

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemagglutinin (Influenza A virus (A/Shangdong/9/93(H3N2))) | BDBM4934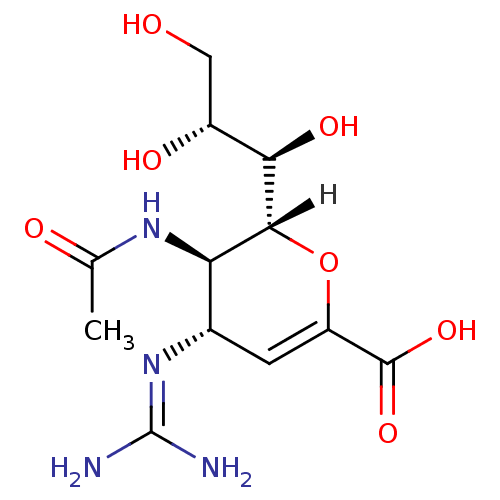 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[(1R,2R...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 1 | -53.4 | 5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 7: 1837-42 (1997) Article DOI: 10.1016/S0960-894X(97)00333-8 BindingDB Entry DOI: 10.7270/Q2FX77MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282061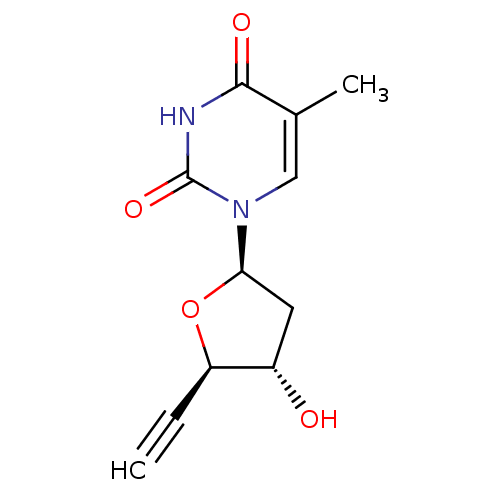 (1-((2R,4S,5R)-5-Ethynyl-4-hydroxy-tetrahydro-furan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282067 (1-((2R,4S,5S)-4-Hydroxy-5-methoxy-tetrahydro-furan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282063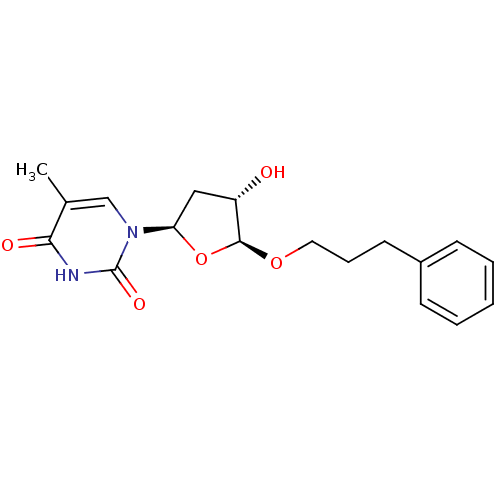 (1-[(2R,4S,5S)-4-Hydroxy-5-(3-phenyl-propoxy)-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282072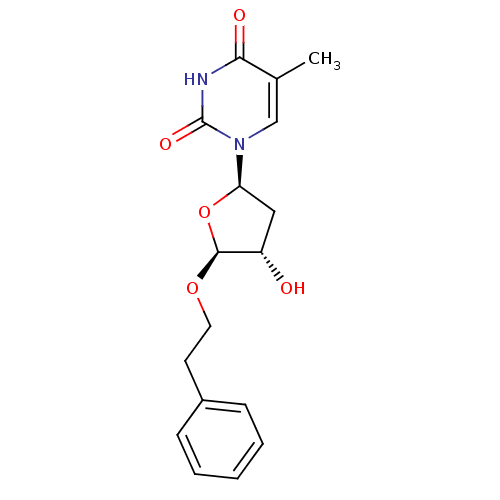 (1-((2R,4S,5S)-4-Hydroxy-5-phenethyloxy-tetrahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (A/Shangdong/9/93(H3N2))) | BDBM5210 (3-carbamimidamido-4-acetamido-5-[(1S,2R)-1,2,3-tri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | >1.00E+5 | >-23.7 | >1.00E+5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 7: 1837-42 (1997) Article