 Found 117 hits with Last Name = 'kim' and Initial = 'yj'
Found 117 hits with Last Name = 'kim' and Initial = 'yj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379308
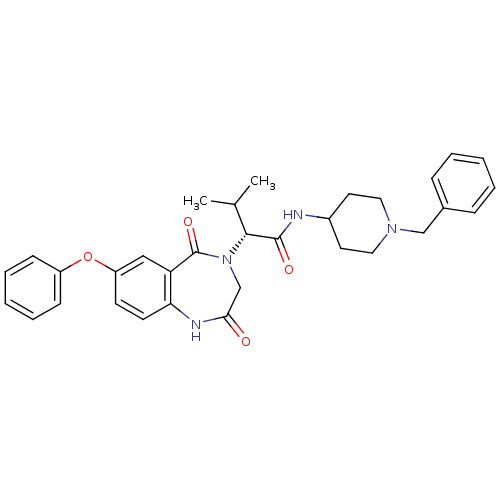
(CHEMBL2011816)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C32H36N4O4/c1-22(2)30(31(38)33-24-15-17-35(18-16-24)20-23-9-5-3-6-10-23)36-21-29(37)34-28-14-13-26(19-27(28)32(36)39)40-25-11-7-4-8-12-25/h3-14,19,22,24,30H,15-18,20-21H2,1-2H3,(H,33,38)(H,34,37)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a receptor |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Mast cell carboxypeptidase A
(Homo sapiens (Human)) | BDBM50281176
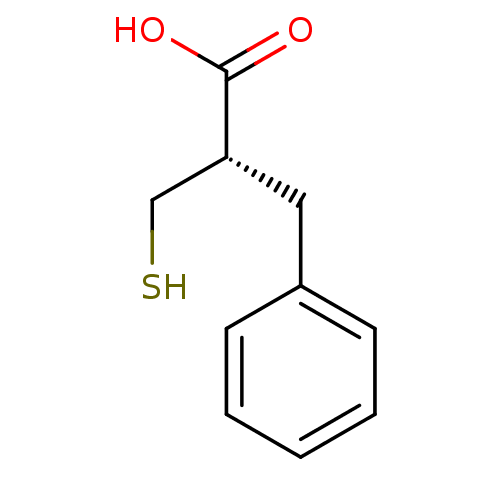
((S)-2-Mercaptomethyl-3-phenyl-propionic acid | 2-T...)Show InChI InChI=1S/C10H12O2S/c11-10(12)9(7-13)6-8-4-2-1-3-5-8/h1-5,9,13H,6-7H2,(H,11,12)/t9-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
UniChem
Similars
| Article
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against carboxypeptidase A |
Bioorg Med Chem Lett 3: 2681-2684 (1993)
Article DOI: 10.1016/S0960-894X(01)80741-1
BindingDB Entry DOI: 10.7270/Q2GM876S |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085045
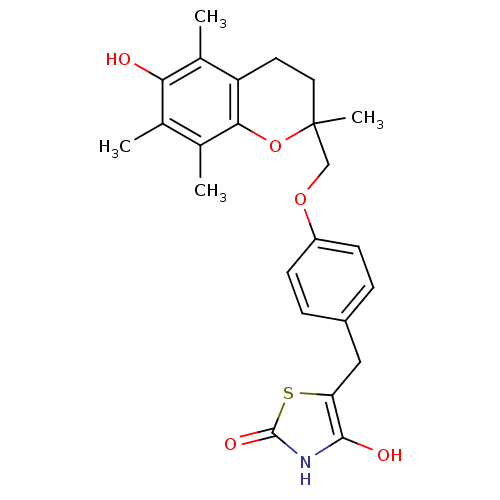
(5-((4-((6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-...)Show SMILES Cc1c(C)c2OC(C)(COc3ccc(Cc4sc(=O)[nH]c4O)cc3)CCc2c(C)c1O Show InChI InChI=1S/C24H27NO5S/c1-13-14(2)21-18(15(3)20(13)26)9-10-24(4,30-21)12-29-17-7-5-16(6-8-17)11-19-22(27)25-23(28)31-19/h5-8,26-27H,9-12H2,1-4H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant PPARgamma by Cheng-Prusoff equation based competitive binding TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.10.010
BindingDB Entry DOI: 10.7270/Q28919F4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM24566
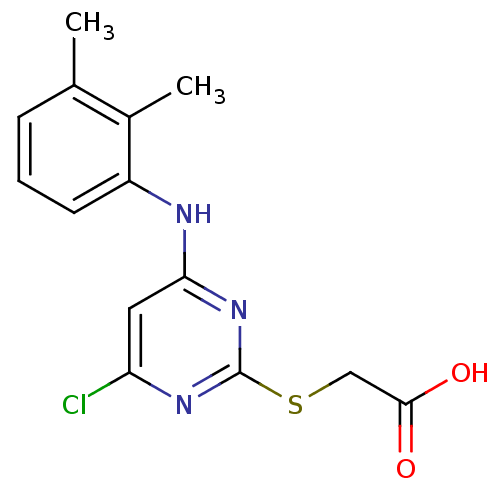
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant PPARalpha by Cheng-Prusoff equation based competitive binding TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.10.010
BindingDB Entry DOI: 10.7270/Q28919F4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50548252
(CHEMBL4765137) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant PPARgamma by Cheng-Prusoff equation based competitive binding TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.10.010
BindingDB Entry DOI: 10.7270/Q28919F4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50548251

