

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930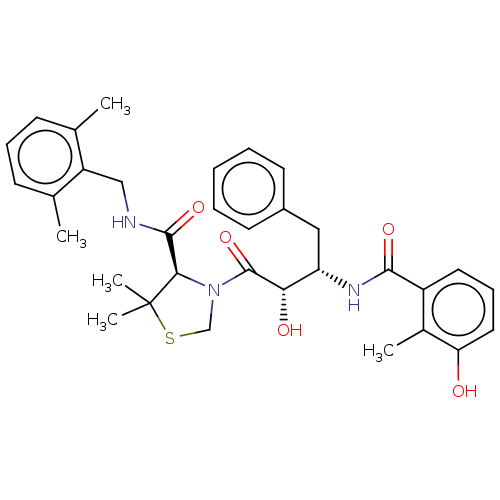 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323472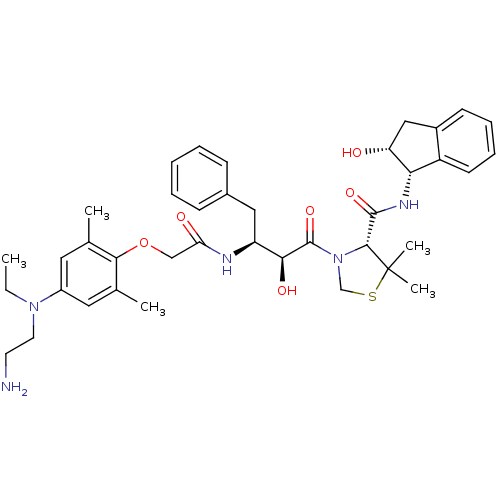 ((R)-3-((2S,3S)-3-(2-(4-((2-aminoethyl)(ethyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323469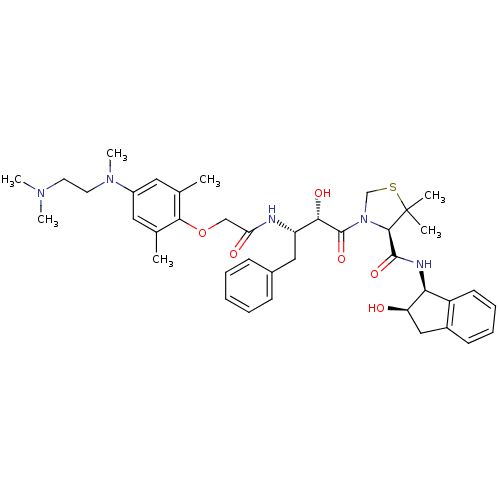 ((R)-3-((2S,3S)-3-(2-(4-((2-(dimethylamino)ethyl)(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065562 (CHEMBL96279 | [2-(6-Fluoro-2,2-dimethyl-chroman-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065578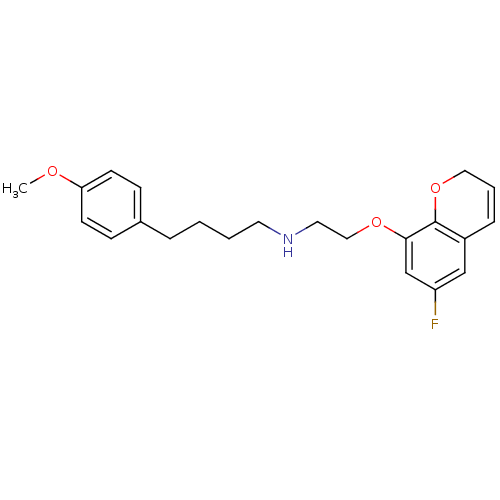 (CHEMBL419147 | [2-(6-Fluoro-2H-chromen-8-yloxy)-et...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121729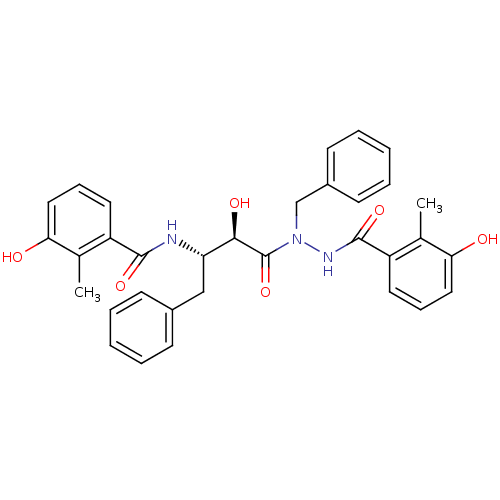 (CHEMBL368169 | KNI-1167 | N-[(S)-3-[N-Benzyl-N'-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194658 ((2R)-N-(1-cyclopropylmethyl-4-piperidinylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065555 (CHEMBL96578 