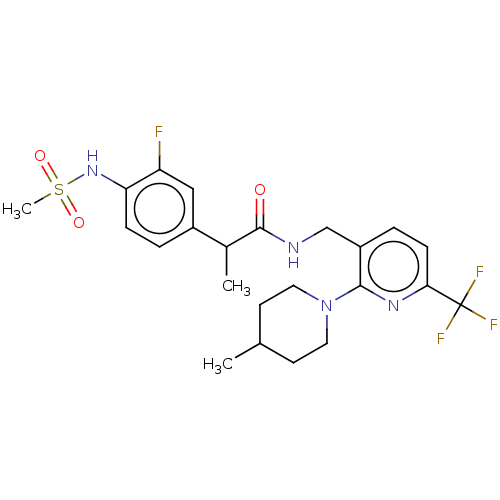 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM729 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0310 | -62.4 | 4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM85776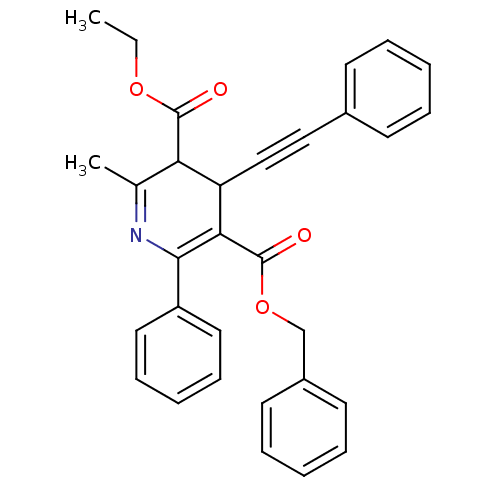 (CAS_393594 | CHEMBL89852 | MRS1191 | NSC_393594) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176555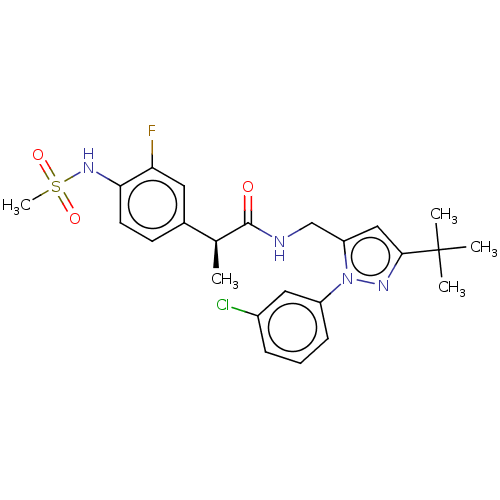 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of NADA-induced intracellular calcium level preincubated with cells ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50450981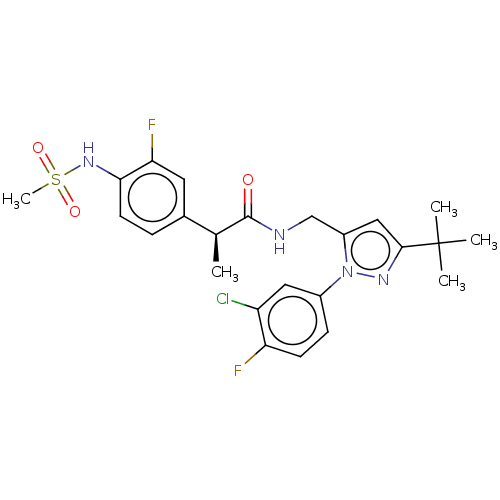 (CHEMBL4215829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50088020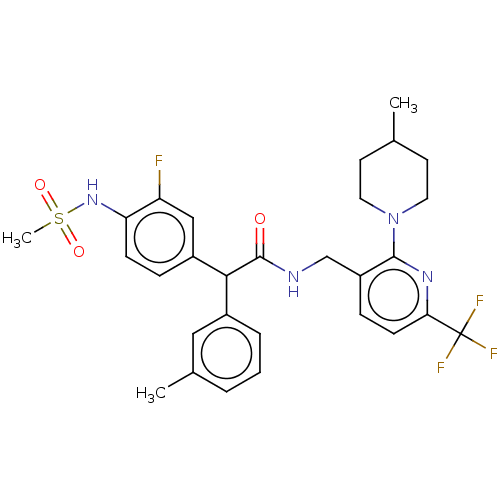 (CHEMBL3427109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytidine deaminase (Mus musculus) | BDBM50367349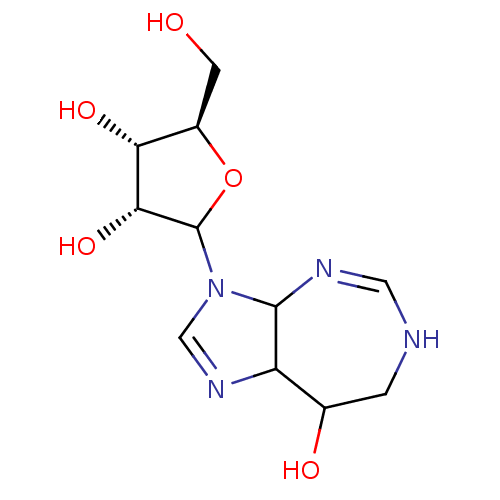 (CHEMBL604436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase | J Med Chem 29: 1374-80 (1986) BindingDB Entry DOI: 10.7270/Q26T0N69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284986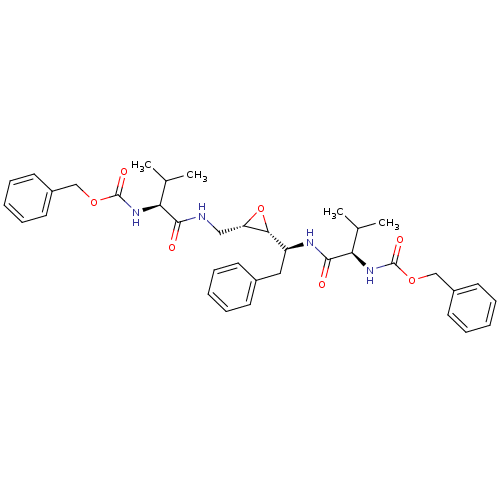 (CHEMBL55197 | [(R)-1-((S)-1-{(2R,3S)-3-[((S)-2-Ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50256547 (CHEMBL4104073) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin induced calcium influx pretreated for 6 mins followed... | Bioorg Med Chem 25: 2451-2462 (2017) Article DOI: 10.1016/j.bmc.2017.03.004 BindingDB Entry DOI: 10.7270/Q2BZ68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176555 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176555 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 mi... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50275762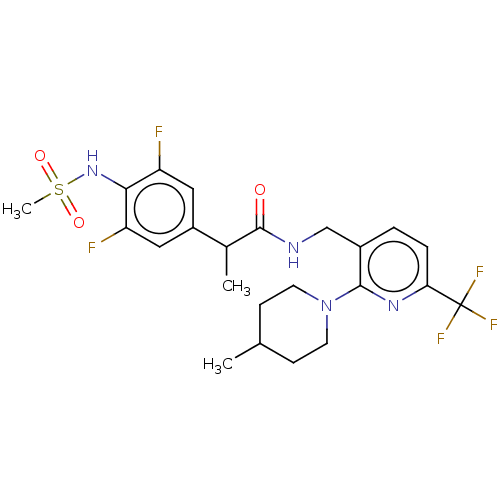 (CHEMBL4125690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat TRPV1 expressed in HEK293 cells assessed as reduction in capsaicin-induced Ca2+ flux preincubated for 3 mins f... | Bioorg Med Chem Lett 28: 2539-2542 (2018) Article DOI: 10.1016/j.bmcl.2018.05.043 BindingDB Entry DOI: 10.7270/Q2MP55RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50463472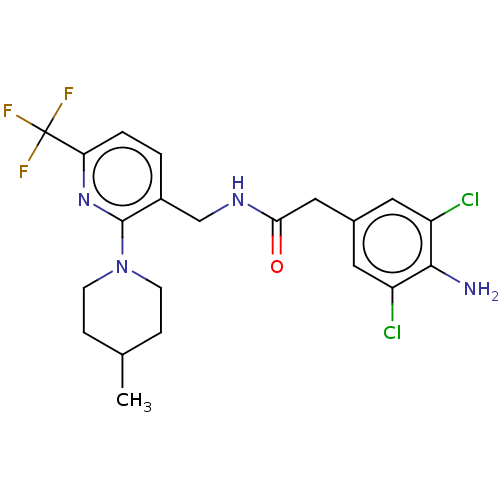 (CHEMBL4242293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as reduction in NADA-induced intracellular calcium level preincubated with cells... | Bioorg Med Chem 26: 4509-4517 (2018) Article DOI: 10.1016/j.bmc.2018.07.040 BindingDB Entry DOI: 10.7270/Q2348P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176564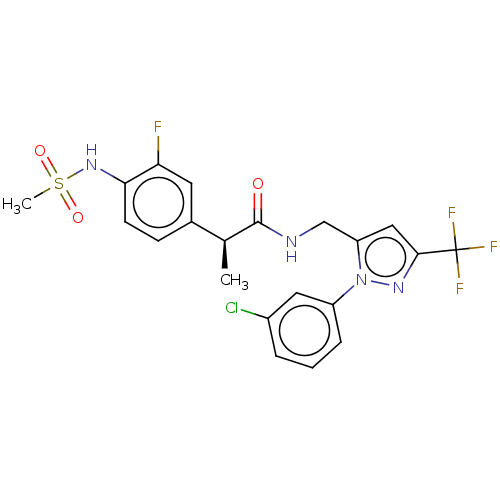 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 mi... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176564 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of NADA-induced intracellular calcium level preincubated with cells ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176564 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50053927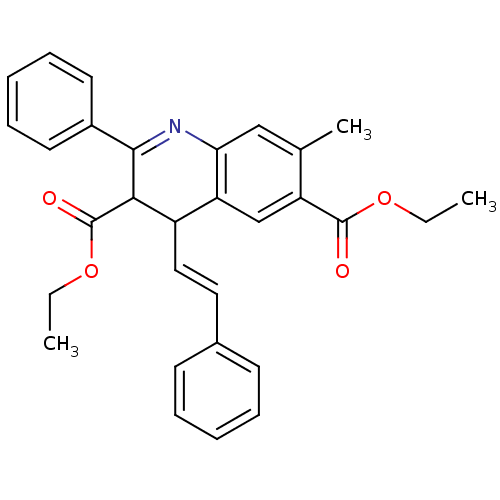 (7-Methyl-2-phenyl-4-((E)-styryl)-1,4-dihydro-quino...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro binding affinity at human Adenosine A3 receptor from HEK-293 cells by [125I]-AB-MECA displacement. | J Med Chem 39: 4142-8 (1996) Article DOI: 10.1021/jm960482i BindingDB Entry DOI: 10.7270/Q2FQ9VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288943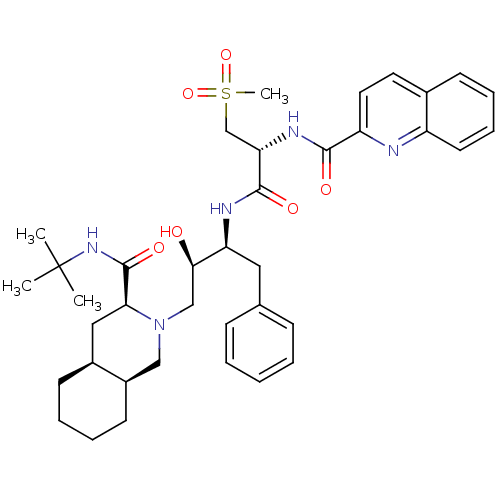 (CHEMBL154519 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM729 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | -58.7 | 10 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288941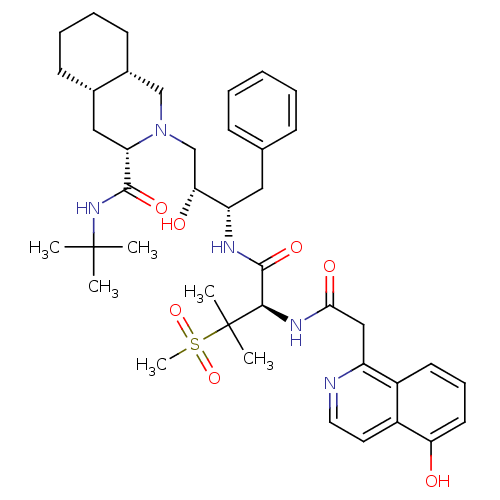 ((3S,4aS,8aS)-2-((2R,3S)-2-Hydroxy-3-{(R)-2-[2-(5-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288942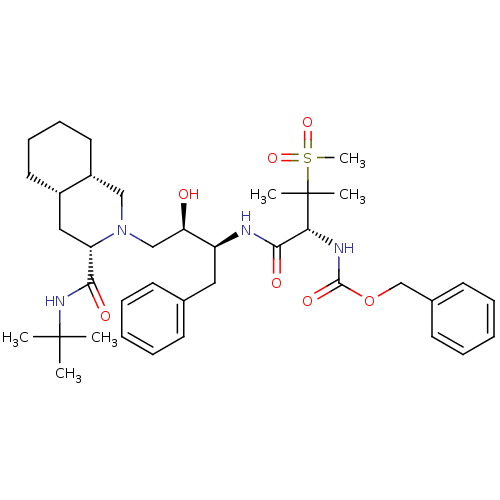 (CHEMBL154692 | {(R)-1-[(1S,2R)-1-Benzyl-3-((3S,4aS...