 Found 42 hits with Last Name = 'kim' and Initial = 'wg'
Found 42 hits with Last Name = 'kim' and Initial = 'wg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50157911

(13a-hydroxy-10-(4-methoxyphenyl)-5,5,5a,7a,13b-pen...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@@]4(O)[C@](C)(CCC(=O)OC4(C)C)[C@]3(O)Cc2c(=O)o1 Show InChI InChI=1S/C27H32O8/c1-23(2)26(30)13-12-25(4)27(31,24(26,3)11-10-21(28)35-23)15-18-20(34-25)14-19(33-22(18)29)16-6-8-17(32-5)9-7-16/h6-9,14,30-31H,10-13,15H2,1-5H3/t24-,25+,26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Binding affinity for Acetylcholinesterase |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Butyrylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptide deformylase
(Staphylococcus aureus) | BDBM50089194

((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...)Show SMILES CCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1CO Show InChI InChI=1S/C19H35N3O5/c1-4-5-6-8-14(11-16(24)21-27)18(25)20-17(13(2)3)19(26)22-10-7-9-15(22)12-23/h13-15,17,23,27H,4-12H2,1-3H3,(H,20,25)(H,21,24)/t14-,15+,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase using N-formylmethionine-alanine-serine as substrate by spectrophotometry |
J Nat Prod 75: 271-4 (2012)
Article DOI: 10.1021/np200720v
BindingDB Entry DOI: 10.7270/Q24B3299 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50157914

(4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@]4(O)C(C)(C)C(=O)CC[C@]4(C)[C@]3(O)Cc2c(=O)o1 Show InChI InChI=1S/C27H32O7/c1-23(2)21(28)10-11-24(3)26(23,30)13-12-25(4)27(24,31)15-18-20(34-25)14-19(33-22(18)29)16-6-8-17(32-5)9-7-16/h6-9,14,30-31H,10-13,15H2,1-5H3/t24-,25+,26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50157915
(4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@]4(O)C(C)(C)C(=O)C=C[C@]4(C)[C@]3(O)Cc2c(=O)o1 |c:24| Show InChI InChI=1S/C27H30O7/c1-23(2)21(28)10-11-24(3)26(23,30)13-12-25(4)27(24,31)15-18-20(34-25)14-19(33-22(18)29)16-6-8-17(32-5)9-7-16/h6-11,14,30-31H,12-13,15H2,1-5H3/t24-,25+,26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50157912
(13-hydroxy-16-methoxy-8-(4-methoxyphenyl)-4,14,19,...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@]45OC(=O)[C@](OC)(C(=O)[C@]4(C)[C@]3(O)Cc2c(=O)o1)C5(C)C |THB:19:18:34:25.23| Show InChI InChI=1S/C28H30O9/c1-23(2)27-12-11-24(3)26(32,25(27,4)21(30)28(23,34-6)22(31)37-27)14-17-19(36-24)13-18(35-20(17)29)15-7-9-16(33-5)10-8-15/h7-10,13,32H,11-12,14H2,1-6H3/t24-,25-,26+,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50157913
(4a,12a-dihydroxy-3-methoxy-4,4,6a,12b-tetramethyl-...)Show SMILES COC1=CC(=O)[C@@]2(C)[C@](O)(CC[C@@]3(C)Oc4cc(oc(=O)c4C[C@@]23O)-c2cc(OC)c(OC)c(OC)c2)C1(C)C |t:2| Show InChI InChI=1S/C30H36O10/c1-26(2)23(37-7)14-22(31)28(4)29(26,33)10-9-27(3)30(28,34)15-17-19(40-27)13-18(39-25(17)32)16-11-20(35-5)24(38-8)21(12-16)36-6/h11-14,33-34H,9-10,15H2,1-8H3/t27-,28+,29+,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50157911

