 Found 4837 hits with Last Name = 'king' and Initial = 'm'
Found 4837 hits with Last Name = 'king' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by PDSP Ki Database
| |
Behav Brain Res 73: 157-61 (1996)
Article DOI: 10.1016/0166-4328(96)00089-7
BindingDB Entry DOI: 10.7270/Q2N58JX0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1D receptor beta |
J Med Chem 37: 2253-7 (1994)
Article DOI: 10.1021/jm00041a001
BindingDB Entry DOI: 10.7270/Q29889QB |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM112780
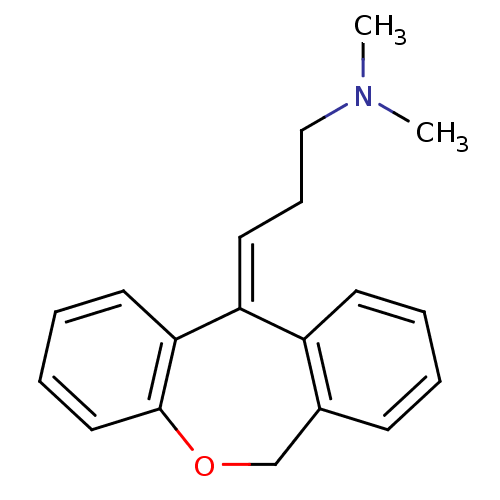
(US8629135, SW-07)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209809
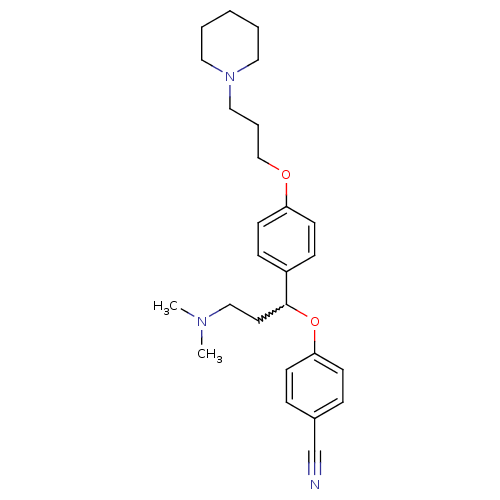
(4-(3-(dimethylamino)-1-(4-(3-(piperidin-1-yl)propo...)Show SMILES CN(C)CCC(Oc1ccc(cc1)C#N)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C26H35N3O2/c1-28(2)19-15-26(31-25-11-7-22(21-27)8-12-25)23-9-13-24(14-10-23)30-20-6-18-29-16-4-3-5-17-29/h7-14,26H,3-6,15-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217586
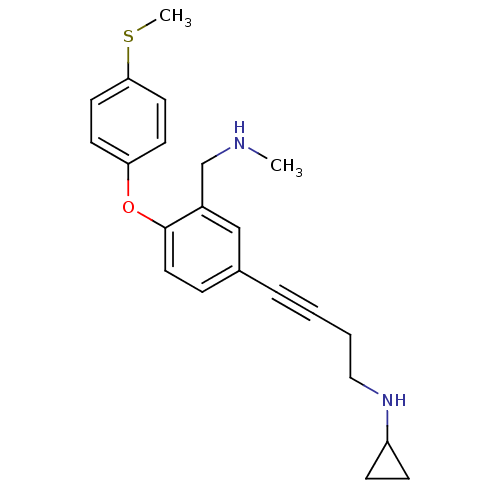
(CHEMBL442080 | N-(4-(3-((methylamino)methyl)-4-(4-...)Show InChI InChI=1S/C22H26N2OS/c1-23-16-18-15-17(5-3-4-14-24-19-7-8-19)6-13-22(18)25-20-9-11-21(26-2)12-10-20/h6,9-13,15,19,23-24H,4,7-8,14,16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217575
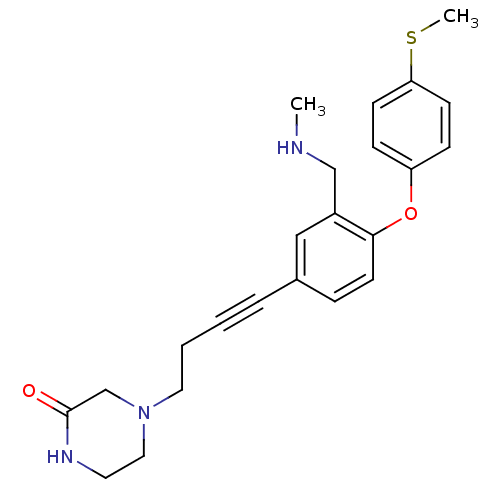