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194659 ((2R)-N-(1-cyclopentylmethyl-4-piperidinylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480931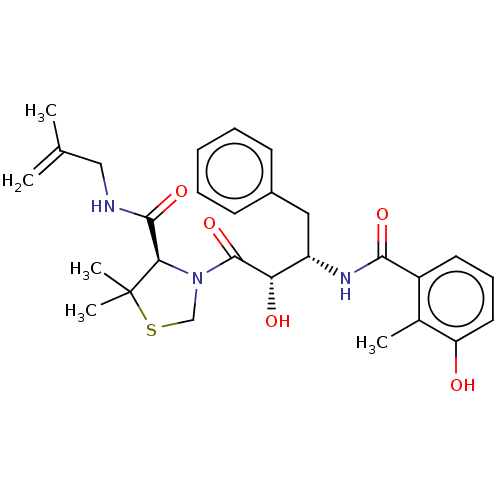 (CHEMBL575512 | KNI-1614) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209553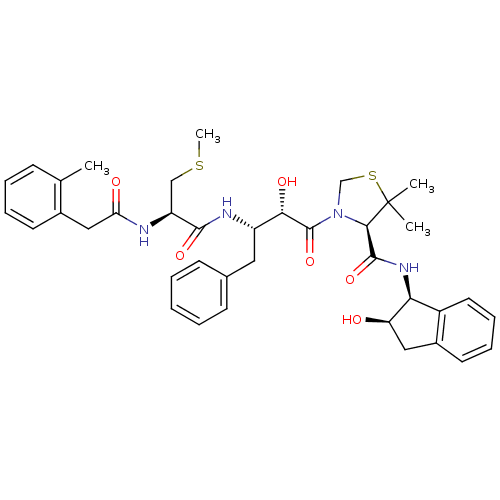 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121730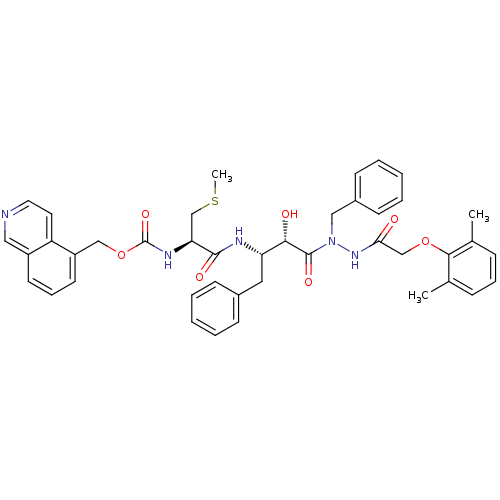 (CHEMBL366433 | {1-[(S)-3-{N-Benzyl-N'-[2-(2,6-dime...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50057436 (CHEMBL25213 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065573 (CHEMBL96222 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065578 (CHEMBL419147 | [2-(6-Fluoro-2H-chromen-8-yloxy)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194640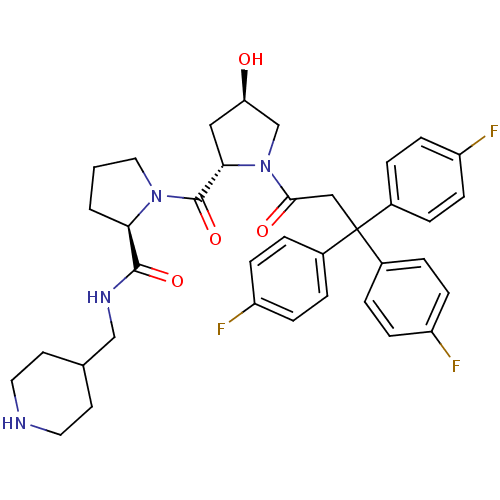 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065573 (CHEMBL96222 | [2-(6-Fluoro-2-methyl-chroman-8-ylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194641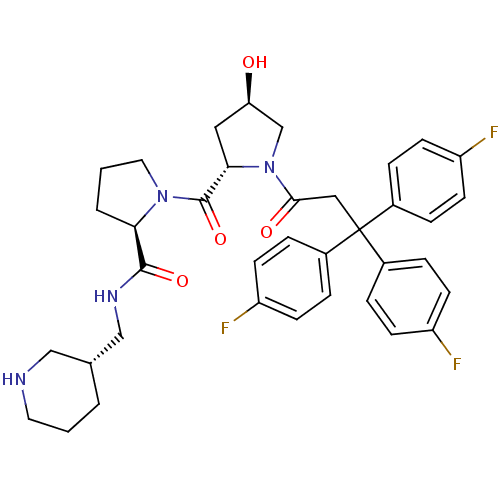 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194654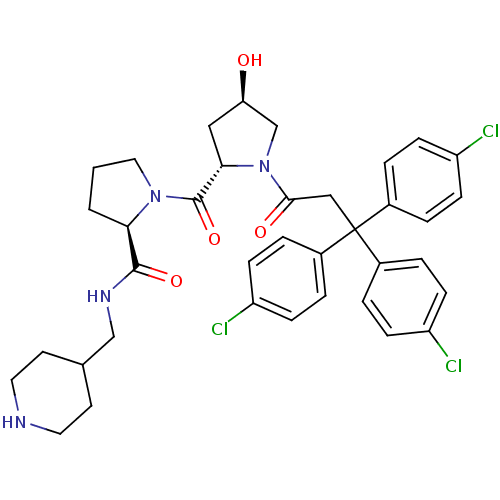 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50065562 (CHEMBL96279 | [2-(6-Fluoro-2,2-dimethyl-chroman-8-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121732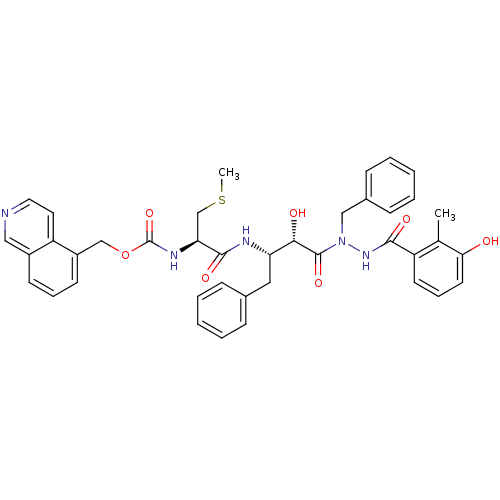 (CHEMBL172850 | {1-[(S)-3-[N-Benzyl-N'-(3-hydroxy-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194645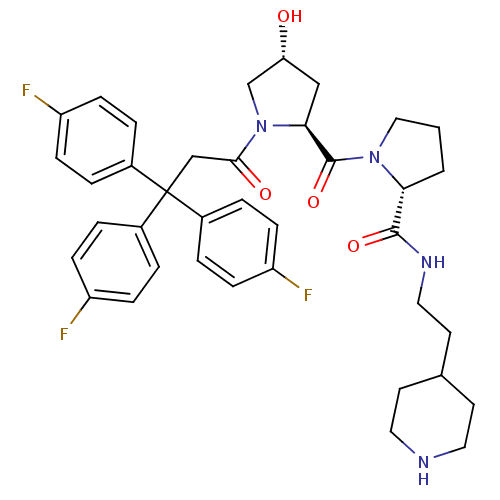 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM82372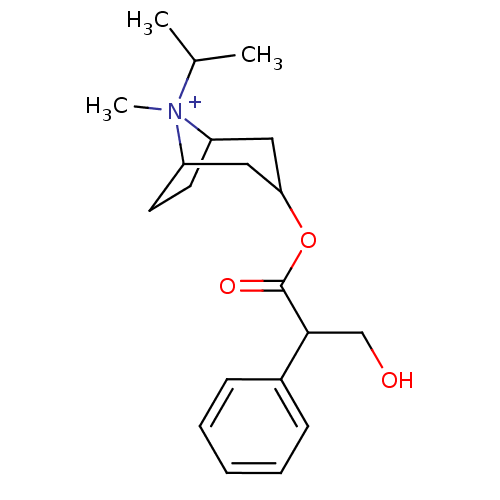 (CAS_22254-24-6 | Ipratropium | NSC_3746) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559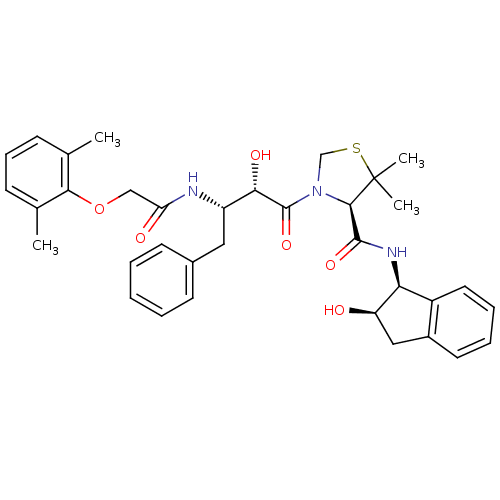 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem 16: 10049-60 (2008) Article DOI: 10.1016/j.bmc.2008.10.011 BindingDB Entry DOI: 10.7270/Q2G160PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM82372 (CAS_22254-24-6 | Ipratropium | NSC_3746) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194642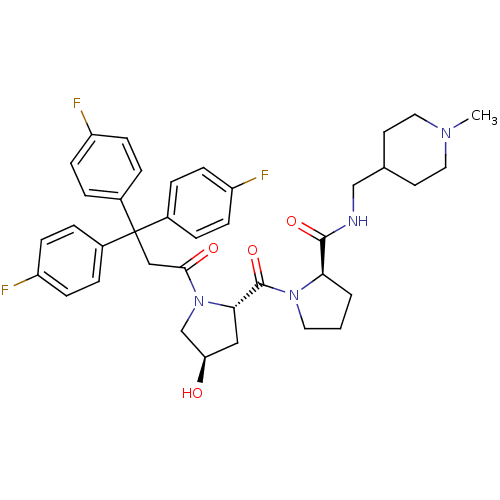 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194655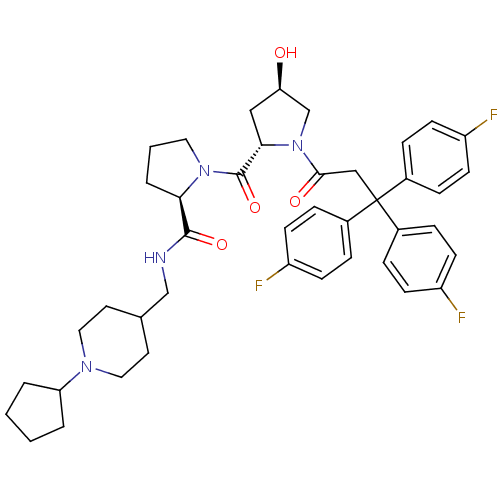 ((2R)-N-(1-cyclopentyl-4-piperidinylmethyl)-1-((2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194639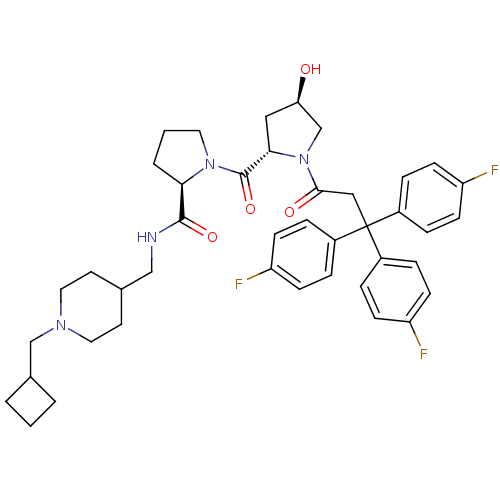 ((2R)-N-(1-cyclobutylmethyl-4-piperidinylmethyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated against Dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand; ND = Not determined | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM82372 (CAS_22254-24-6 | Ipratropium | NSC_3746) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323470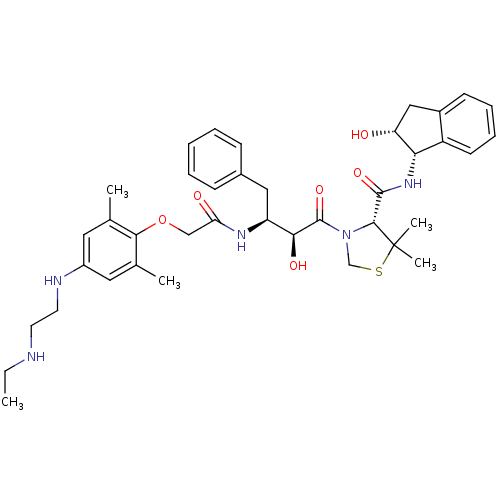 ((R)-3-((2S,3S)-3-(2-(4-(2-(ethylamino)ethylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065563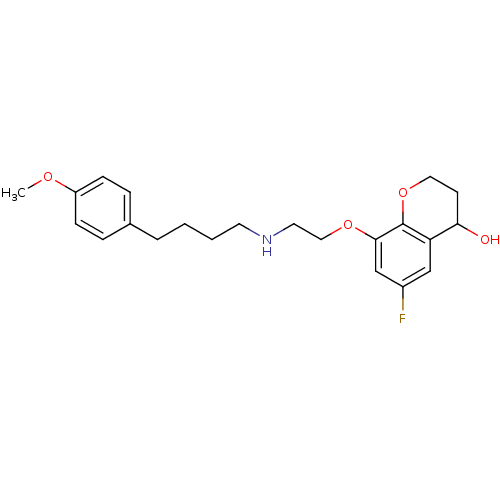 (6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50065563 (6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand. | J Med Chem 41: 2765-78 (1998) Article DOI: 10.1021/jm9707840 BindingDB Entry DOI: 10.7270/Q2K073CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480929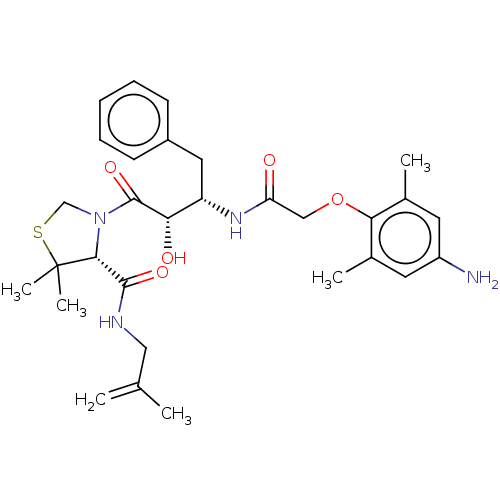 (CHEMBL573975 | KNI-1689) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50109647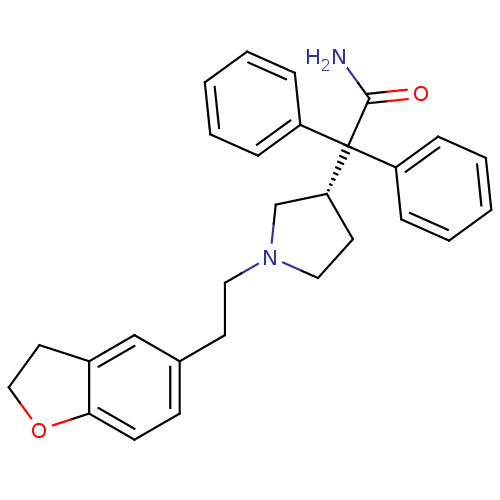 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity (Ki) against binding of [3H]NMS using membranes from CHO cells expressing cloned human Muscarinic acetylcholine receptor M3 | J Med Chem 45: 984-7 (2002) BindingDB Entry DOI: 10.7270/Q2P26ZVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50109647 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50095656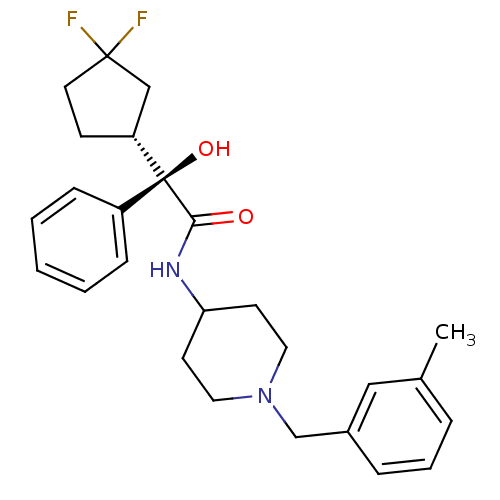 (2-(3,3-Difluoro-cyclopentyl)-2-hydroxy-N-[1-(3-met...