(13a-hydroxy-10-(4-methoxyphenyl)-5,5,5a,7a,13b-pen...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@@]4(O)[C@](C)(CCC(=O)OC4(C)C)[C@]3(O)Cc2c(=O)o1 Show InChI InChI=1S/C27H32O8/c1-23(2)26(30)13-12-25(4)27(31,24(26,3)11-10-21(28)35-23)15-18-20(34-25)14-19(33-22(18)29)16-6-8-17(32-5)9-7-16/h6-9,14,30-31H,10-13,15H2,1-5H3/t24-,25+,26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346613
(CHEMBL470029 | Oleanonic Acid)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CCC(=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H46O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-22H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241553
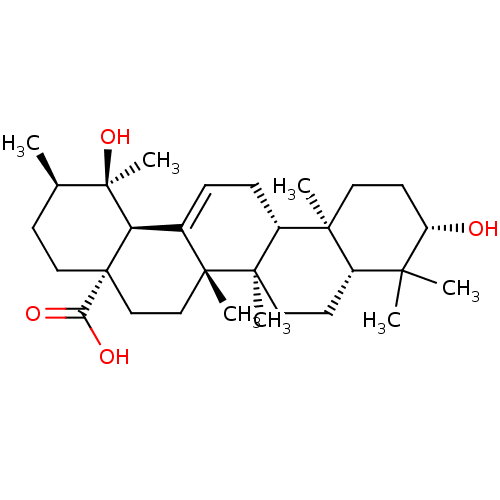
(CHEMBL486986 | pomolic acid)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@]1(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O4/c1-18-10-15-30(24(32)33)17-16-27(5)19(23(30)29(18,7)34)8-9-21-26(4)13-12-22(31)25(2,3)20(26)11-14-28(21,27)6/h8,18,20-23,31,34H,9-17H2,1-7H3,(H,32,33)/t18-,20+,21-,22+,23-,26+,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694
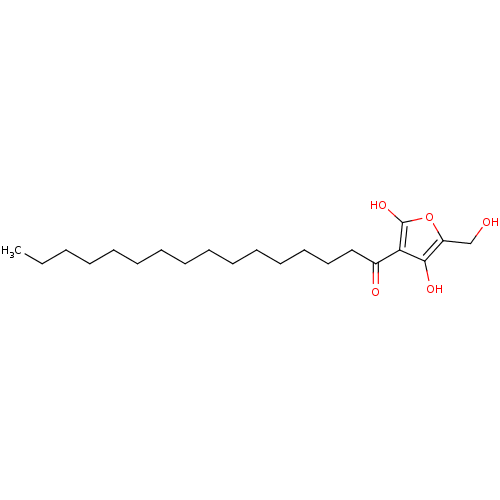
((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli FabI using trans-2-octanoyl-N-acetylcysteamine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127651
BindingDB Entry DOI: 10.7270/Q2NC64T0 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251]
(Staphylococcus aureus) | BDBM50338300
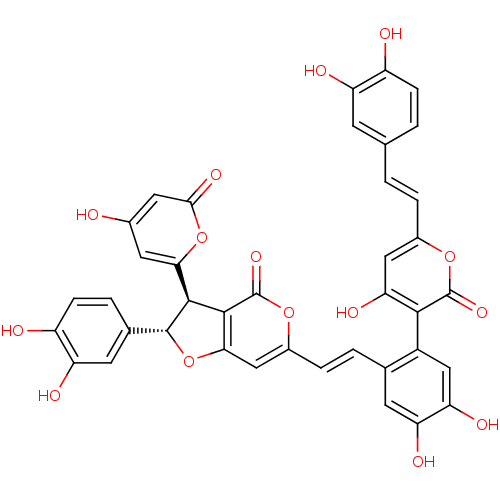
(CHEMBL1682259 | phellinstatin)Show SMILES Oc1cc(oc(=O)c1)[C@@H]1[C@H](Oc2cc(\C=C\c3cc(O)c(O)cc3-c3c(O)cc(\C=C\c4ccc(O)c(O)c4)oc3=O)oc(=O)c12)c1ccc(O)c(O)c1 |r,wU:8.8,wD:9.51,(-3.09,.5,;-1.55,.47,;-.81,-.89,;.72,-.91,;1.53,.41,;.78,1.75,;1.59,3.07,;-.76,1.79,;1.47,-2.26,;.55,-3.5,;1.46,-4.75,;2.92,-4.29,;4.25,-5.07,;5.59,-4.3,;6.92,-5.07,;8.25,-4.3,;9.58,-5.06,;10.92,-4.3,;12.25,-5.06,;13.59,-4.29,;12.26,-6.61,;13.59,-7.38,;10.92,-7.38,;9.58,-6.61,;7.48,-7.73,;6.17,-6.92,;6.11,-5.65,;4.82,-7.66,;4.79,-9.21,;3.44,-9.95,;2.12,-9.15,;.77,-9.89,;-.54,-9.09,;-1.88,-9.83,;-1.92,-11.37,;-3.27,-12.11,;-.59,-12.17,;-.62,-13.71,;.75,-11.42,;6.12,-10.01,;7.46,-9.26,;8.78,-10.06,;5.59,-2.75,;4.26,-1.98,;4.26,-.44,;2.93,-2.74,;-.98,-3.49,;-1.76,-4.81,;-3.3,-4.81,;-4.06,-3.47,;-5.61,-3.45,;-3.28,-2.14,;-4.04,-.79,;-1.74,-2.15,)| Show InChI InChI=1S/C39H26O15/c40-20-12-31(53-33(48)13-20)35-36-32(54-37(35)19-4-8-25(42)27(44)11-19)15-22(52-39(36)50)6-3-18-10-28(45)29(46)16-23(18)34-30(47)14-21(51-38(34)49)5-1-17-2-7-24(41)26(43)9-17/h1-16,35,37,40-47H/b5-1+,6-3+/t35-,37+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus enoyl-ACP reductase assessed as increase of NADPH level |
