 Found 713 hits with Last Name = 'kimura' and Initial = 'h'
Found 713 hits with Last Name = 'kimura' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 60 mins by activity based 100 fold dilution assay |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50351401

(CHEMBL1819091)Show SMILES CC[C@@]1(N)CCCN(C1)c1cc2n(C)c(=O)n(C)c(=O)c2n1Cc1cc(F)ccc1C#N |r| Show InChI InChI=1S/C23H27FN6O2/c1-4-23(26)8-5-9-29(14-23)19-11-18-20(21(31)28(3)22(32)27(18)2)30(19)13-16-10-17(24)7-6-15(16)12-25/h6-7,10-11H,4-5,8-9,13-14,26H2,1-3H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis |
Bioorg Med Chem 19: 5490-9 (2011)
Article DOI: 10.1016/j.bmc.2011.07.042
BindingDB Entry DOI: 10.7270/Q2T72HT6 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50327046
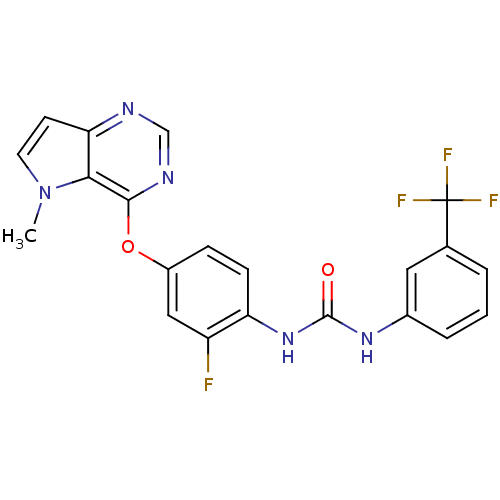
(1-(2-fluoro-4-(5-methyl-5H-pyrrolo[3,2-d]pyrimidin...)Show SMILES Cn1ccc2ncnc(Oc3ccc(NC(=O)Nc4cccc(c4)C(F)(F)F)c(F)c3)c12 Show InChI InChI=1S/C21H15F4N5O2/c1-30-8-7-17-18(30)19(27-11-26-17)32-14-5-6-16(15(22)10-14)29-20(31)28-13-4-2-3-12(9-13)21(23,24)25/h2-11H,1H3,(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 60 mins by activity based 100 fold dilution assay |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50351399
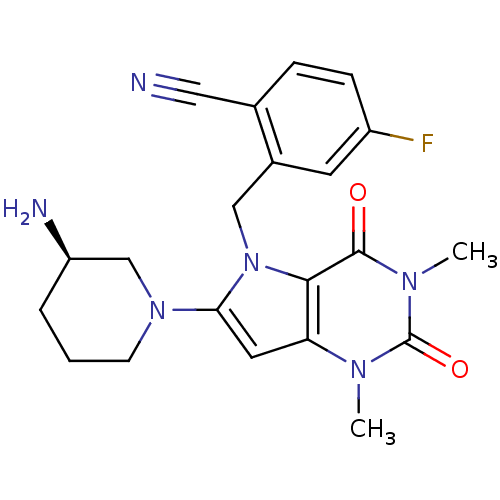
(CHEMBL1819089)Show SMILES Cn1c2cc(N3CCC[C@@H](N)C3)n(Cc3cc(F)ccc3C#N)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H23FN6O2/c1-25-17-9-18(27-7-3-4-16(24)12-27)28(19(17)20(29)26(2)21(25)30)11-14-8-15(22)6-5-13(14)10-23/h5-6,8-9,16H,3-4,7,11-12,24H2,1-2H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis |
Bioorg Med Chem 19: 5490-9 (2011)
Article DOI: 10.1016/j.bmc.2011.07.042
BindingDB Entry DOI: 10.7270/Q2T72HT6 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50327046