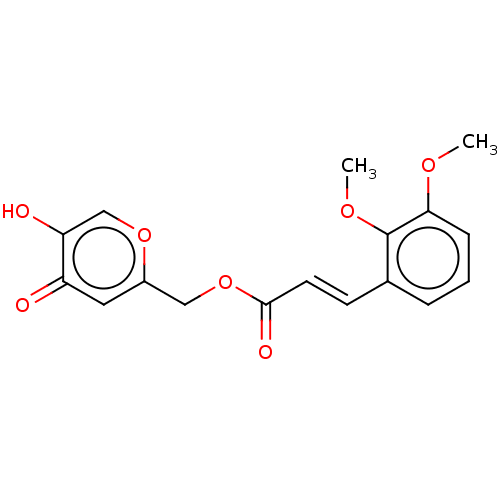
(CHEMBL4797877)Show SMILES COc1ccc(OC)c(\C=C\C(=O)OCc2cc(=O)c(O)co2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant PPARgamma by Cheng-Prusoff equation based competitive binding TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.10.010
BindingDB Entry DOI: 10.7270/Q28919F4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50548252

(CHEMBL4765137) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant PPARalpha by Cheng-Prusoff equation based competitive binding TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.10.010
BindingDB Entry DOI: 10.7270/Q28919F4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50548251

(CHEMBL4797877)Show SMILES COc1ccc(OC)c(\C=C\C(=O)OCc2cc(=O)c(O)co2)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant PPARalpha by Cheng-Prusoff equation based competitive binding TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.10.010
BindingDB Entry DOI: 10.7270/Q28919F4 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379328

(CHEMBL2011825)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccc(F)cc3C)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C33H37FN4O4/c1-21(2)31(32(40)35-25-13-15-37(16-14-25)19-23-7-5-4-6-8-23)38-20-30(39)36-28-11-10-26(18-27(28)33(38)41)42-29-12-9-24(34)17-22(29)3/h4-12,17-18,21,25,31H,13-16,19-20H2,1-3H3,(H,35,40)(H,36,39)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379309

(CHEMBL2011826)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3c(C)cccc3C)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C34H40N4O4/c1-22(2)31(33(40)35-26-15-17-37(18-16-26)20-25-11-6-5-7-12-25)38-21-30(39)36-29-14-13-27(19-28(29)34(38)41)42-32-23(3)9-8-10-24(32)4/h5-14,19,22,26,31H,15-18,20-21H2,1-4H3,(H,35,40)(H,36,39)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379312
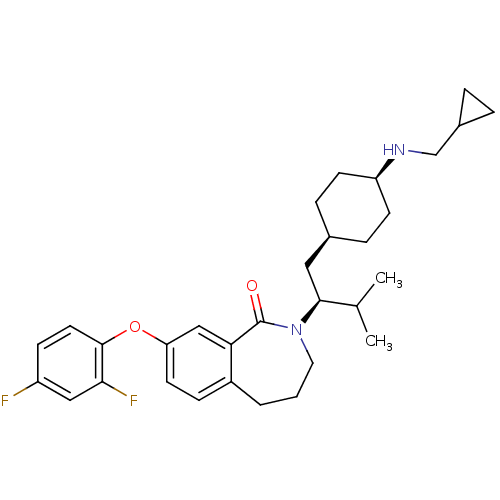
(CHEMBL2011892)Show SMILES CC(C)[C@H](C[C@H]1CC[C@H](CC1)NCC1CC1)N1CCCc2ccc(Oc3ccc(F)cc3F)cc2C1=O |r,wU:8.11,5.4,wD:3.17,(36.58,-32.93,;35.81,-34.26,;34.27,-34.25,;36.57,-35.59,;38.11,-35.6,;39.01,-34.36,;38.22,-33.06,;38.97,-31.7,;40.5,-31.69,;41.29,-33,;40.55,-34.34,;41.25,-30.34,;40.46,-29.01,;41.21,-27.66,;42.53,-26.86,;41.18,-26.11,;35.79,-36.92,;36.59,-38.39,;35.93,-39.87,;34.36,-40.25,;33.03,-39.23,;31.7,-40,;30.36,-39.23,;30.36,-37.7,;29.03,-36.94,;27.7,-37.71,;27.71,-39.26,;26.37,-40.03,;25.04,-39.26,;23.7,-40.03,;25.04,-37.72,;26.37,-36.95,;26.36,-35.41,;31.68,-36.92,;33.01,-37.68,;34.24,-36.67,;33.83,-35.18,)| Show InChI InChI=1S/C31H40F2N2O2/c1-20(2)29(16-21-7-11-25(12-8-21)34-19-22-5-6-22)35-15-3-4-23-9-13-26(18-27(23)31(35)36)37-30-14-10-24(32)17-28(30)33/h9-10,13-14,17-18,20-22,25,29,34H,3-8,11-12,15-16,19H2,1-2H3/t21-,25+,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379320