Bioorg Med Chem Lett 21: 1716-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.080
BindingDB Entry DOI: 10.7270/Q29W0FRM |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50411101
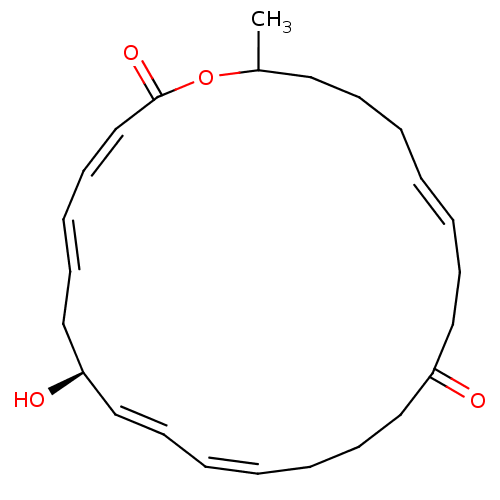
(CHEMBL425430)Show SMILES CC1CCC\C=C\CCC(=O)CCC\C=C/C=C/[C@@H](O)C\C=C\C=C/C(=O)O1 |c:14,23,t:5,16,21| Show InChI InChI=1S/C24H34O4/c1-21-15-9-4-2-5-10-16-22(25)17-11-6-3-7-12-18-23(26)19-13-8-14-20-24(27)28-21/h2-3,5,7-8,12-14,18,20-21,23,26H,4,6,9-11,15-17,19H2,1H3/b5-2+,7-3-,13-8+,18-12+,20-14-/t21?,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase in presence of substrate f-MAS |
Bioorg Med Chem Lett 16: 4889-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.058
BindingDB Entry DOI: 10.7270/Q2JW8G3Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253117
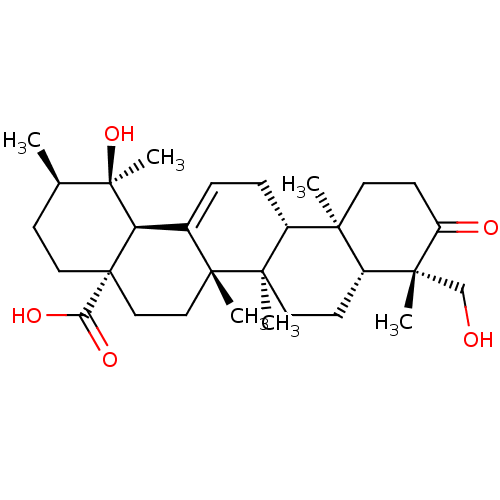
(19alpha,24-dihydroxyurs-12-en-3-on-28-oic acid | C...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CCC(=O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@]1(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C30H46O5/c1-18-9-14-30(24(33)34)16-15-27(4)19(23(30)29(18,6)35)7-8-21-25(2)12-11-22(32)26(3,17-31)20(25)10-13-28(21,27)5/h7,18,20-21,23,31,35H,8-17H2,1-6H3,(H,33,34)/t18-,20-,21-,23-,25+,26-,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253053
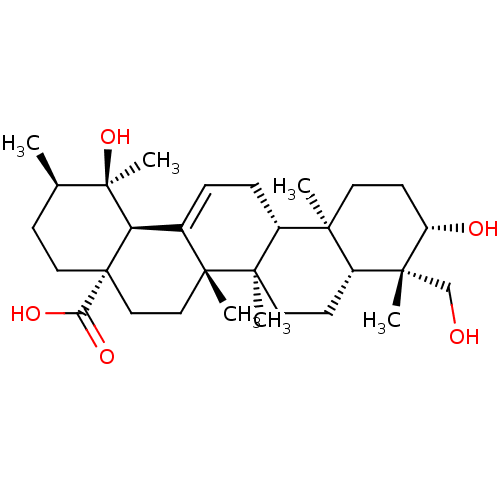
(CHEMBL493908 | rotungenic acid)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@]1(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O5/c1-18-9-14-30(24(33)34)16-15-27(4)19(23(30)29(18,6)35)7-8-21-25(2)12-11-22(32)26(3,17-31)20(25)10-13-28(21,27)5/h7,18,20-23,31-32,35H,8-17H2,1-6H3,(H,33,34)/t18-,20-,21-,22+,23-,25+,26-,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50222945
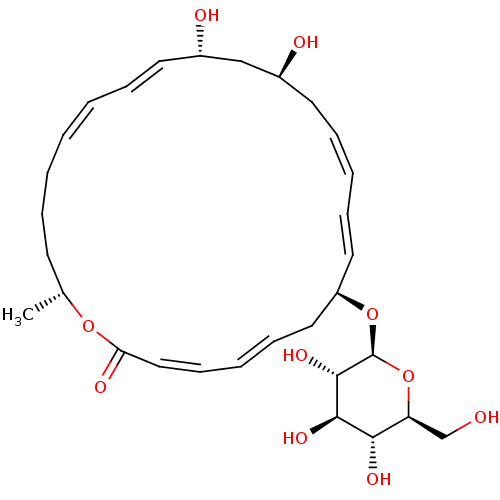