(1-(2-fluoro-4-(5-methyl-5H-pyrrolo[3,2-d]pyrimidin...)Show SMILES Cn1ccc2ncnc(Oc3ccc(NC(=O)Nc4cccc(c4)C(F)(F)F)c(F)c3)c12 Show InChI InChI=1S/C21H15F4N5O2/c1-30-8-7-17-18(30)19(27-11-26-17)32-14-5-6-16(15(22)10-14)29-20(31)28-13-4-2-3-12(9-13)21(23,24)25/h2-11H,1H3,(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 60 mins by ligand displacement based enzyme-inhibitor dilution assay |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 60 mins by ligand displacement based enzyme-inhibitor dilution assay |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50384537
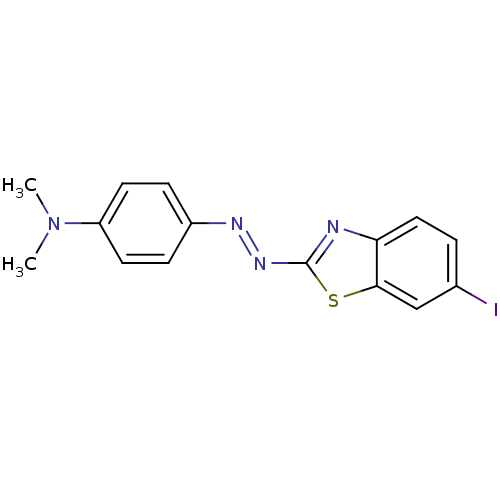
(CHEMBL2036430)Show InChI InChI=1S/C15H13IN4S/c1-20(2)12-6-4-11(5-7-12)18-19-15-17-13-8-3-10(16)9-14(13)21-15/h3-9H,1-2H3/b19-18+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of Thioflavin S from recombinant human tau expressed in Escherichia coli after 30 mins by fluorescence assay |
ACS Med Chem Lett 3: 58-62 (2012)
Article DOI: 10.1021/ml200230e
BindingDB Entry DOI: 10.7270/Q2PR7X1P |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492526
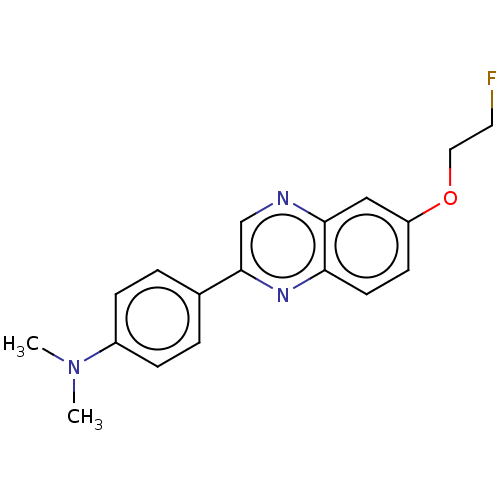
(CHEMBL2407616)Show InChI InChI=1S/C18H18FN3O/c1-22(2)14-5-3-13(4-6-14)18-12-20-17-11-15(23-10-9-19)7-8-16(17)21-18/h3-8,11-12H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.895 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492522

(CHEMBL2407622)Show InChI InChI=1S/C18H18FN3O/c1-22(2)14-5-3-13(4-6-14)18-12-20-17-11-15(23-10-9-19)7-8-16(17)21-18/h3-8,11-12H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.895 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to beta-amyloid plaque in Alzheimer's disease patient brain cortex after 1 hr by thioflavin-S based autoradiography |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 10 mins by Global fit analysis |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441236
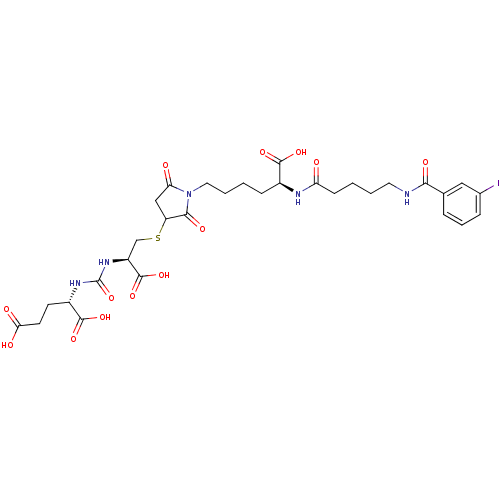
(CHEMBL2431341)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CSC1CC(=O)N(CCCC[C@H](NC(=O)CCCCNC(=O)c2cccc(I)c2)C(O)=O)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C31H40IN5O13S/c32-18-7-5-6-17(14-18)26(42)33-12-3-1-9-23(38)34-19(28(44)45)8-2-4-13-37-24(39)15-22(27(37)43)51-16-21(30(48)49)36-31(50)35-20(29(46)47)10-11-25(40)41/h5-7,14,19-22H,1-4,8-13,15-16H2,(H,33,42)(H,34,38)(H,40,41)(H,44,45)(H,46,47)(H,48,49)(H2,35,36,50)/t19-,20-,21-,22?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441235
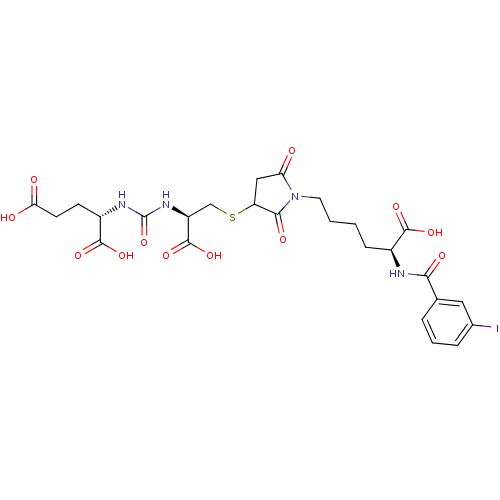
(CHEMBL2431330)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CSC1CC(=O)N(CCCC[C@H](NC(=O)c2cccc(I)c2)C(O)=O)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C26H31IN4O12S/c27-14-5-3-4-13(10-14)21(35)28-15(23(37)38)6-1-2-9-31-19(32)11-18(22(31)36)44-12-17(25(41)42)30-26(43)29-16(24(39)40)7-8-20(33)34/h3-5,10,15-18H,1-2,6-9,11-12H2,(H,28,35)(H,33,34)(H,37,38)(H,39,40)(H,41,42)(H2,29,30,43)/t15-,16-,17-,18?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 10 mins by Global fit analysis |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441237