(CHEMBL2011834)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)N1CCC(CC1)N[C@H](C)C1CCCCC1 |r| Show InChI InChI=1S/C33H44N4O4/c1-22(2)31(33(40)36-18-16-25(17-19-36)34-23(3)24-10-6-4-7-11-24)37-21-30(38)35-29-15-14-27(20-28(29)32(37)39)41-26-12-8-5-9-13-26/h5,8-9,12-15,20,22-25,31,34H,4,6-7,10-11,16-19,21H2,1-3H3,(H,35,38)/t23-,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379322
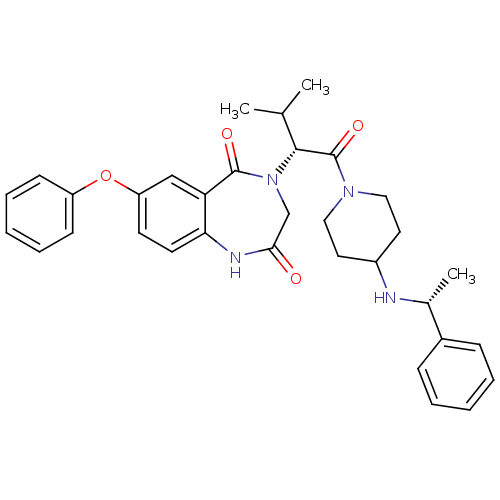
(CHEMBL2011832)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)N1CCC(CC1)N[C@H](C)c1ccccc1 |r| Show InChI InChI=1S/C33H38N4O4/c1-22(2)31(33(40)36-18-16-25(17-19-36)34-23(3)24-10-6-4-7-11-24)37-21-30(38)35-29-15-14-27(20-28(29)32(37)39)41-26-12-8-5-9-13-26/h4-15,20,22-23,25,31,34H,16-19,21H2,1-3H3,(H,35,38)/t23-,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379310

(CHEMBL2011894)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccc(F)cc3F)cc2C1=O)C(=O)N1CCC(CC1)NCc1ccccc1 |r| Show InChI InChI=1S/C32H34F2N4O4/c1-20(2)30(32(41)37-14-12-23(13-15-37)35-18-21-6-4-3-5-7-21)38-19-29(39)36-27-10-9-24(17-25(27)31(38)40)42-28-11-8-22(33)16-26(28)34/h3-11,16-17,20,23,30,35H,12-15,18-19H2,1-2H3,(H,36,39)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379334

(CHEMBL2011819)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3C)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C33H38N4O4/c1-22(2)31(32(39)34-25-15-17-36(18-16-25)20-24-10-5-4-6-11-24)37-21-30(38)35-28-14-13-26(19-27(28)33(37)40)41-29-12-8-7-9-23(29)3/h4-14,19,22,25,31H,15-18,20-21H2,1-3H3,(H,34,39)(H,35,38)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379319

(CHEMBL2011835)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)N1CCC(CC1)NCCC1CCCCC1 |r| Show InChI InChI=1S/C33H44N4O4/c1-23(2)31(33(40)36-19-16-25(17-20-36)34-18-15-24-9-5-3-6-10-24)37-22-30(38)35-29-14-13-27(21-28(29)32(37)39)41-26-11-7-4-8-12-26/h4,7-8,11-14,21,23-25,31,34H,3,5-6,9-10,15-20,22H2,1-2H3,(H,35,38)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379314