(CHEMBL252148 | macrolactin Q)Show SMILES C[C@@H]1CCC\C=C/C=C/[C@H](O)C[C@@H](O)C\C=C/C=C/[C@H](C\C=C\C=C/C(=O)O1)O[C@H]1O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]1O |c:5,15,23,t:7,17,21| Show InChI InChI=1S/C30H44O10/c1-21-13-7-3-2-4-8-14-22(32)19-23(33)15-9-5-10-16-24(17-11-6-12-18-26(34)38-21)39-30-29(37)28(36)27(35)25(20-31)40-30/h2,4-6,8-12,14,16,18,21-25,27-33,35-37H,3,7,13,15,17,19-20H2,1H3/b4-2-,9-5-,11-6+,14-8+,16-10+,18-12-/t21-,22+,23+,24-,25+,27+,28-,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase |
J Nat Prod 70: 1632-5 (2007)
Article DOI: 10.1021/np0701327
BindingDB Entry DOI: 10.7270/Q2K07406 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253075
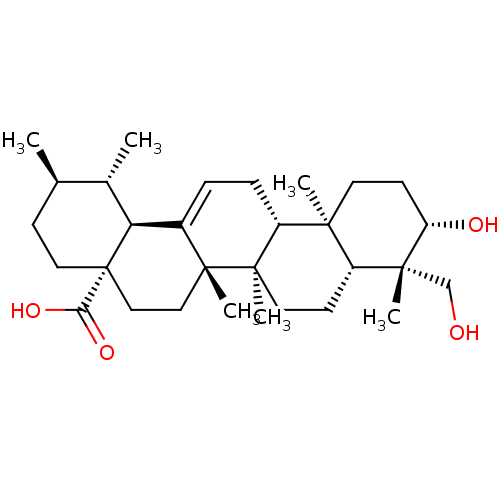
(24-hydroxyursolic acid | CHEMBL522373)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O4/c1-18-9-14-30(25(33)34)16-15-28(5)20(24(30)19(18)2)7-8-22-26(3)12-11-23(32)27(4,17-31)21(26)10-13-29(22,28)6/h7,18-19,21-24,31-32H,8-17H2,1-6H3,(H,33,34)/t18-,19+,21-,22-,23+,24+,26+,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251]
(Staphylococcus aureus) | BDBM50338301
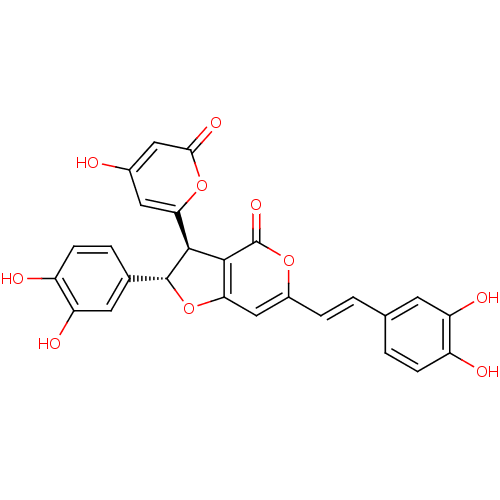
(CHEMBL1682260 | hypholomine B)Show SMILES Oc1cc(oc(=O)c1)[C@@H]1[C@H](Oc2cc(\C=C\c3ccc(O)c(O)c3)oc(=O)c12)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C26H18O10/c27-14-9-20(35-22(32)10-14)23-24-21(36-25(23)13-3-6-17(29)19(31)8-13)11-15(34-26(24)33)4-1-12-2-5-16(28)18(30)7-12/h1-11,23,25,27-31H/b4-1+/t23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus enoyl-ACP reductase assessed as increase of NADPH level |
Bioorg Med Chem Lett 21: 1716-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.080
BindingDB Entry DOI: 10.7270/Q29W0FRM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50218196
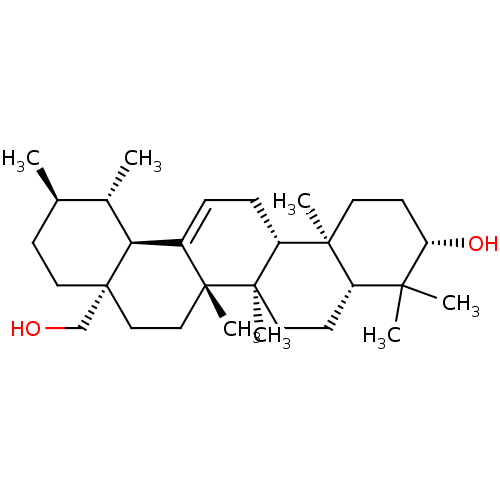
(3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | US...)Show SMILES C[C@@H]1CC[C@]2(CO)CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C |r,c:11| Show InChI InChI=1S/C30H50O2/c1-19-10-15-30(18-31)17-16-28(6)21(25(30)20(19)2)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h8,19-20,22-25,31-32H,9-18H2,1-7H3/t19-,20+,22+,23-,24+,25+,27+,28-,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM50548726
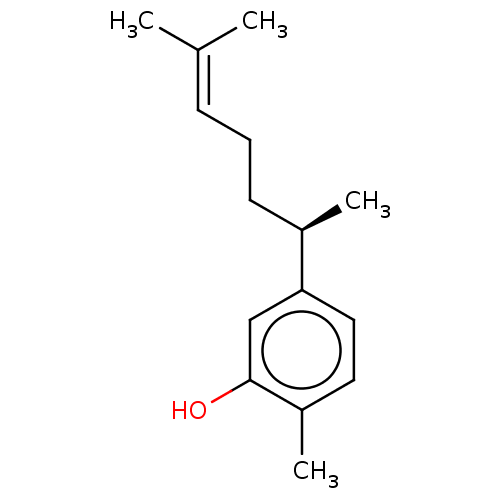
(Xanthorrizol)Show SMILES [#6]-[#6@H](-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-c1ccc(-[#6])c(-[#8])c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type Escherichia coli FabI |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127651
BindingDB Entry DOI: 10.7270/Q2NC64T0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253200