(CHEMBL2431340)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CSC1CC(=O)N(CCCC[C@H](NC(=O)CCCNC(=O)c2cccc(I)c2)C(O)=O)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C30H38IN5O13S/c31-17-6-3-5-16(13-17)25(41)32-11-4-8-22(37)33-18(27(43)44)7-1-2-12-36-23(38)14-21(26(36)42)50-15-20(29(47)48)35-30(49)34-19(28(45)46)9-10-24(39)40/h3,5-6,13,18-21H,1-2,4,7-12,14-15H2,(H,32,41)(H,33,37)(H,39,40)(H,43,44)(H,45,46)(H,47,48)(H2,34,35,49)/t18-,19-,20-,21?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC as substrate by fluorimetric analysis |
Bioorg Med Chem 20: 5864-83 (2012)
Article DOI: 10.1016/j.bmc.2012.07.046
BindingDB Entry DOI: 10.7270/Q2FX7BJX |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50327046

(1-(2-fluoro-4-(5-methyl-5H-pyrrolo[3,2-d]pyrimidin...)Show SMILES Cn1ccc2ncnc(Oc3ccc(NC(=O)Nc4cccc(c4)C(F)(F)F)c(F)c3)c12 Show InChI InChI=1S/C21H15F4N5O2/c1-30-8-7-17-18(30)19(27-11-26-17)32-14-5-6-16(15(22)10-14)29-20(31)28-13-4-2-3-12(9-13)21(23,24)25/h2-11H,1H3,(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 10 mins by Global fit analysis |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441241