(CHEMBL2011890)Show SMILES CC(C)[C@H](C[C@H]1CC[C@H](CC1)NCc1ccccc1)N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O |r,wU:5.4,8.11,wD:3.20,(.88,-6.3,;.11,-7.62,;-1.43,-7.61,;.86,-8.95,;2.4,-8.96,;3.18,-7.63,;2.41,-6.3,;3.2,-4.97,;4.74,-4.98,;5.5,-6.33,;4.72,-7.65,;5.53,-3.65,;7.07,-3.67,;7.83,-5.01,;7.04,-6.33,;7.8,-7.68,;9.34,-7.69,;10.13,-6.35,;9.37,-5.02,;.09,-10.28,;.89,-11.74,;.23,-13.22,;1.27,-14.47,;-1.34,-13.6,;-2.66,-12.58,;-4,-13.35,;-5.33,-12.59,;-5.33,-11.06,;-6.66,-10.29,;-7.99,-11.06,;-9.31,-10.31,;-10.64,-11.07,;-10.64,-12.61,;-9.31,-13.38,;-7.98,-12.61,;-4.01,-10.28,;-2.68,-11.04,;-1.46,-10.03,;-1.86,-8.54,)| Show InChI InChI=1S/C33H39N3O3/c1-23(2)31(19-24-13-15-26(16-14-24)34-21-25-9-5-3-6-10-25)36-22-32(37)35-30-18-17-28(20-29(30)33(36)38)39-27-11-7-4-8-12-27/h3-12,17-18,20,23-24,26,31,34H,13-16,19,21-22H2,1-2H3,(H,35,37)/t24-,26+,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379308

(CHEMBL2011816)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C32H36N4O4/c1-22(2)30(31(38)33-24-15-17-35(18-16-24)20-23-9-5-3-6-10-23)36-21-29(37)34-28-14-13-26(19-27(28)32(36)39)40-25-11-7-4-8-12-25/h3-14,19,22,24,30H,15-18,20-21H2,1-2H3,(H,33,38)(H,34,37)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse GHSR1 receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379311

(CHEMBL2011893)Show SMILES CC(C)[C@H](C[C@H]1CC[C@H](CC1)NCC1CC1)N1CCc2ccc(Oc3ccc(F)cc3F)cc2C1=O |r,wU:5.4,8.11,wD:3.17,(13.01,-46.57,;11.68,-47.35,;10.34,-46.58,;11.68,-48.88,;13.01,-49.65,;14.42,-49.01,;14.37,-47.49,;15.69,-46.68,;17.04,-47.43,;17.07,-48.96,;15.76,-49.75,;18.35,-46.62,;18.32,-45.08,;19.64,-44.27,;21.19,-44.23,;20.38,-42.91,;10.35,-49.65,;10.37,-51.2,;9.04,-51.98,;7.7,-51.23,;6.37,-52,;5.03,-51.24,;5.03,-49.7,;3.7,-48.94,;2.37,-49.71,;2.37,-51.26,;1.04,-52.04,;-.3,-51.26,;-1.64,-52.03,;-.29,-49.72,;1.04,-48.95,;1.03,-47.41,;6.35,-48.92,;7.68,-49.68,;9,-48.89,;8.99,-47.35,)| Show InChI InChI=1S/C30H38F2N2O2/c1-19(2)28(15-20-5-9-24(10-6-20)33-18-21-3-4-21)34-14-13-22-7-11-25(17-26(22)30(34)35)36-29-12-8-23(31)16-27(29)32/h7-8,11-12,16-17,19-21,24,28,33H,3-6,9-10,13-15,18H2,1-2H3/t20-,24+,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379335

(CHEMBL2011818)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccc(C)cc3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C33H38N4O4/c1-22(2)31(32(39)34-25-15-17-36(18-16-25)20-24-7-5-4-6-8-24)37-21-30(38)35-29-14-13-27(19-28(29)33(37)40)41-26-11-9-23(3)10-12-26/h4-14,19,22,25,31H,15-18,20-21H2,1-3H3,(H,34,39)(H,35,38)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379323
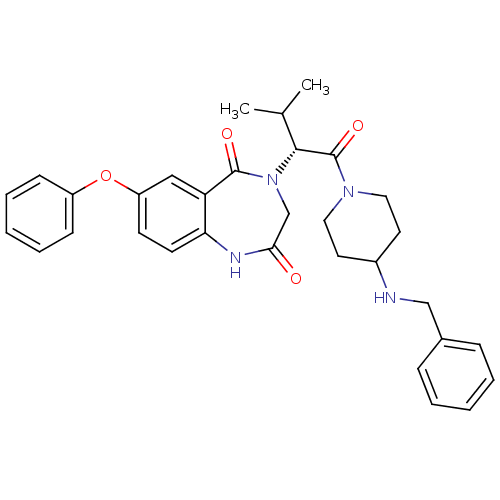
(CHEMBL2011831)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)N1CCC(CC1)NCc1ccccc1 |r| Show InChI InChI=1S/C32H36N4O4/c1-22(2)30(32(39)35-17-15-24(16-18-35)33-20-23-9-5-3-6-10-23)36-21-29(37)34-28-14-13-26(19-27(28)31(36)38)40-25-11-7-4-8-12-25/h3-14,19,22,24,30,33H,15-18,20-21H2,1-2H3,(H,34,37)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50153547