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288939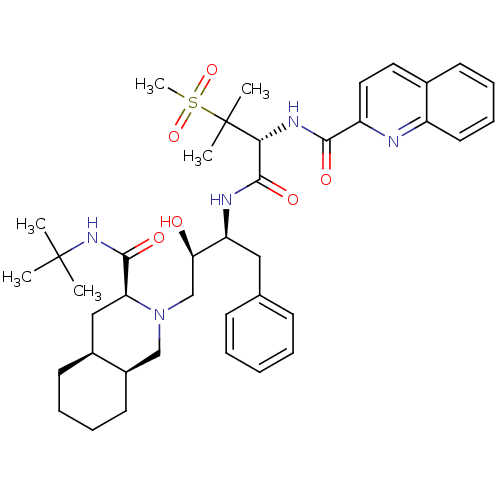 (CHEMBL345187 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as reduction in capsaicin-induced intracellular calcium level preincubated for 6... | Bioorg Med Chem 26: 4509-4517 (2018) Article DOI: 10.1016/j.bmc.2018.07.040 BindingDB Entry DOI: 10.7270/Q2348P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50275762 (CHEMBL4125690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV1 expressed in CHOK1 cells assessed as reduction in capsaicin-induced Ca2+ flux after 15 mins by fluo-4-... | Bioorg Med Chem Lett 28: 2539-2542 (2018) Article DOI: 10.1016/j.bmcl.2018.05.043 BindingDB Entry DOI: 10.7270/Q2MP55RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50088020 (CHEMBL3427109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17B (Homo sapiens (Human)) | BDBM50166121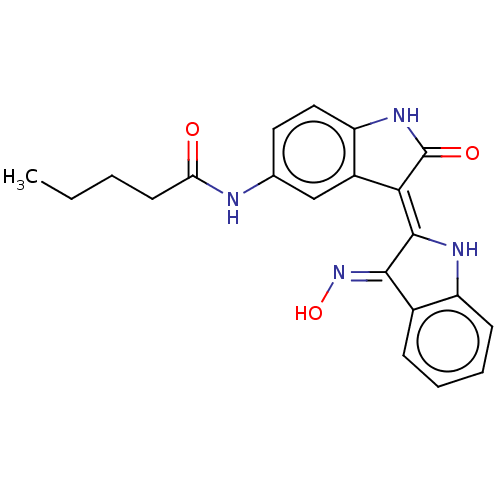 (CHEMBL3797480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176562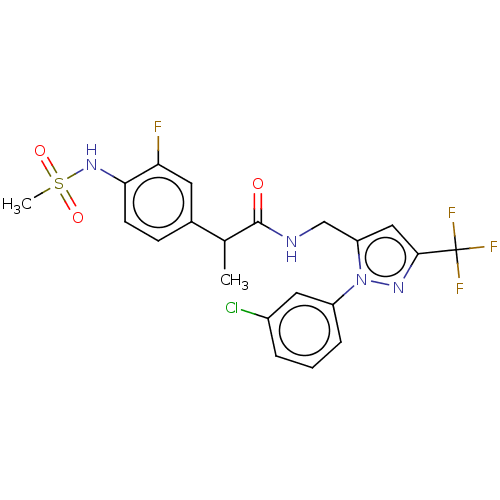 (US9120756, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50463472 (CHEMBL4242293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as reduction