(CHEMBL2431333 | CHEMBL2431336)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CSC1CC(=O)N(CCCC[C@H](NC(=O)CNC(=O)c2cccc(I)c2)C(O)=O)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H34IN5O13S/c29-15-5-3-4-14(10-15)23(39)30-12-20(35)31-16(25(41)42)6-1-2-9-34-21(36)11-19(24(34)40)48-13-18(27(45)46)33-28(47)32-17(26(43)44)7-8-22(37)38/h3-5,10,16-19H,1-2,6-9,11-13H2,(H,30,39)(H,31,35)(H,37,38)(H,41,42)(H,43,44)(H,45,46)(H2,32,33,47)/t16-,17-,18-,19?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441238
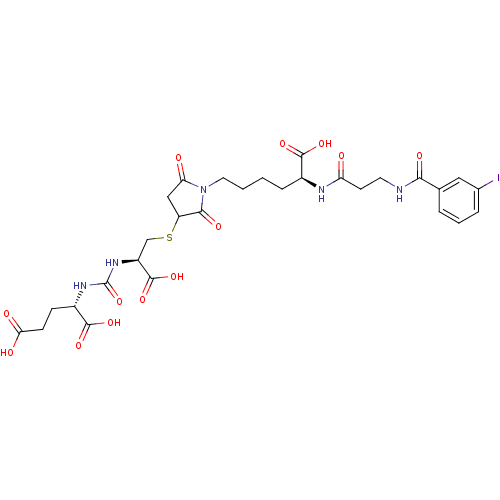
(CHEMBL2431339)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CSC1CC(=O)N(CCCC[C@H](NC(=O)CCNC(=O)c2cccc(I)c2)C(O)=O)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H36IN5O13S/c30-16-5-3-4-15(12-16)24(40)31-10-9-21(36)32-17(26(42)43)6-1-2-11-35-22(37)13-20(25(35)41)49-14-19(28(46)47)34-29(48)33-18(27(44)45)7-8-23(38)39/h3-5,12,17-20H,1-2,6-11,13-14H2,(H,31,40)(H,32,36)(H,38,39)(H,42,43)(H,44,45)(H,46,47)(H2,33,34,48)/t17-,18-,19-,20?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50122787
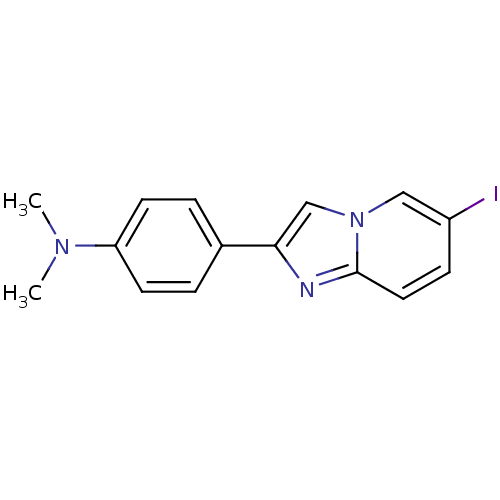
(2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...)Show InChI InChI=1S/C15H14IN3/c1-18(2)13-6-3-11(4-7-13)14-10-19-9-12(16)5-8-15(19)17-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441239
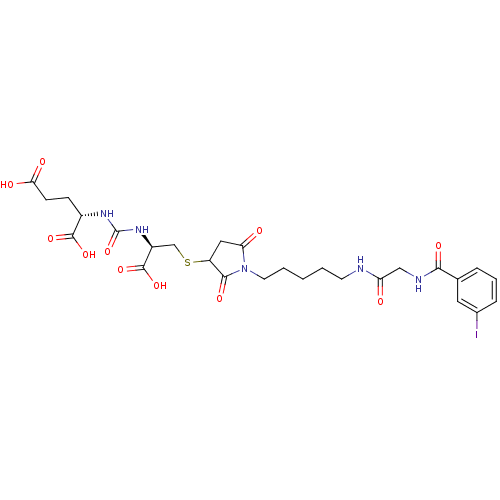
(CHEMBL2431338)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CSC1CC(=O)N(CCCCCNC(=O)CNC(=O)c2cccc(I)c2)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C27H34IN5O11S/c28-16-6-4-5-15(11-16)23(38)30-13-20(34)29-9-2-1-3-10-33-21(35)12-19(24(33)39)45-14-18(26(42)43)32-27(44)31-17(25(40)41)7-8-22(36)37/h4-6,11,17-19H,1-3,7-10,12-14H2,(H,29,34)(H,30,38)(H,36,37)(H,40,41)(H,42,43)(H2,31,32,44)/t17-,18-,19?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441240
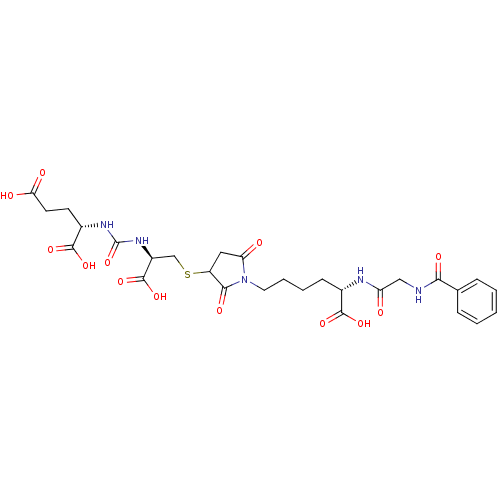
(CHEMBL2431337)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CSC1CC(=O)N(CCCC[C@H](NC(=O)CNC(=O)c2ccccc2)C(O)=O)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H35N5O13S/c34-20(13-29-23(38)15-6-2-1-3-7-15)30-16(25(40)41)8-4-5-11-33-21(35)12-19(24(33)39)47-14-18(27(44)45)32-28(46)31-17(26(42)43)9-10-22(36)37/h1-3,6-7,16-19H,4-5,8-14H2,(H,29,38)(H,30,34)(H,36,37)(H,40,41)(H,42,43)(H,44,45)(H2,31,32,46)/t16-,17-,18-,19?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492518
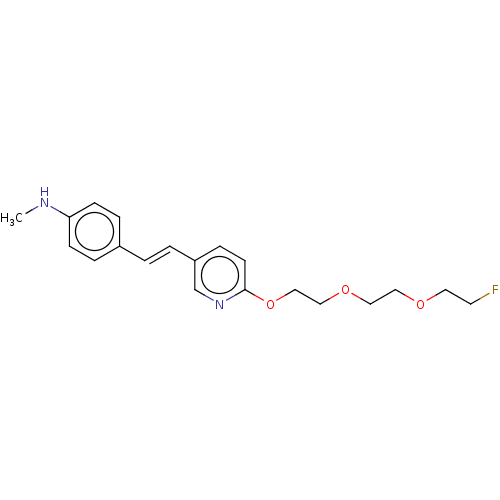
(Florbetapir | US10906900, AV45)Show InChI InChI=1S/C20H25FN2O3/c1-22-19-7-4-17(5-8-19)2-3-18-6-9-20(23-16-18)26-15-14-25-13-12-24-11-10-21/h2-9,16,22H,10-15H2,1H3/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50384538
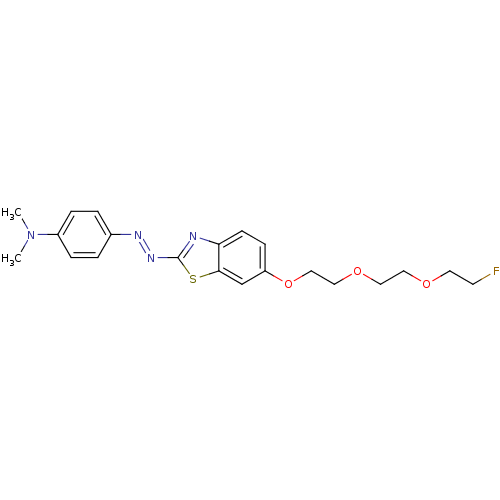
(CHEMBL2036419 | CHEMBL2036420)Show SMILES CN(C)c1ccc(cc1)\N=N\c1nc2ccc(OCCOCCOCCF)cc2s1 Show InChI InChI=1S/C21H25FN4O3S/c1-26(2)17-5-3-16(4-6-17)24-25-21-23-19-8-7-18(15-20(19)30-21)29-14-13-28-12-11-27-10-9-22/h3-8,15H,9-14H2,1-2H3/b25-24+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of Thioflavin S from recombinant human tau expressed in Escherichia coli after 30 mins by fluorescence assay |
ACS Med Chem Lett 3: 58-62 (2012)
Article DOI: 10.1021/ml200230e
BindingDB Entry DOI: 10.7270/Q2PR7X1P |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492519