(3-(2,6-Dichloro-phenyl)-5-[2-(2-oxo-piperidin-1-yl...)Show SMILES CCN(CC)c1ccc(NC(=O)c2c(CCN3CCCCC3=O)onc2-c2c(Cl)cccc2Cl)cc1 |(8.86,-1.12,;7.38,-.65,;6.25,-1.69,;6.56,-3.2,;8.05,-3.65,;4.77,-1.21,;3.64,-2.25,;2.18,-1.77,;1.85,-.28,;.4,.2,;-.75,-.84,;-.42,-2.33,;-2.22,-.36,;-2.7,1.1,;-1.8,2.35,;-2.43,3.77,;-1.53,5.01,;.01,4.83,;.89,6.06,;.28,7.49,;-1.25,7.63,;-2.16,6.39,;-3.7,6.57,;-4.24,1.1,;-4.71,-.37,;-3.45,-1.26,;-3.45,-2.8,;-4.78,-3.58,;-6.13,-2.8,;-4.78,-5.12,;-3.45,-5.88,;-2.11,-5.12,;-2.11,-3.57,;-.58,-3.57,;2.99,.76,;4.44,.3,)| Show InChI InChI=1S/C27H30Cl2N4O3/c1-3-32(4-2)19-13-11-18(12-14-19)30-27(35)25-22(15-17-33-16-6-5-10-23(33)34)36-31-26(25)24-20(28)8-7-9-21(24)29/h7-9,11-14H,3-6,10,15-17H2,1-2H3,(H,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as blockade of intracellular Ca2+ mobilization |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379308

(CHEMBL2011816)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C32H36N4O4/c1-22(2)30(31(38)33-24-15-17-35(18-16-24)20-23-9-5-3-6-10-23)36-21-29(37)34-28-14-13-26(19-27(28)32(36)39)40-25-11-7-4-8-12-25/h3-14,19,22,24,30H,15-18,20-21H2,1-2H3,(H,33,38)(H,34,37)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379336
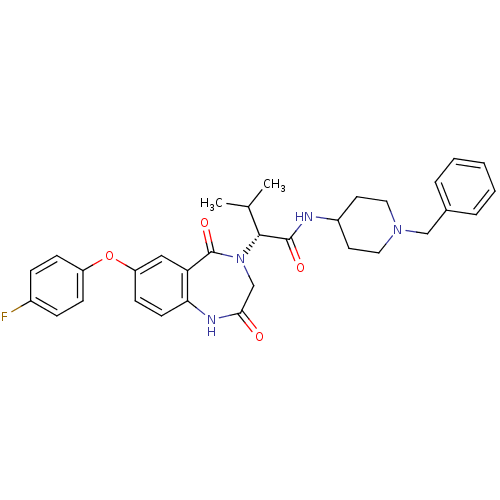
(CHEMBL2011817)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccc(F)cc3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C32H35FN4O4/c1-21(2)30(31(39)34-24-14-16-36(17-15-24)19-22-6-4-3-5-7-22)37-20-29(38)35-28-13-12-26(18-27(28)32(37)40)41-25-10-8-23(33)9-11-25/h3-13,18,21,24,30H,14-17,19-20H2,1-2H3,(H,34,39)(H,35,38)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379326

(CHEMBL2011828)Show SMILES O=C(NC1CCN(Cc2ccccc2)CC1)[C@@H](C1CC1)N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O |r| Show InChI InChI=1S/C32H34N4O4/c37-29-21-36(32(39)27-19-26(13-14-28(27)34-29)40-25-9-5-2-6-10-25)30(23-11-12-23)31(38)33-24-15-17-35(18-16-24)20-22-7-3-1-4-8-22/h1-10,13-14,19,23-24,30H,11-12,15-18,20-21H2,(H,33,38)(H,34,37)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Probable G-protein coupled receptor 142
(Mus musculus) | BDBM50395775
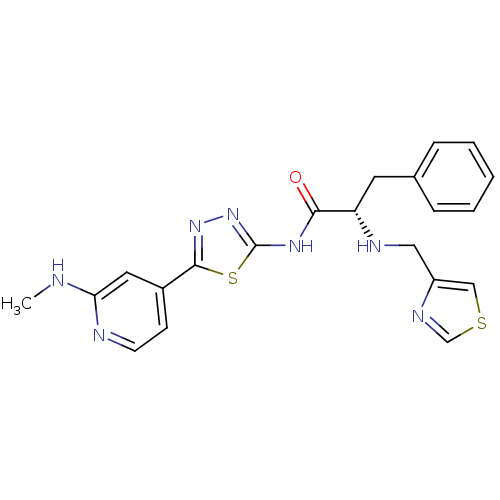
(CHEMBL2164857)Show SMILES CNc1cc(ccn1)-c1nnc(NC(=O)[C@H](Cc2ccccc2)NCc2cscn2)s1 |r| Show InChI InChI=1S/C21H21N7OS2/c1-22-18-10-15(7-8-23-18)20-27-28-21(31-20)26-19(29)17(9-14-5-3-2-4-6-14)24-11-16-12-30-13-25-16/h2-8,10,12-13,17,24H,9,11H2,1H3,(H,22,23)(H,26,28,29)/t17-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Partial agonist activity at mouse GPR142 expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6218-23 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.015
BindingDB Entry DOI: 10.7270/Q2CN751R |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50158327