(CHEMBL493893 | spathodic acid)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@@H]1O)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O5/c1-25(2)13-15-30(24(34)35)16-14-28(5)18(22(30)23(25)33)7-8-20-26(3)11-10-21(32)27(4,17-31)19(26)9-12-29(20,28)6/h7,19-23,31-33H,8-17H2,1-6H3,(H,34,35)/t19-,20-,21+,22-,23+,26+,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253118

(24-hydroxy-3-epi-oleanolic acid | CHEMBL492472 | S...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O4/c1-25(2)13-15-30(24(33)34)16-14-28(5)19(20(30)17-25)7-8-22-26(3)11-10-23(32)27(4,18-31)21(26)9-12-29(22,28)6/h7,20-23,31-32H,8-18H2,1-6H3,(H,33,34)/t20-,21+,22+,23+,26-,27+,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253119
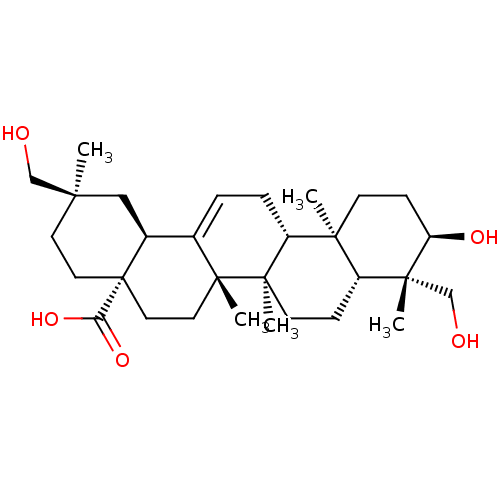
(3alpha,24,29-Trihydroxyolean-12-en-28-oic acid | C...)Show SMILES C[C@@]1(CO)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:11| Show InChI InChI=1S/C30H48O5/c1-25(17-31)12-14-30(24(34)35)15-13-28(4)19(20(30)16-25)6-7-22-26(2)10-9-23(33)27(3,18-32)21(26)8-11-29(22,28)5/h6,20-23,31-33H,7-18H2,1-5H3,(H,34,35)/t20-,21+,22+,23+,25+,26-,27+,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253052
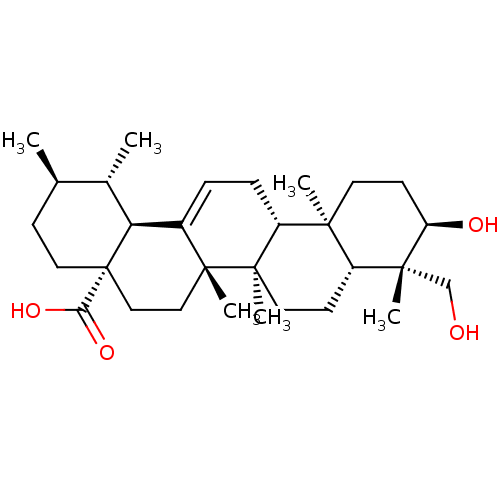
(24-hydroxy-3-epi-ursolic acid | CHEMBL493907)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O4/c1-18-9-14-30(25(33)34)16-15-28(5)20(24(30)19(18)2)7-8-22-26(3)12-11-23(32)27(4,17-31)21(26)10-13-29(22,28)6/h7,18-19,21-24,31-32H,8-17H2,1-6H3,(H,33,34)/t18-,19+,21-,22-,23-,24+,26+,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253027
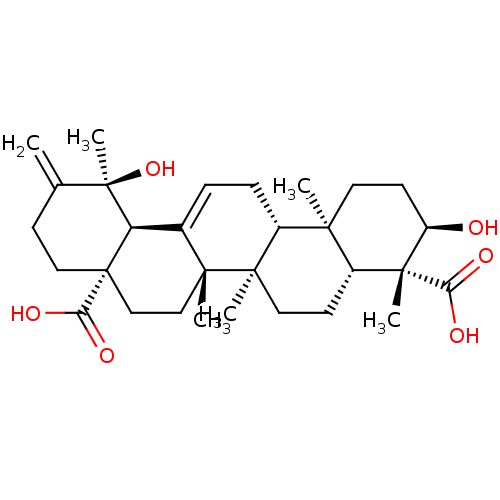
(3alpha,19alpha-dihydroxyurs-12,20(30)-dien-24,28-d...)Show SMILES C[C@]1(O)[C@H]2C3=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@@](C)([C@@H]5CC[C@@]4(C)[C@]3(C)CC[C@]2(CCC1=C)C(O)=O)C(O)=O |r,t:4| Show InChI InChI=1S/C30H44O6/c1-17-9-14-30(24(34)35)16-15-26(3)18(22(30)29(17,6)36)7-8-19-25(2)12-11-21(31)28(5,23(32)33)20(25)10-13-27(19,26)4/h7,19-22,31,36H,1,8-16H2,2-6H3,(H,32,33)(H,34,35)/t19-,20-,21-,22-,25-,26-,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253028