DOI: 10.1016/S0960-894X(97)00333-8 BindingDB Entry DOI: 10.7270/Q2FX77MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50052738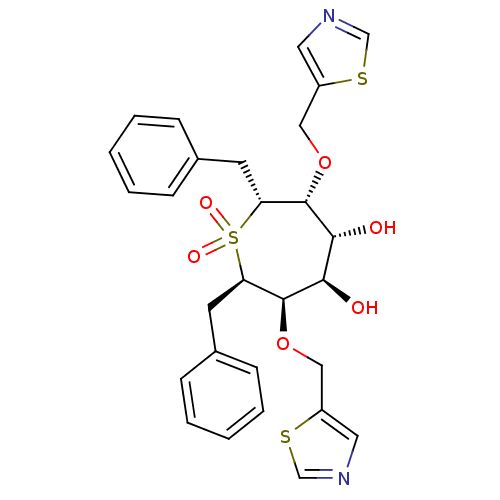 ((2R,3R,4R,5R,6R,7R)-2,7-Dibenzyl-1,1-dioxo-3,6-bis...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. Curated by ChEMBL | Assay Description Concentration needed to inhibit HIV- protease activity by 50%. | J Med Chem 39: 3431-4 (1996) Article DOI: 10.1021/jm960340o BindingDB Entry DOI: 10.7270/Q22N51DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5003 ((3R,4R,5S)-5-amino-4-acetamido-3-{[(3S)-1-phenylpe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137405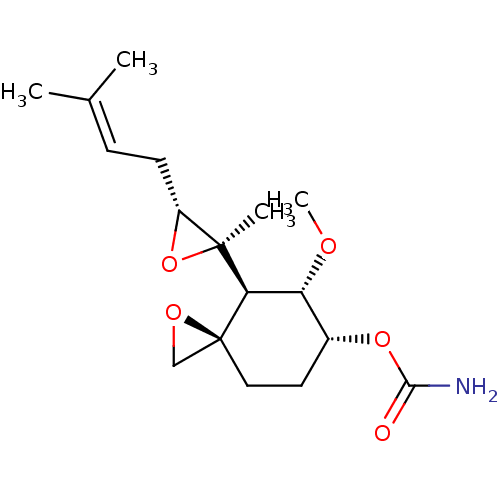 (CHEMBL176975 | Carbamic acid (3R,4S,5S,6R)-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 91-4 (2003) BindingDB Entry DOI: 10.7270/Q27P8ZXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137403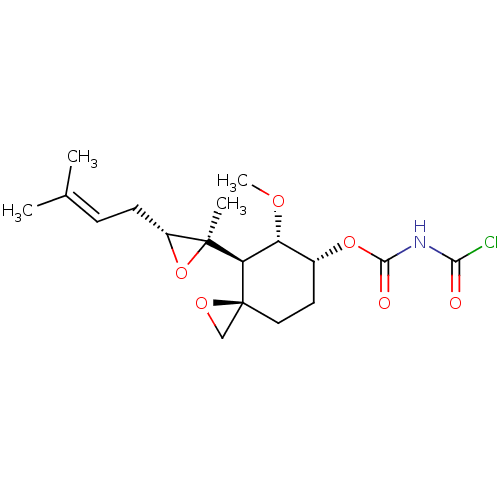 (Acetyl-carbamic acid (3R,4S,5S,6R)-5-methoxy-4-[(R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 91-4 (2003) BindingDB Entry DOI: 10.7270/Q27P8ZXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5013 ((3R,4R,5S)-4-Acetamido-5-guanidinyl-3-(1-ethylprop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5012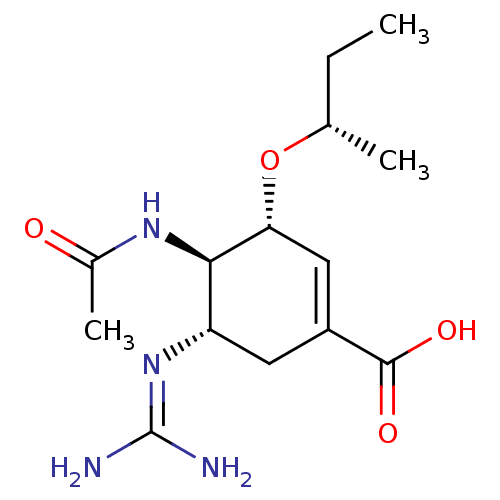 ((3R,4R,5S)-3-[(2S)-butan-2-yloxy]-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5011 ((3R,4R,5S)-3-[(2R)-butan-2-yloxy]-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137404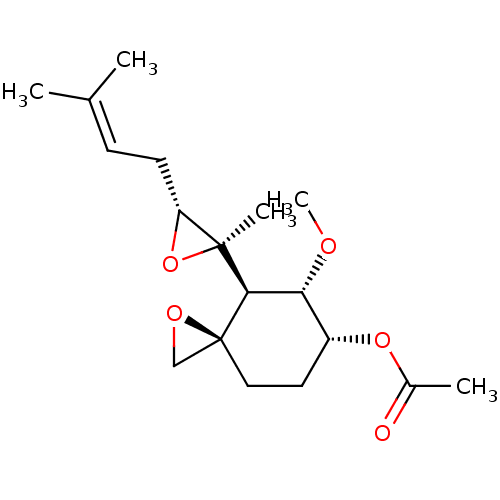 (Acetic acid (3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 91-4 (2003) BindingDB Entry DOI: 10.7270/Q27P8ZXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50052740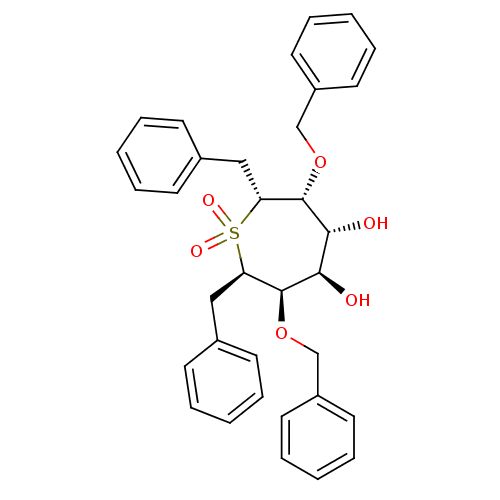 ((2R,3R,4R,5R,6R,7R)-2,7-Dibenzyl-3,6-bis-benzyloxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. Curated by ChEMBL | Assay Description Concentration needed to inhibit HIV- protease activity by 50%. | J Med Chem 39: 3431-4 (1996) Article DOI: 10.1021/jm960340o BindingDB Entry DOI: 10.7270/Q22N51DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50113436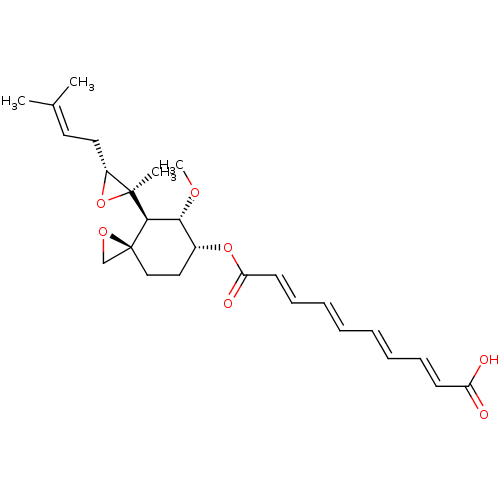 (CHEMBL32838 | fumagillin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 91-4 (2003) BindingDB Entry DOI: 10.7270/Q27P8ZXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137402 (4-Ethyl-piperazine-1-carboxylic acid (3R,4S,5S,6R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 91-4 (2003) BindingDB Entry DOI: 10.7270/Q27P8ZXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 8: 3321-4 (1998) Article DOI: 10.1016/s0960-894x(98)00587-3 