((+/-)isobutyl 2-((4-(diethylamino)phenyl)carbamoyl...)Show SMILES CCN(CC)c1ccc(NC(=O)C2(CCc3cccc(OC)c3C2)N(C)C(=O)OCC(C)C)cc1 Show InChI InChI=1S/C28H39N3O4/c1-7-31(8-2)23-14-12-22(13-15-23)29-26(32)28(30(5)27(33)35-19-20(3)4)17-16-21-10-9-11-25(34-6)24(21)18-28/h9-15,20H,7-8,16-19H2,1-6H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as blockade of intracellular Ca2+ mobilization |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379321

(CHEMBL2011833)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)N1CCC(CC1)NC(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C34H40N4O4/c1-23(2)31(33(41)37-19-17-25(18-20-37)36-34(3,4)24-11-7-5-8-12-24)38-22-30(39)35-29-16-15-27(21-28(29)32(38)40)42-26-13-9-6-10-14-26/h5-16,21,23,25,31,36H,17-20,22H2,1-4H3,(H,35,39)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379313

(CHEMBL2011891)Show SMILES CC(C)[C@H](C[C@H]1CC[C@H](CC1)NCC1CC1)N1CC(=O)Nc2ccc(Oc3ccc(F)cc3F)cc2C1=O |r,wU:8.11,5.4,wD:3.17,(17.11,-33.7,;16.33,-35.03,;14.8,-35.02,;17.09,-36.36,;18.63,-36.37,;19.54,-35.12,;18.74,-33.82,;19.49,-32.47,;21.03,-32.45,;21.82,-33.77,;21.07,-35.1,;21.78,-31.11,;20.98,-29.78,;21.73,-28.43,;23.06,-27.63,;21.7,-26.87,;16.32,-37.69,;17.12,-39.15,;16.45,-40.64,;17.5,-41.89,;14.88,-41.02,;13.56,-40,;12.22,-40.77,;10.88,-40,;10.88,-38.47,;9.55,-37.7,;8.22,-38.47,;8.23,-40.02,;6.9,-40.8,;5.56,-40.02,;4.22,-40.79,;5.57,-38.49,;6.89,-37.72,;6.89,-36.17,;12.21,-37.69,;13.54,-38.45,;14.76,-37.43,;14.36,-35.95,)| Show InChI InChI=1S/C30H37F2N3O3/c1-18(2)27(13-19-5-8-22(9-6-19)33-16-20-3-4-20)35-17-29(36)34-26-11-10-23(15-24(26)30(35)37)38-28-12-7-21(31)14-25(28)32/h7,10-12,14-15,18-20,22,27,33H,3-6,8-9,13,16-17H2,1-2H3,(H,34,36)/t19-,22+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379331

(CHEMBL2011822)Show SMILES COc1ccccc1Oc1ccc2NC(=O)CN([C@H](C(C)C)C(=O)NC3CCN(Cc4ccccc4)CC3)C(=O)c2c1 |r| Show InChI InChI=1S/C33H38N4O5/c1-22(2)31(32(39)34-24-15-17-36(18-16-24)20-23-9-5-4-6-10-23)37-21-30(38)35-27-14-13-25(19-26(27)33(37)40)42-29-12-8-7-11-28(29)41-3/h4-14,19,22,24,31H,15-18,20-21H2,1-3H3,(H,34,39)(H,35,38)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379332

(CHEMBL2011821)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccc(Cl)cc3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C32H35ClN4O4/c1-21(2)30(31(39)34-24-14-16-36(17-15-24)19-22-6-4-3-5-7-22)37-20-29(38)35-28-13-12-26(18-27(28)32(37)40)41-25-10-8-23(33)9-11-25/h3-13,18,21,24,30H,14-17,19-20H2,1-2H3,(H,34,39)(H,35,38)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379317

(CHEMBL2011887)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)N1CCC(CC1)NCC1CC1 |r| Show InChI InChI=1S/C29H36N4O4/c1-19(2)27(29(36)32-14-12-21(13-15-32)30-17-20-8-9-20)33-18-26(34)31-25-11-10-23(16-24(25)28(33)35)37-22-6-4-3-5-7-22/h3-7,10-11,16,19-21,27,30H,8-9,12-15,17-18H2,1-2H3,(H,31,34)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <62 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50435297