(CHEMBL2407615)Show InChI InChI=1S/C17H16FN3O/c1-19-13-4-2-12(3-5-13)17-11-20-16-10-14(22-9-8-18)6-7-15(16)21-17/h2-7,10-11,19H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492516
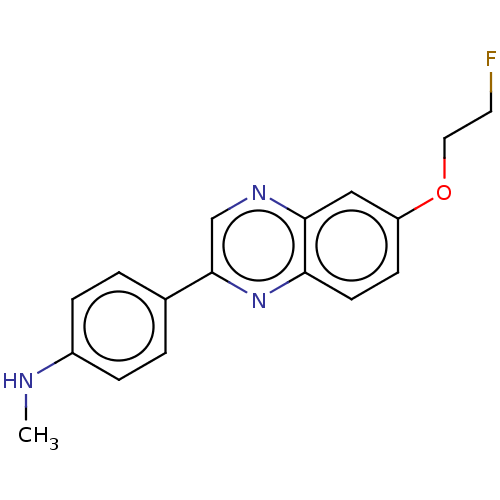
(CHEMBL2407621)Show InChI InChI=1S/C17H16FN3O/c1-19-13-4-2-12(3-5-13)17-11-20-16-10-14(22-9-8-18)6-7-15(16)21-17/h2-7,10-11,19H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to beta-amyloid plaque in Alzheimer's disease patient brain cortex after 1 hr by thioflavin-S based autoradiography |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50390102
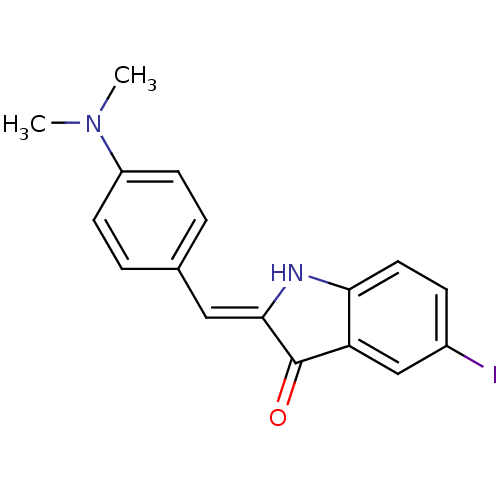
(CHEMBL2069433)Show InChI InChI=1S/C17H15IN2O/c1-20(2)13-6-3-11(4-7-13)9-16-17(21)14-10-12(18)5-8-15(14)19-16/h3-10,19H,1-2H3/b16-9- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to human Tau aggregates after 30 mins by Thioflavin-S-based fluorescence assay |
Bioorg Med Chem Lett 22: 5700-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.086
BindingDB Entry DOI: 10.7270/Q2TQ62K1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged non-phosphorylated VEGFR2 after 10 mins by Global fit analysis |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50327046

(1-(2-fluoro-4-(5-methyl-5H-pyrrolo[3,2-d]pyrimidin...)Show SMILES Cn1ccc2ncnc(Oc3ccc(NC(=O)Nc4cccc(c4)C(F)(F)F)c(F)c3)c12 Show InChI InChI=1S/C21H15F4N5O2/c1-30-8-7-17-18(30)19(27-11-26-17)32-14-5-6-16(15(22)10-14)29-20(31)28-13-4-2-3-12(9-13)21(23,24)25/h2-11H,1H3,(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged phosphorylated VEGFR2 in presence of ATP measured without preincubation by AlphaScreen analysis |
Bioorg Med Chem 19: 5342-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.002
BindingDB Entry DOI: 10.7270/Q21R6QW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441234

(CHEMBL2431331)Show SMILES CN1C(=O)CC(SC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C1=O |r| Show InChI InChI=1S/C14H19N3O9S/c1-17-9(18)4-8(11(17)21)27-5-7(13(24)25)16-14(26)15-6(12(22)23)2-3-10(19)20/h6-8H,2-5H2,1H3,(H,19,20)(H,22,23)(H,24,25)(H2,15,16,26)/t6-,7-,8?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50441233
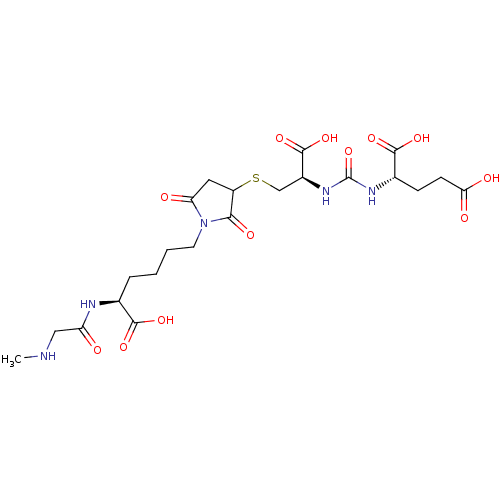
(CHEMBL2431332)Show SMILES CNCC(=O)N[C@@H](CCCCN1C(=O)CC(SC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C1=O)C(O)=O |r| Show InChI InChI=1S/C22H33N5O12S/c1-23-9-15(28)24-11(19(33)34)4-2-3-7-27-16(29)8-14(18(27)32)40-10-13(21(37)38)26-22(39)25-12(20(35)36)5-6-17(30)31/h11-14,23H,2-10H2,1H3,(H,24,28)(H,30,31)(H,33,34)(H,35,36)(H,37,38)(H2,25,26,39)/t11-,12-,13-,14?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem 20: 5864-83 (2012)
Article DOI: 10.1016/j.bmc.2012.07.046
BindingDB Entry DOI: 10.7270/Q2FX7BJX |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492523
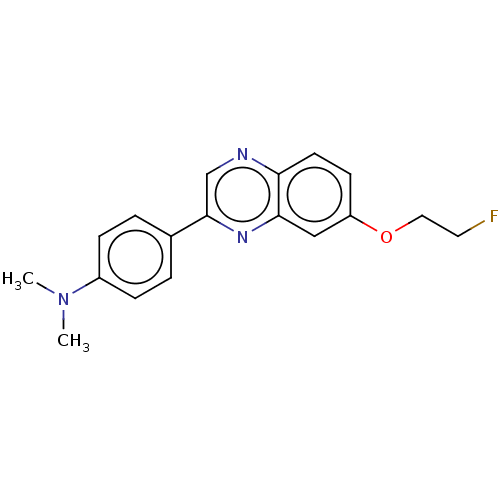
(CHEMBL2407623)Show InChI InChI=1S/C18H18FN3O/c1-22(2)14-5-3-13(4-6-14)18-12-20-16-8-7-15(23-10-9-19)11-17(16)21-18/h3-8,11-12H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to beta-amyloid plaque in Alzheimer's disease patient brain cortex after 1 hr by thioflavin-S based autoradiography |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492523