BindingDB Entry DOI: 10.7270/Q2B56GXM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 7: 1837-42 (1997) Article DOI: 10.1016/S0960-894X(97)00333-8 BindingDB Entry DOI: 10.7270/Q2FX77MK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 10: 1257-60 (2000) Article DOI: 10.1016/s0960-894x(00)00214-6 BindingDB Entry DOI: 10.7270/Q22R3PWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50052739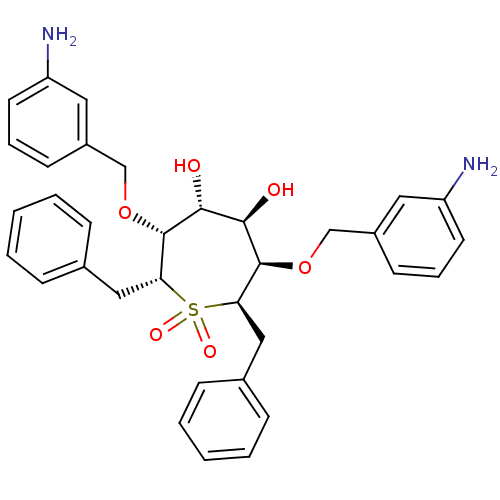 ((2R,3R,4R,5R,6R,7R)-3,6-Bis-(3-amino-benzyloxy)-2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. Curated by ChEMBL | Assay Description Concentration needed to inhibit HIV- protease activity by 50%. | J Med Chem 39: 3431-4 (1996) Article DOI: 10.1021/jm960340o BindingDB Entry DOI: 10.7270/Q22N51DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5000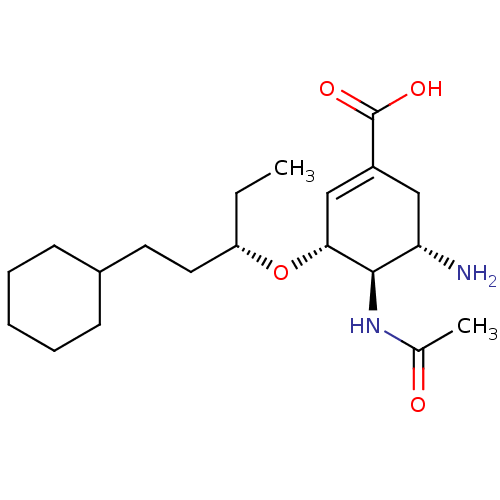 ((3R,4R,5S)-4-Acetamido-5-amino-3-(1(S)-(2-cyclohex...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4997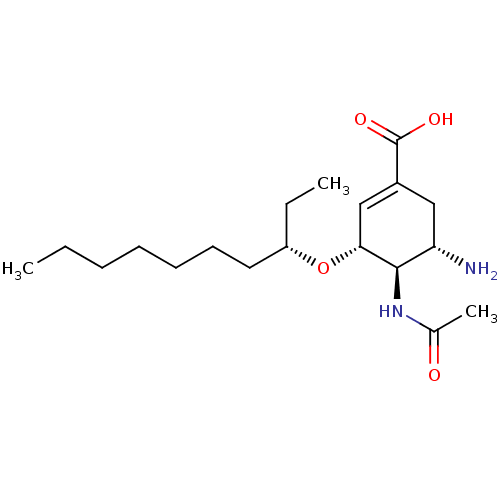 ((3R,4R,5S)-4-Acetamido-5-amino-3-[(3S)-decyloxy]-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 2811-4 (1999) Article DOI: 10.1016/s0960-894x(99)00479-5 BindingDB Entry DOI: 10.7270/Q26H4FMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4995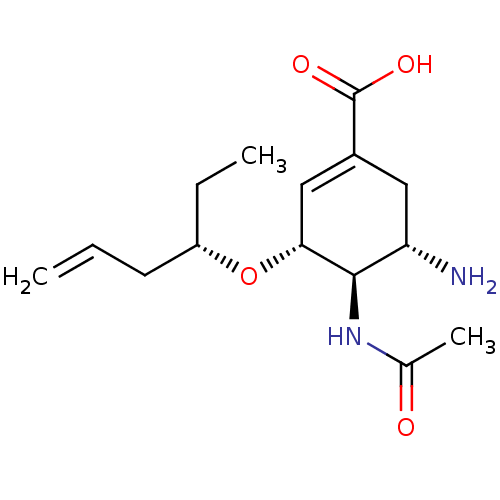 ((3R,4R,5S)-4-Acetamido-5-amino-3-[[(1S)-1-ethylbut...