(CHEMBL2393244)Show InChI InChI=1S/C21H21FN4/c22-16-7-4-8-17(14-16)24-21-18-9-11-23-12-10-19(18)25-20(26-21)13-15-5-2-1-3-6-15/h1-8,14,23H,9-13H2,(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D2L receptor (unknown origin) after 60 mins |
Eur J Med Chem 63: 558-69 (2013)
Article DOI: 10.1016/j.ejmech.2013.02.020
BindingDB Entry DOI: 10.7270/Q2RX9DGD |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379329
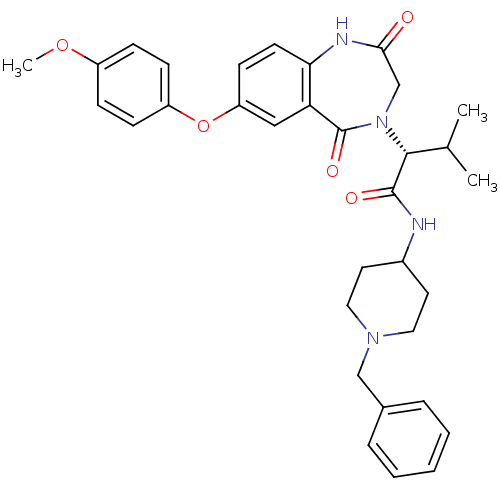
(CHEMBL2011824)Show SMILES COc1ccc(Oc2ccc3NC(=O)CN([C@H](C(C)C)C(=O)NC4CCN(Cc5ccccc5)CC4)C(=O)c3c2)cc1 |r| Show InChI InChI=1S/C33H38N4O5/c1-22(2)31(32(39)34-24-15-17-36(18-16-24)20-23-7-5-4-6-8-23)37-21-30(38)35-29-14-13-27(19-28(29)33(37)40)42-26-11-9-25(41-3)10-12-26/h4-14,19,22,24,31H,15-18,20-21H2,1-3H3,(H,34,39)(H,35,38)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50435300

(CHEMBL2393245)Show InChI InChI=1S/C21H21ClN4/c22-16-7-4-8-17(14-16)24-21-18-9-11-23-12-10-19(18)25-20(26-21)13-15-5-2-1-3-6-15/h1-8,14,23H,9-13H2,(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D2L receptor (unknown origin) after 60 mins |
Eur J Med Chem 63: 558-69 (2013)
Article DOI: 10.1016/j.ejmech.2013.02.020
BindingDB Entry DOI: 10.7270/Q2RX9DGD |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379330

(CHEMBL2011823)Show SMILES COc1cccc(Oc2ccc3NC(=O)CN([C@H](C(C)C)C(=O)NC4CCN(Cc5ccccc5)CC4)C(=O)c3c2)c1 |r| Show InChI InChI=1S/C33H38N4O5/c1-22(2)31(32(39)34-24-14-16-36(17-15-24)20-23-8-5-4-6-9-23)37-21-30(38)35-29-13-12-27(19-28(29)33(37)40)42-26-11-7-10-25(18-26)41-3/h4-13,18-19,22,24,31H,14-17,20-21H2,1-3H3,(H,34,39)(H,35,38)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50435299

(CHEMBL2393243)Show InChI InChI=1S/C21H22N4/c1-3-7-16(8-4-1)15-20-24-19-12-14-22-13-11-18(19)21(25-20)23-17-9-5-2-6-10-17/h1-10,22H,11-15H2,(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D2L receptor (unknown origin) after 60 mins |
Eur J Med Chem 63: 558-69 (2013)
Article DOI: 10.1016/j.ejmech.2013.02.020
BindingDB Entry DOI: 10.7270/Q2RX9DGD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50435298

(CHEMBL2393246)Show InChI InChI=1S/C22H24N4O/c1-27-18-9-5-8-17(15-18)24-22-19-10-12-23-13-11-20(19)25-21(26-22)14-16-6-3-2-4-7-16/h2-9,15,23H,10-14H2,1H3,(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D2L receptor (unknown origin) after 60 mins |
Eur J Med Chem 63: 558-69 (2013)
Article DOI: 10.1016/j.ejmech.2013.02.020
BindingDB Entry DOI: 10.7270/Q2RX9DGD |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379333

(CHEMBL2011820)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3cccc(Cl)c3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C32H35ClN4O4/c1-21(2)30(31(39)34-24-13-15-36(16-14-24)19-22-7-4-3-5-8-22)37-20-29(38)35-28-12-11-26(18-27(28)32(37)40)41-25-10-6-9-23(33)17-25/h3-12,17-18,21,24,30H,13-16,19-20H2,1-2H3,(H,34,39)(H,35,38)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677
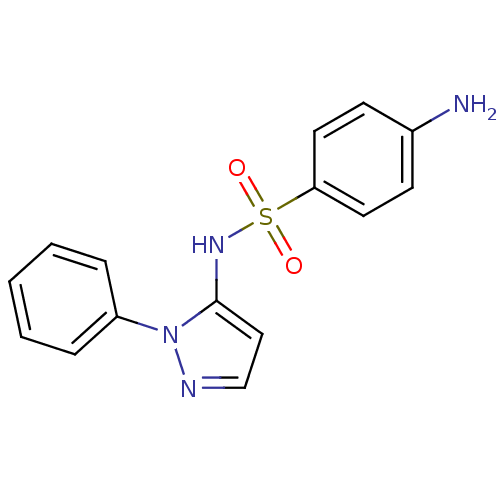
(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379325