(3alpha,19alpha-dihydroxyurs-12-en-24,28-dioic acid...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@@](C)([C@@H]5CC[C@@]34C)C(O)=O)[C@@H]2[C@]1(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C30H46O6/c1-17-9-14-30(24(34)35)16-15-26(3)18(22(30)29(17,6)36)7-8-19-25(2)12-11-21(31)28(5,23(32)33)20(25)10-13-27(19,26)4/h7,17,19-22,31,36H,8-16H2,1-6H3,(H,32,33)(H,34,35)/t17-,19-,20-,21-,22-,25-,26-,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253029

(CHEMBL492458 | coussaric acid)Show SMILES C[C@]1(O)[C@H]2C3=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@](C)(CO)[C@@H]5CC[C@@]4(C)[C@]3(C)CC[C@]2(CCC1=C)C(O)=O |r,t:4| Show InChI InChI=1S/C30H46O5/c1-18-9-14-30(24(33)34)16-15-27(4)19(23(30)29(18,6)35)7-8-21-25(2)12-11-22(32)26(3,17-31)20(25)10-13-28(21,27)5/h7,20-23,31-32,35H,1,8-17H2,2-6H3,(H,33,34)/t20-,21-,22-,23-,25+,26-,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50253051

(CHEMBL523209 | barbinervic acid)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@]1(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O5/c1-18-9-14-30(24(33)34)16-15-27(4)19(23(30)29(18,6)35)7-8-21-25(2)12-11-22(32)26(3,17-31)20(25)10-13-28(21,27)5/h7,18,20-23,31-32,35H,8-17H2,1-6H3,(H,33,34)/t18-,20-,21-,22-,23-,25+,26-,27-,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B after 30 mins |
J Nat Prod 71: 1775-8 (2008)
Article DOI: 10.1021/np800298w
BindingDB Entry DOI: 10.7270/Q2TQ62F7 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50379509
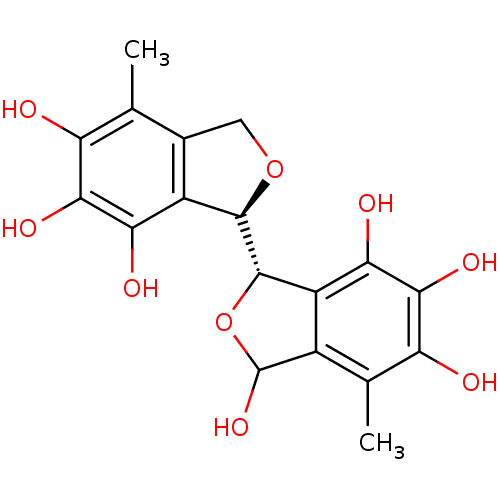
(CHEMBL2012550)Show SMILES Cc1c2CO[C@@H]([C@H]3OC(O)c4c3c(O)c(O)c(O)c4C)c2c(O)c(O)c1O |r| Show InChI InChI=1S/C18H18O9/c1-4-6-3-26-16(8(6)12(21)14(23)10(4)19)17-9-7(18(25)27-17)5(2)11(20)15(24)13(9)22/h16-25H,3H2,1-2H3/t16-,17+,18?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase using N-formylmethionine-alanine-serine as substrate by spectrophotometry |
J Nat Prod 75: 271-4 (2012)
Article DOI: 10.1021/np200720v
BindingDB Entry DOI: 10.7270/Q24B3299 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50222944