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137406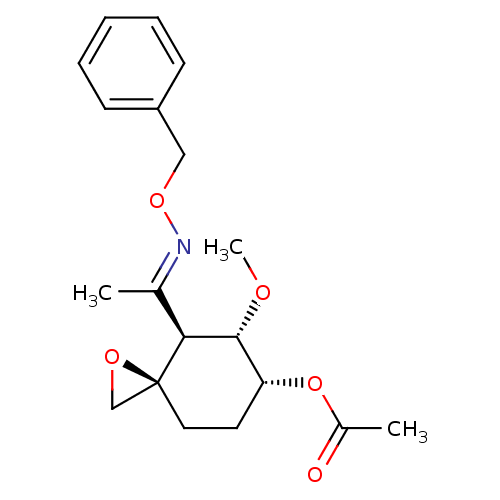 (Acetic acid (3R,4S,5S,6R)-4-{1-[(E)-benzyloxyimino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 91-4 (2003) BindingDB Entry DOI: 10.7270/Q27P8ZXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137408 (CHEMBL172883 | Carbamic acid (3R,4S,5S,6R)-4-{1-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 91-4 (2003) BindingDB Entry DOI: 10.7270/Q27P8ZXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137409 (CHEMBL369152 | Carbamic acid (3R,4S,5S,6R)-4-((E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 91-4 (2003) BindingDB Entry DOI: 10.7270/Q27P8ZXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5009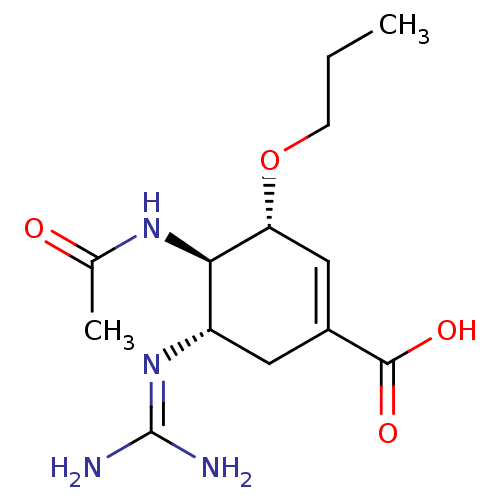 ((3R,4R,5S)-4-Acetamido-5-guanidinyl-3-propoxy-1-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 7: 1837-42 (1997) Article DOI: 10.1016/S0960-894X(97)00333-8 BindingDB Entry DOI: 10.7270/Q2FX77MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50289699 ((3R,4R,5S)-4-Acetylamino-5-guanidino-3-propoxy-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description Inhibition of neuraminidase of influenza A/PR/8/34(H1N1) | Bioorg Med Chem Lett 7: 1837-42 (1997) Article