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-NMS binding to human muscarinic acetylcholine receptor M1 expressed in CHO cells | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194657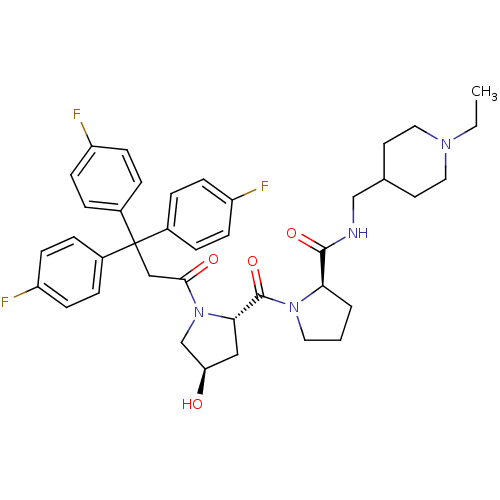 ((2R)-N-(1-ethyl-4-piperidinylmethyl)-1-((2S,4R)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129387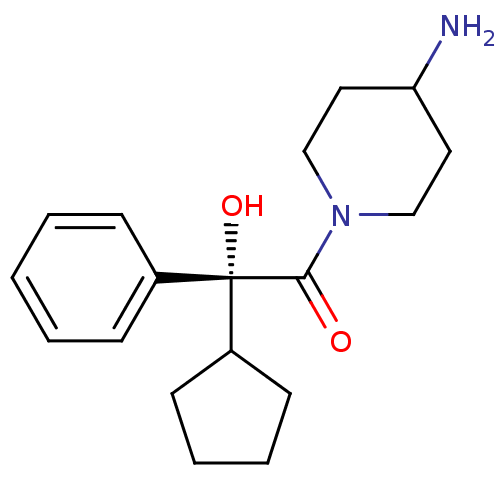 ((R)-1-(4-Amino-piperidin-1-yl)-2-cyclopentyl-2-hyd...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129390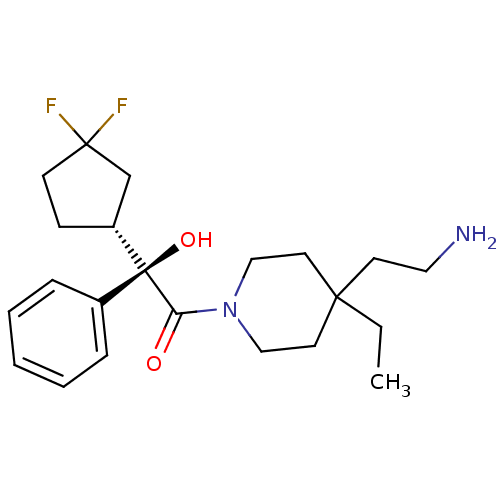 ((R)-1-[4-(2-Amino-ethyl)-4-ethyl-piperidin-1-yl]-2...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50129399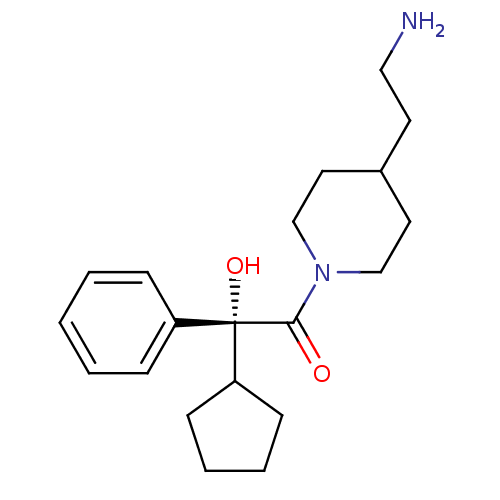 ((R)-1-[4-(2-Amino-ethyl)-piperidin-1-yl]-2-cyclope...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human muscarinic acetylcholine receptor M3 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323468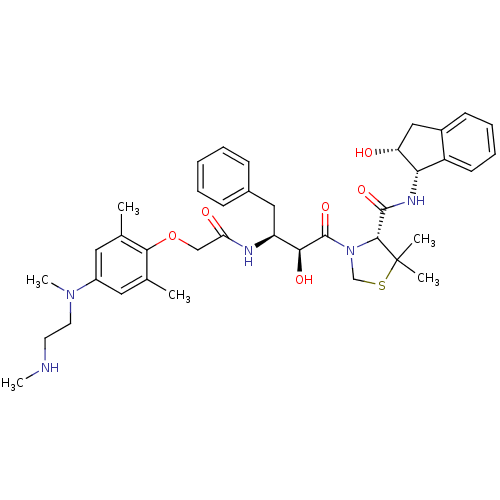 ((R)-3-((2S,3S)-3-(2-(2,6-dimethyl-4-(methyl(2-(met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1346 total ) | Next | Last >> |