(CHEMBL399664 | macrolactin O)Show SMILES C[C@@H]1CCC\C=C\CCC(=O)C[C@@H](O)C\C=C/C=C/[C@H](C\C=C\C=C/C(=O)O1)O[C@H]1O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]1O |c:15,23,t:5,17,21| Show InChI InChI=1S/C30H44O10/c1-21-13-7-3-2-4-8-14-22(32)19-23(33)15-9-5-10-16-24(17-11-6-12-18-26(34)38-21)39-30-29(37)28(36)27(35)25(20-31)40-30/h2,4-6,9-12,16,18,21,23-25,27-31,33,35-37H,3,7-8,13-15,17,19-20H2,1H3/b4-2+,9-5-,11-6+,16-10+,18-12-/t21-,23+,24-,25+,27+,28-,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase |
J Nat Prod 70: 1632-5 (2007)
Article DOI: 10.1021/np0701327
BindingDB Entry DOI: 10.7270/Q2K07406 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50222942
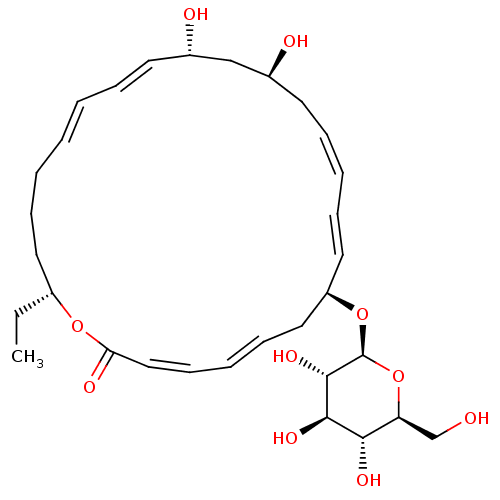
(CHEMBL399435 | macrolactinn P)Show SMILES CC[C@@H]1CCC\C=C\C=C\[C@H](O)C[C@@H](O)C\C=C/C=C/[C@H](C\C=C\C=C/C(=O)O1)O[C@H]1O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]1O |c:16,24,t:6,8,18,22| Show InChI InChI=1S/C31H46O10/c1-2-24-16-10-5-3-4-8-14-22(33)20-23(34)15-9-6-11-17-25(18-12-7-13-19-27(35)39-24)40-31-30(38)29(37)28(36)26(21-32)41-31/h3-4,6-9,11-14,17,19,22-26,28-34,36-38H,2,5,10,15-16,18,20-21H2,1H3/b4-3+,9-6-,12-7+,14-8+,17-11+,19-13-/t22-,23-,24+,25+,26-,28-,29+,30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase |
J Nat Prod 70: 1632-5 (2007)
Article DOI: 10.1021/np0701327
BindingDB Entry DOI: 10.7270/Q2K07406 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50222943
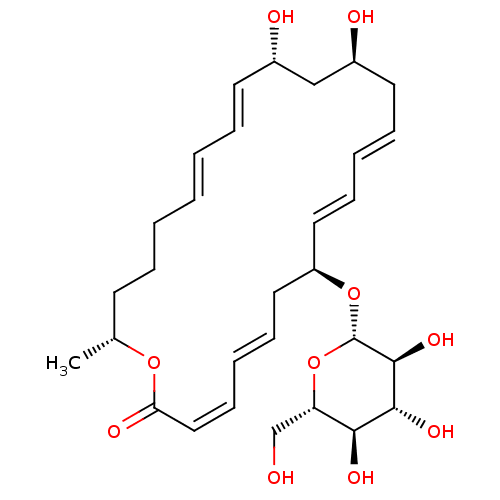
(CHEMBL399871 | macrolactin R)Show SMILES C[C@@H]1CCC\C=C\C=C\[C@H](O)C[C@@H](O)C\C=C\C=C\[C@H](C\C=C\C=C/C(=O)O1)O[C@H]1O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]1O |c:23,t:5,7,15,17,21| Show InChI InChI=1S/C30H44O10/c1-21-13-7-3-2-4-8-14-22(32)19-23(33)15-9-5-10-16-24(17-11-6-12-18-26(34)38-21)39-30-29(37)28(36)27(35)25(20-31)40-30/h2,4-6,8-12,14,16,18,21-25,27-33,35-37H,3,7,13,15,17,19-20H2,1H3/b4-2+,9-5+,11-6+,14-8+,16-10+,18-12-/t21-,22+,23+,24-,25+,27+,28-,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase |
J Nat Prod 70: 1632-5 (2007)
Article DOI: 10.1021/np0701327
BindingDB Entry DOI: 10.7270/Q2K07406 |
More data for this
Ligand-Target Pair | |
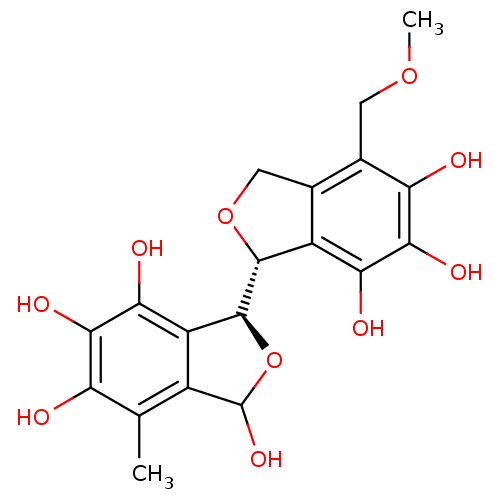
Peptide deformylase
(Staphylococcus aureus) | BDBM50379510
(CHEMBL2012551)Show SMILES COCc1c2CO[C@@H]([C@H]3OC(O)c4c3c(O)c(O)c(O)c4C)c2c(O)c(O)c1O |r| Show InChI InChI=1S/C19H20O10/c1-5-8-10(14(23)15(24)11(5)20)18(29-19(8)26)17-9-6(4-28-17)7(3-27-2)12(21)16(25)13(9)22/h17-26H,3-4H2,1-2H3/t17-,18+,19?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase using N-formylmethionine-alanine-serine as substrate by spectrophotometry |
J Nat Prod 75: 271-4 (2012)
Article DOI: 10.1021/np200720v
BindingDB Entry DOI: 10.7270/Q24B3299 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50157913