DOI: 10.1016/S0960-894X(97)00333-8 BindingDB Entry DOI: 10.7270/Q2FX77MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97761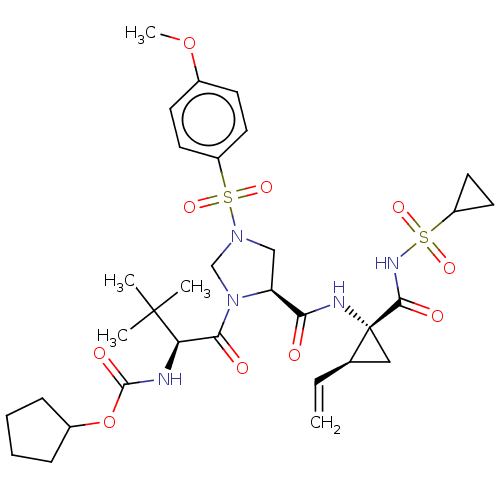 (US8476225, 22) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97758 (US8476225, 19) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4993 ((3R,4R,5S)-5-amino-3-[(2S)-butan-2-yloxy]-4-acetam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5009 ((3R,4R,5S)-4-Acetamido-5-guanidinyl-3-propoxy-1-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97799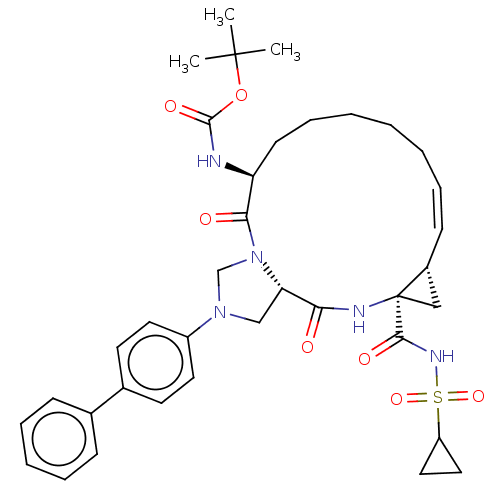 (US8476225, 60) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97798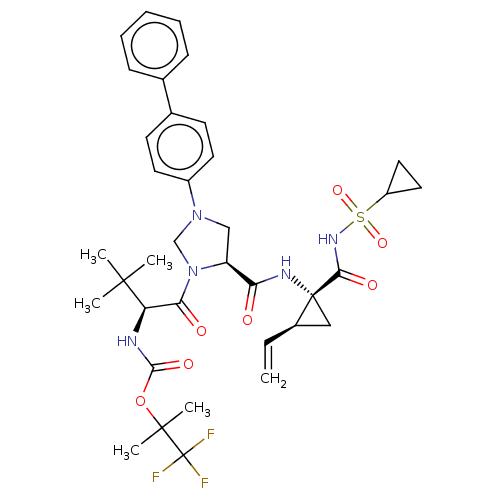 (US8476225, 59) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97773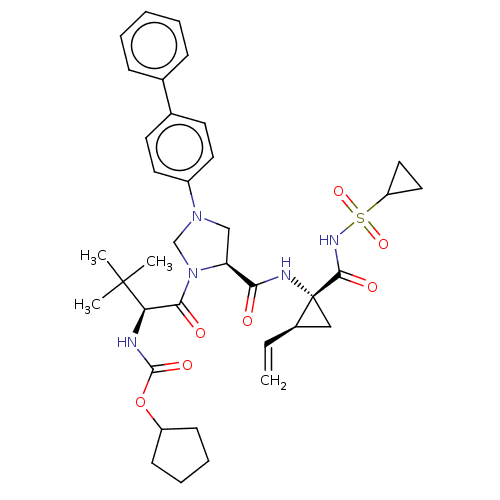 (US8476225, 34) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97753 (US8476225, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97789 (US8476225, 50) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97763 (US8476225, 24) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4996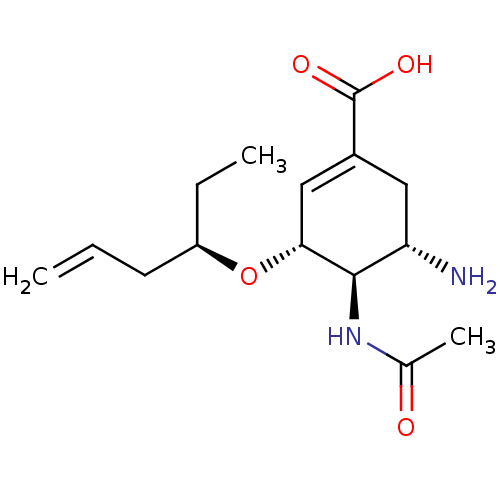 ((3R,4R,5S)-4-Acetamido-5-amino-3-[[(1R)-1-ethylbut...