in capsaicin-induced intracellular calcium level preincubated for 6... | Bioorg Med Chem 26: 4509-4517 (2018) Article DOI: 10.1016/j.bmc.2018.07.040 BindingDB Entry DOI: 10.7270/Q2348P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176555 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176561 (US9120756, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50256581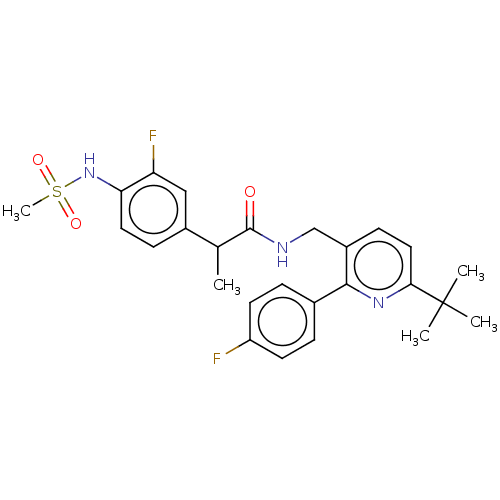 (CHEMBL4078240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin induced calcium influx pretreated for 6 mins followed... | Bioorg Med Chem 25: 2451-2462 (2017) Article DOI: 10.1016/j.bmc.2017.03.004 BindingDB Entry DOI: 10.7270/Q2BZ68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50256546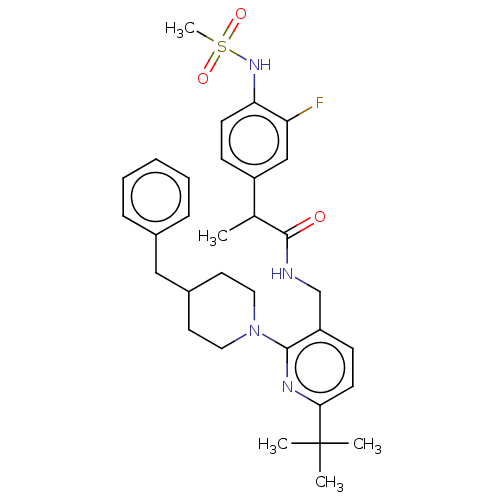 (CHEMBL4105672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin induced calcium influx pretreated for 6 mins followed... | Bioorg Med Chem 25: 2451-2462 (2017) Article DOI: 10.1016/j.bmc.2017.03.004 BindingDB Entry DOI: 10.7270/Q2BZ68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176554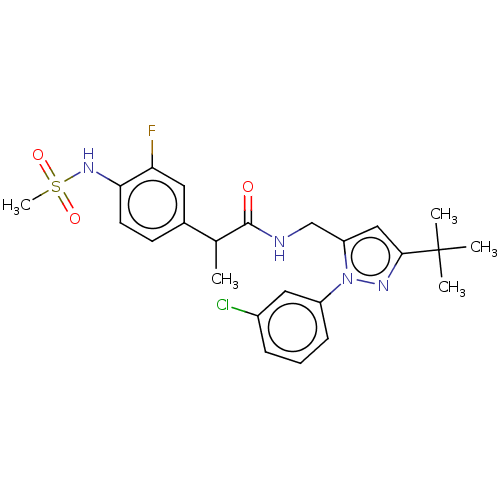 (US9120756, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176564 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50275818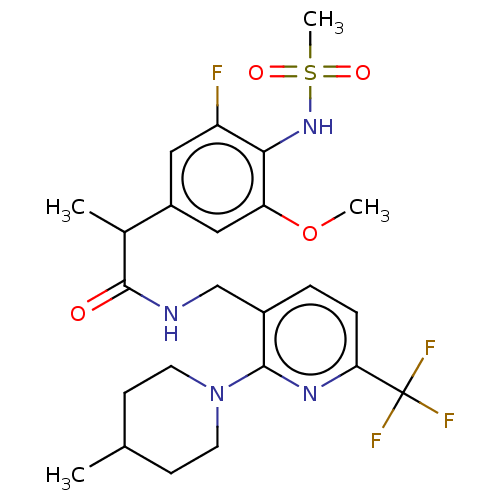 (CHEMBL4129132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV1 expressed in CHOK1 cells assessed as reduction in capsaicin-induced Ca2+ flux after 15 mins by fluo-4-... | Bioorg Med Chem Lett 28: 2539-2542 (2018) Article DOI: 10.1016/j.bmcl.2018.05.043 BindingDB Entry DOI: 10.7270/Q2MP55RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065757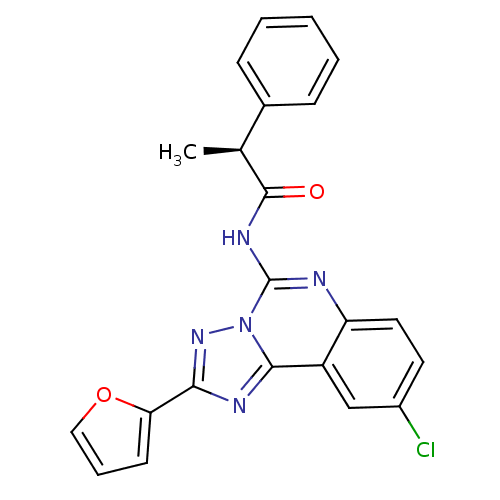 ((S)-N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.362 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176559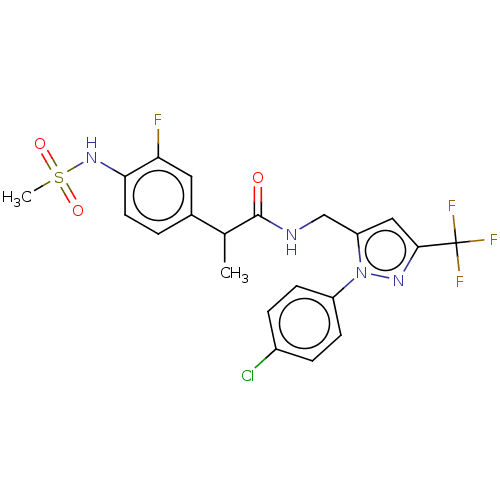 (US9120756, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065774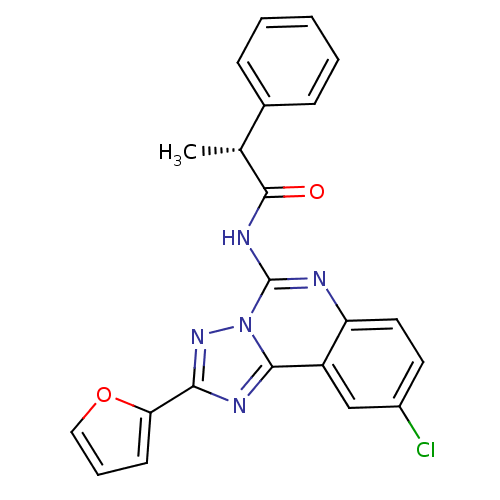 ((R)-N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176556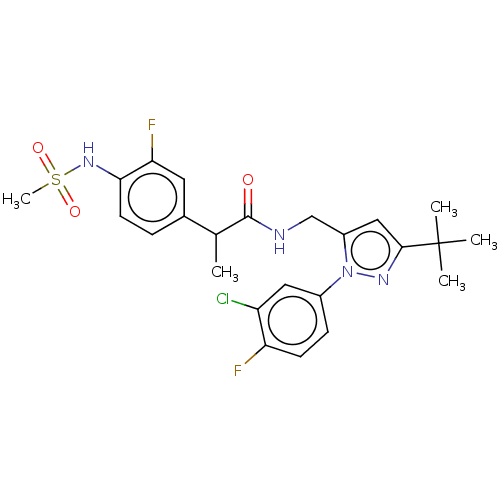 (US9120756, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50256552 (CHEMBL4102090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin induced calcium influx pretreated for 6 mins followed... | Bioorg Med Chem 25: 2451-2462 (2017) Article DOI: 10.1016/j.bmc.2017.03.004 BindingDB Entry DOI: 10.7270/Q2BZ68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50151114 (CHEMBL3770682) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in CHO