(4a,12a-dihydroxy-3-methoxy-4,4,6a,12b-tetramethyl-...)Show SMILES COC1=CC(=O)[C@@]2(C)[C@](O)(CC[C@@]3(C)Oc4cc(oc(=O)c4C[C@@]23O)-c2cc(OC)c(OC)c(OC)c2)C1(C)C |t:2| Show InChI InChI=1S/C30H36O10/c1-26(2)23(37-7)14-22(31)28(4)29(26,33)10-9-27(3)30(28,34)15-17-19(40-27)13-18(39-25(17)32)16-11-20(35-5)24(38-8)21(12-16)36-6/h11-14,33-34H,9-10,15H2,1-8H3/t27-,28+,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Butyrylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50157912

(13-hydroxy-16-methoxy-8-(4-methoxyphenyl)-4,14,19,...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@]45OC(=O)[C@](OC)(C(=O)[C@]4(C)[C@]3(O)Cc2c(=O)o1)C5(C)C |THB:19:18:34:25.23| Show InChI InChI=1S/C28H30O9/c1-23(2)27-12-11-24(3)26(32,25(27,4)21(30)28(23,34-6)22(31)37-27)14-17-19(36-24)13-18(35-20(17)29)15-7-9-16(33-5)10-8-15/h7-10,13,32H,11-12,14H2,1-6H3/t24-,25-,26+,27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Butyrylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50157914

(4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@]4(O)C(C)(C)C(=O)CC[C@]4(C)[C@]3(O)Cc2c(=O)o1 Show InChI InChI=1S/C27H32O7/c1-23(2)21(28)10-11-24(3)26(23,30)13-12-25(4)27(24,31)15-18-20(34-25)14-19(33-22(18)29)16-6-8-17(32-5)9-7-16/h6-9,14,30-31H,10-13,15H2,1-5H3/t24-,25+,26-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Butyrylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50157915

(4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@]4(O)C(C)(C)C(=O)C=C[C@]4(C)[C@]3(O)Cc2c(=O)o1 |c:24| Show InChI InChI=1S/C27H30O7/c1-23(2)21(28)10-11-24(3)26(23,30)13-12-25(4)27(24,31)15-18-20(34-25)14-19(33-22(18)29)16-6-8-17(32-5)9-7-16/h6-11,14,30-31H,12-13,15H2,1-5H3/t24-,25+,26-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Butyrylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50157911

(13a-hydroxy-10-(4-methoxyphenyl)-5,5,5a,7a,13b-pen...)Show SMILES COc1ccc(cc1)-c1cc2O[C@]3(C)CC[C@@]4(O)[C@](C)(CCC(=O)OC4(C)C)[C@]3(O)Cc2c(=O)o1 Show InChI InChI=1S/C27H32O8/c1-23(2)26(30)13-12-25(4)27(31,24(26,3)11-10-21(28)35-23)15-18-20(34-25)14-19(33-22(18)29)16-6-8-17(32-5)9-7-16/h6-9,14,30-31H,10-13,15H2,1-5H3/t24-,25+,26-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Butyrylcholinesterase activity |
Bioorg Med Chem Lett 15: 353-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.067
BindingDB Entry DOI: 10.7270/Q2CN73CD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data