(CHEMBL2407623)Show InChI InChI=1S/C18H18FN3O/c1-22(2)14-5-3-13(4-6-14)18-12-20-16-8-7-15(23-10-9-19)11-17(16)21-18/h3-8,11-12H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50390103
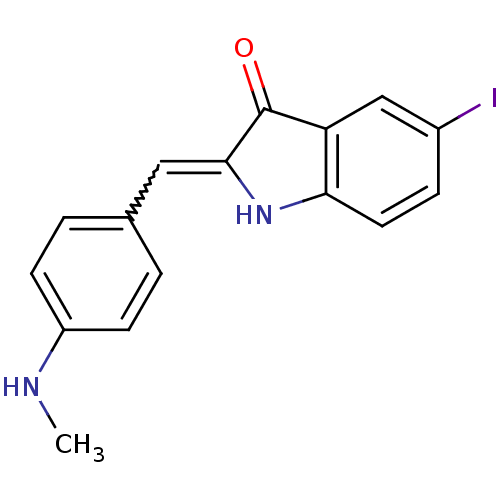
(CHEMBL2069432)Show InChI InChI=1S/C16H13IN2O/c1-18-12-5-2-10(3-6-12)8-15-16(20)13-9-11(17)4-7-14(13)19-15/h2-9,18-19H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to human Tau aggregates after 30 mins by Thioflavin-S-based fluorescence assay |
Bioorg Med Chem Lett 22: 5700-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.086
BindingDB Entry DOI: 10.7270/Q2TQ62K1 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492520
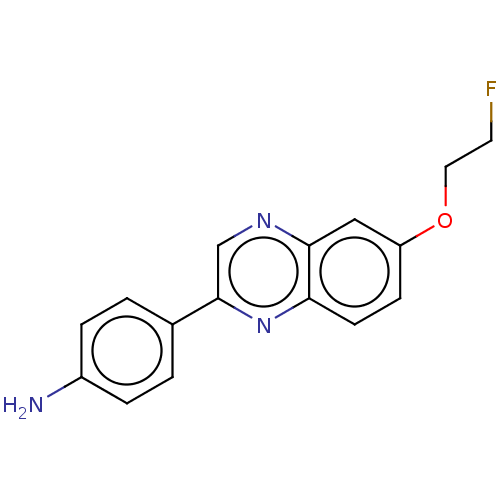
(CHEMBL2407624)Show InChI InChI=1S/C16H14FN3O/c17-7-8-21-13-5-6-14-15(9-13)19-10-16(20-14)11-1-3-12(18)4-2-11/h1-6,9-10H,7-8,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to beta-amyloid plaque in Alzheimer's disease patient brain cortex after 1 hr by thioflavin-S based autoradiography |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492521

(CHEMBL2407617)Show InChI InChI=1S/C16H14FN3O/c17-7-8-21-13-5-6-14-15(9-13)20-16(10-19-14)11-1-3-12(18)4-2-11/h1-6,9-10H,7-8,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to beta-amyloid plaque in Alzheimer's disease patient brain cortex after 1 hr by thioflavin-S based autoradiography |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492520