(CHEMBL2011829)Show SMILES CC(C)[C@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C32H36N4O4/c1-22(2)30(31(38)33-24-15-17-35(18-16-24)20-23-9-5-3-6-10-23)36-21-29(37)34-28-14-13-26(19-27(28)32(36)39)40-25-11-7-4-8-12-25/h3-14,19,22,24,30H,15-18,20-21H2,1-2H3,(H,33,38)(H,34,37)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50100556
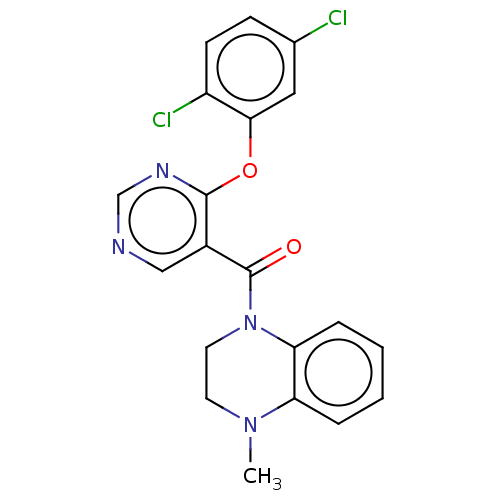
(CHEMBL3321845)Show SMILES CN1CCN(C(=O)c2cncnc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-8-9-26(17-5-3-2-4-16(17)25)20(27)14-11-23-12-24-19(14)28-18-10-13(21)6-7-15(18)22/h2-7,10-12H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50100557
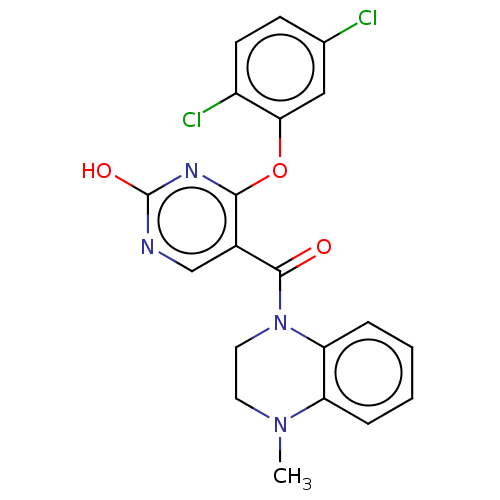
(CHEMBL3321846)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-25-8-9-26(16-5-3-2-4-15(16)25)19(27)13-11-23-20(28)24-18(13)29-17-10-12(21)6-7-14(17)22/h2-7,10-11H,8-9H2,1H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50100558

(CHEMBL3321850)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H19Cl2N3O2/c1-24-10-11-25(17-5-3-2-4-16(17)24)19(27)20(8-9-20)18(26)23-15-12-13(21)6-7-14(15)22/h2-7,12H,8-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379318

(CHEMBL2011836)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)N1CCC(CC1)NCCC1CCOCC1 |r| Show InChI InChI=1S/C32H42N4O5/c1-22(2)30(32(39)35-16-11-24(12-17-35)33-15-10-23-13-18-40-19-14-23)36-21-29(37)34-28-9-8-26(20-27(28)31(36)38)41-25-6-4-3-5-7-25/h3-9,20,22-24,30,33H,10-19,21H2,1-2H3,(H,34,37)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379327

(CHEMBL2011827)Show SMILES O=C(CN1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)NC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C29H30N4O4/c34-27(30-22-13-15-32(16-14-22)18-21-7-3-1-4-8-21)19-33-20-28(35)31-26-12-11-24(17-25(26)29(33)36)37-23-9-5-2-6-10-23/h1-12,17,22H,13-16,18-20H2,(H,30,34)(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1a receptor assessed as intracellular Ca2+ concentration by aequorin luminescent assay |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50100558

(CHEMBL3321850)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H19Cl2N3O2/c1-24-10-11-25(17-5-3-2-4-16(17)24)19(27)20(8-9-20)18(26)23-15-12-13(21)6-7-14(15)22/h2-7,12H,8-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50100557

(CHEMBL3321846)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-25-8-9-26(16-5-3-2-4-15(16)25)19(27)13-11-23-20(28)24-18(13)29-17-10-12(21)6-7-14(17)22/h2-7,10-11H,8-9H2,1H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data