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5010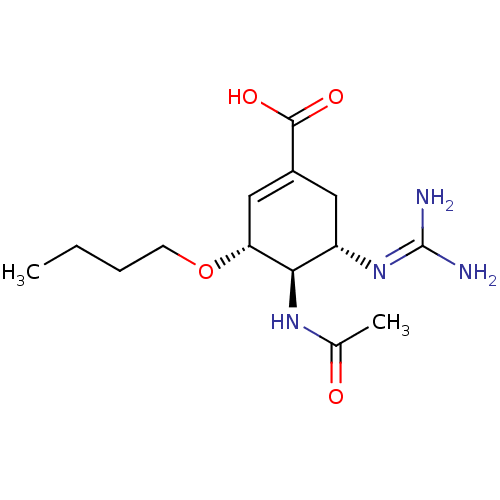 ((3R,4R,5S)-3-butoxy-5-carbamimidamido-4-acetamidoc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5015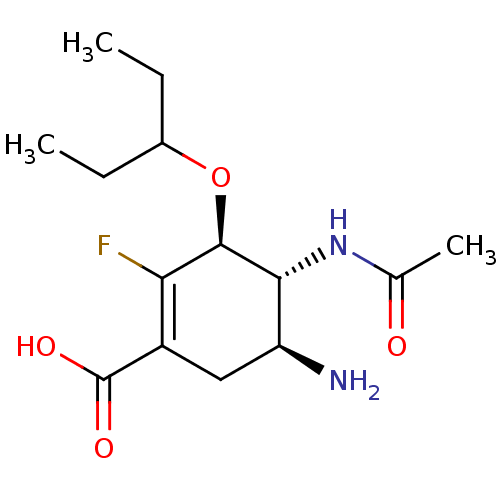 ((3S,4R,5S)-5-amino-4-acetamido-2-fluoro-3-(pentan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4995 ((3R,4R,5S)-4-Acetamido-5-amino-3-[[(1S)-1-ethylbut...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1657] (Hepatitis C virus genotype 1a (isolate 1) (HCV)) | BDBM97749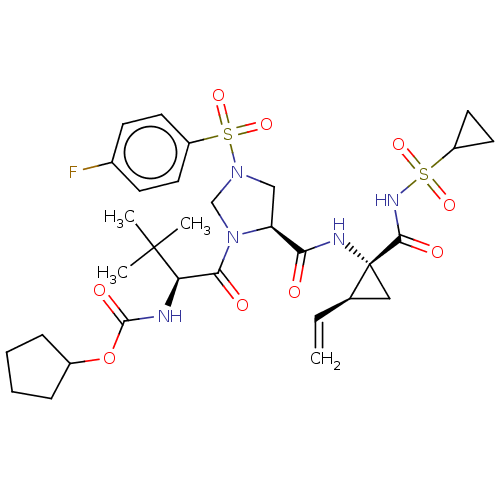 (US8476225, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Gilead Sciences, Inc. US Patent | Assay Description Inhibition assay using HCV protease. | US Patent US8476225 (2013) BindingDB Entry DOI: 10.7270/Q2M32TD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4997 ((3R,4R,5S)-4-Acetamido-5-amino-3-[(3S)-decyloxy]-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 2451-60 (1998) Article DOI: 10.1021/jm980162u BindingDB Entry DOI: 10.7270/Q2MP51GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 8: 3321-4 (1998) Article DOI: 10.1016/s0960-894x(98)00587-3 BindingDB Entry DOI: 10.7270/Q2B56GXM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 2811-4 (1999) Article DOI: 10.1016/s0960-894x(99)00479-5 BindingDB Entry DOI: 10.7270/Q26H4FMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 328 total ) | Next | Last >> |