(CHEMBL2407624)Show InChI InChI=1S/C16H14FN3O/c17-7-8-21-13-5-6-14-15(9-13)19-10-16(20-14)11-1-3-12(18)4-2-11/h1-6,9-10H,7-8,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492517
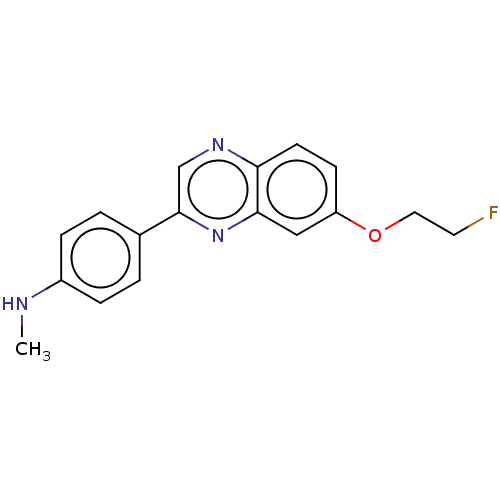
(CHEMBL2407618)Show InChI InChI=1S/C17H16FN3O/c1-19-13-4-2-12(3-5-13)17-11-20-15-7-6-14(22-9-8-18)10-16(15)21-17/h2-7,10-11,19H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to beta-amyloid plaque in Alzheimer's disease patient brain cortex after 1 hr by thioflavin-S based autoradiography |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50102256
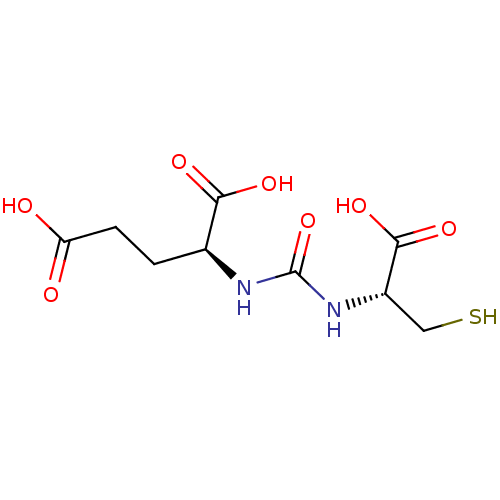
(2-[3-(1-Carboxy-2-mercapto-ethyl)-ureido]-pentaned...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CS)C(O)=O)C(O)=O Show InChI InChI=1S/C9H14N2O7S/c12-6(13)2-1-4(7(14)15)10-9(18)11-5(3-19)8(16)17/h4-5,19H,1-3H2,(H,12,13)(H,14,15)(H,16,17)(H2,10,11,18)/t4-,5-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [123I]-DCIT from PSMA in human LNCaP cells after 1 hr by gamma counting |
J Med Chem 56: 7890-901 (2013)
Article DOI: 10.1021/jm400895s
BindingDB Entry DOI: 10.7270/Q21G0NPK |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50390104
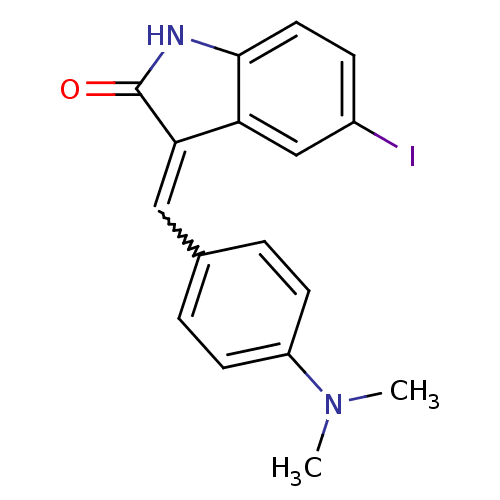
(CHEMBL2069431)Show InChI InChI=1S/C17H15IN2O/c1-20(2)13-6-3-11(4-7-13)9-15-14-10-12(18)5-8-16(14)19-17(15)21/h3-10H,1-2H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 599 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to human Tau aggregates after 30 mins by Thioflavin-S-based fluorescence assay |
Bioorg Med Chem Lett 22: 5700-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.086
BindingDB Entry DOI: 10.7270/Q2TQ62K1 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492524

(CHEMBL2407625)Show InChI InChI=1S/C17H16FN3O/c1-19-13-4-2-12(3-5-13)17-11-20-15-7-6-14(22-9-8-18)10-16(15)21-17/h2-7,10-11,19H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 758 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem 20: 5864-83 (2012)
Article DOI: 10.1016/j.bmc.2012.07.046
BindingDB Entry DOI: 10.7270/Q2FX7BJX |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492525

(CHEMBL2407626)Show InChI InChI=1S/C16H14FN3O/c17-7-8-21-13-5-6-14-15(9-13)20-16(10-19-14)11-1-3-12(18)4-2-11/h1-6,9-10H,7-8,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
ACS Med Chem Lett 4: 596-600 (2013)
Article DOI: 10.1021/ml4000707
BindingDB Entry DOI: 10.7270/Q2RX9G0Z |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50390105