cells after 60 mins by scintillation counting analysis | Bioorg Med Chem 24: 1231-40 (2016) Article DOI: 10.1016/j.bmc.2016.01.051 BindingDB Entry DOI: 10.7270/Q25D8TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9294 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50053924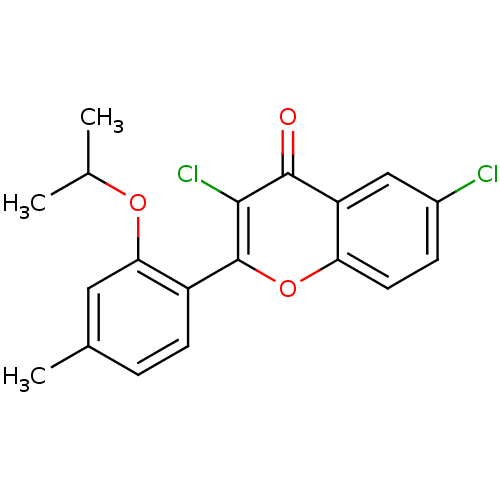 (3,6-Dichloro-2-(2-isopropoxy-4-methyl-phenyl)-chro...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro binding affinity at human Adenosine A3 receptor from HEK-293 cells by [125I]-AB-MECA displacement. | J Med Chem 39: 4142-8 (1996) Article DOI: 10.1021/jm960482i BindingDB Entry DOI: 10.7270/Q2FQ9VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065756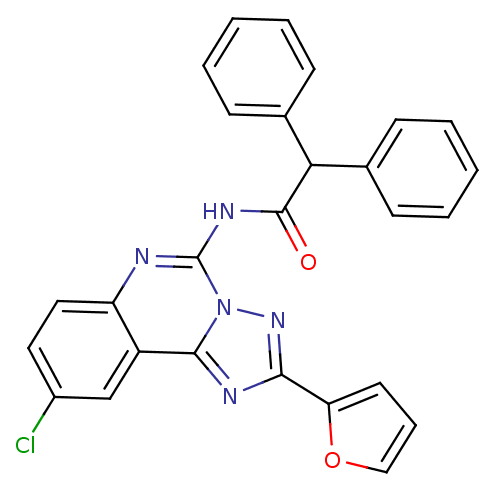 (CHEMBL317580 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tri...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.586 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50256624 (CHEMBL4070760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin induced calcium influx pretreated for 6 mins followed... | Bioorg Med Chem 25: 2451-2462 (2017) Article DOI: 10.1016/j.bmc.2017.03.004 BindingDB Entry DOI: 10.7270/Q2BZ68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5000 total ) | Next | Last >> |