(CHEMBL2069430)Show InChI InChI=1S/C16H13IN2O/c1-18-12-5-2-10(3-6-12)8-14-13-9-11(17)4-7-15(13)19-16(14)20/h2-9,18H,1H3,(H,19,20) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Binding affinity to human Tau aggregates after 30 mins by Thioflavin-S-based fluorescence assay |
Bioorg Med Chem Lett 22: 5700-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.086
BindingDB Entry DOI: 10.7270/Q2TQ62K1 |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 8
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of MTX uptake in OAT3-expressing S2 cells |
J Pharmacol Exp Ther 302: 666-71 (2002)
Article DOI: 10.1124/jpet.102.034330
BindingDB Entry DOI: 10.7270/Q2HH6MBN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389606
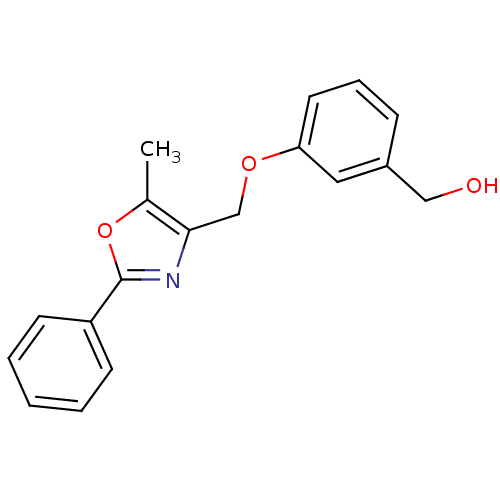
(CHEMBL2069625)Show InChI InChI=1S/C18H17NO3/c1-13-17(12-21-16-9-5-6-14(10-16)11-20)19-18(22-13)15-7-3-2-4-8-15/h2-10,20H,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human unphosphorylated N-terminal-His6 tagged VEGFR2 catalytic domain using 5-FAM-EEPLYWSFPAKKK-CONH2 as substrate after 60 mins by mob... |
ACS Med Chem Lett 3: 342-346 (2012)
Article DOI: 10.1021/ml3000403
BindingDB Entry DOI: 10.7270/Q2FN1782 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Solute carrier family 22 member 8
(Homo sapiens (Human)) | BDBM50206509
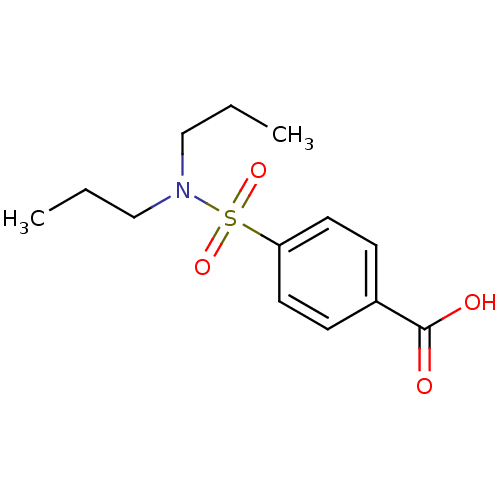
(4-Dipropylsulfamoyl-benzoic acid | 4-Dipropylsulfa...)Show InChI InChI=1S/C13H19NO4S/c1-3-9-14(10-4-2)19(17,18)12-7-5-11(6-8-12)13(15)16/h5-8H,3-4,9-10H2,1-2H3,(H,15,16) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of MTX uptake in OAT3-expressing S2 cells |
J Pharmacol Exp Ther 302: 666-71 (2002)
Article DOI: 10.1124/jpet.102.034330
BindingDB Entry DOI: 10.7270/Q2HH6MBN |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 8
(Homo sapiens (Human)) | BDBM50022309
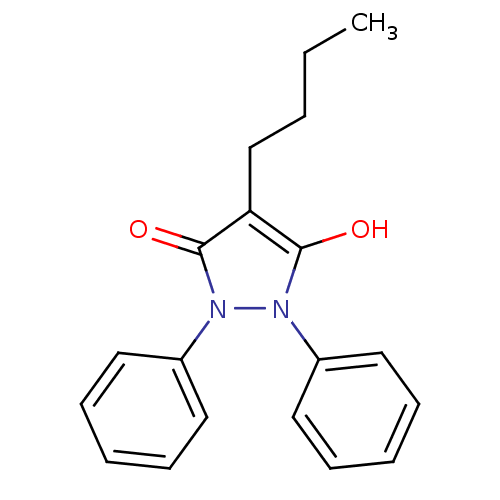
(3,5-Dioxo-1,2-diphenyl-4-n-butylpyrazolidine | 4-b...)Show InChI InChI=1S/C19H20N2O2/c1-2-3-14-17-18(22)20(15-10-6-4-7-11-15)21(19(17)23)16-12-8-5-9-13-16/h4-13,22H,2-3,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of MTX uptake in OAT3-expressing S2 cells |
J Pharmacol Exp Ther 302: 666-71 (2002)
Article DOI: 10.1124/jpet.102.034330
BindingDB Entry DOI: 10.7270/Q2HH6MBN |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 8
(Homo sapiens (Human)) | BDBM50022787
((+)-3,3-Dimethyl-7-oxo-6-phenylacetylamino-4-thia-...)Show SMILES CC1(C)S[C@@H]2[C@H](NC(=O)Cc3ccccc3)C(=O)N2[C@H]1C(O)=O |r| Show InChI InChI=1S/C16H18N2O4S/c1-16(2)12(15(21)22)18-13(20)11(14(18)23-16)17-10(19)8-9-6-4-3-5-7-9/h3-7,11-12,14H,8H2,1-2H3,(H,17,19)(H,21,22)/t11-,12+,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of MTX uptake in OAT3-expressing S2 cells |
J Pharmacol Exp Ther 302: 666-71 (2002)
Article DOI: 10.1124/jpet.102.034330
BindingDB Entry DOI: 10.7270/Q2HH6MBN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data