(4-(4-(3-((methylamino)methyl)-4-(4-(methylthio)phe...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCNC(=O)C1 Show InChI InChI=1S/C23H27N3O2S/c1-24-16-19-15-18(5-3-4-13-26-14-12-25-23(27)17-26)6-11-22(19)28-20-7-9-21(29-2)10-8-20/h6-11,15,24H,4,12-14,16-17H2,1-2H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217584

((5-(4-(4-isopropylpiperazin-1-yl)butyl)-2-(4-(meth...)Show SMILES CNCc1cc(CCCCN2CCN(CC2)C(C)C)ccc1Oc1ccc(SC)cc1 Show InChI InChI=1S/C26H39N3OS/c1-21(2)29-17-15-28(16-18-29)14-6-5-7-22-8-13-26(23(19-22)20-27-3)30-24-9-11-25(31-4)12-10-24/h8-13,19,21,27H,5-7,14-18,20H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217593
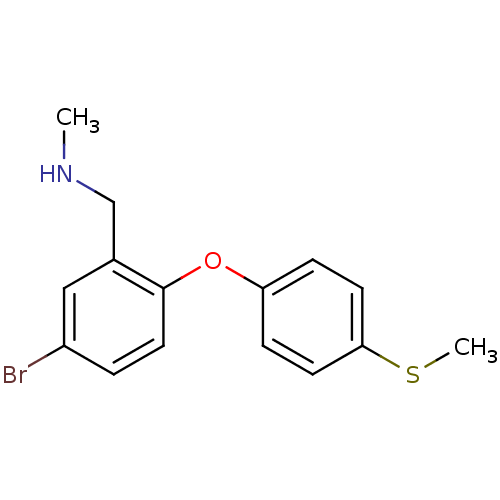
((5-bromo-2-(4-(methylthio)phenoxy)phenyl)-N-methyl...)Show InChI InChI=1S/C15H16BrNOS/c1-17-10-11-9-12(16)3-8-15(11)18-13-4-6-14(19-2)7-5-13/h3-9,17H,10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371305
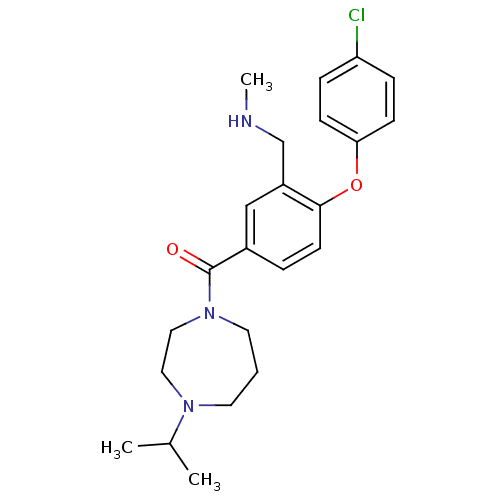
(CHEMBL272077)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-11-4-12-27(14-13-26)23(28)18-5-10-22(19(15-18)16-25-3)29-21-8-6-20(24)7-9-21/h5-10,15,17,25H,4,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217592
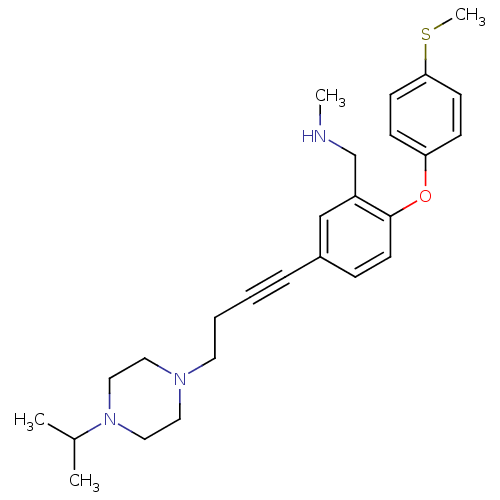
((5-(4-(4-isopropylpiperazin-1-yl)but-1-ynyl)-2-(4-...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCN(CC1)C(C)C Show InChI InChI=1S/C26H35N3OS/c1-21(2)29-17-15-28(16-18-29)14-6-5-7-22-8-13-26(23(19-22)20-27-3)30-24-9-11-25(31-4)12-10-24/h8-13,19,21,27H,6,14-18,20H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217590
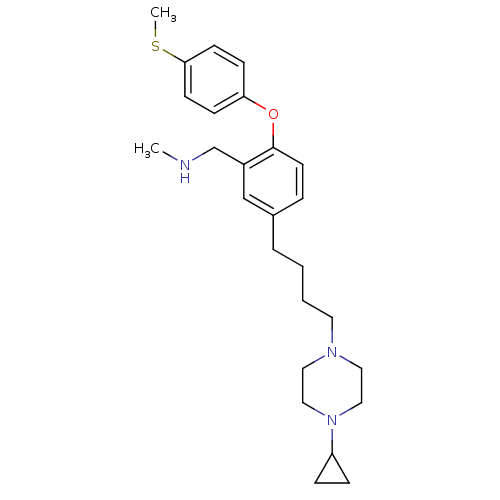
((5-(4-(4-cyclopropylpiperazin-1-yl)butyl)-2-(4-(me...)Show SMILES CNCc1cc(CCCCN2CCN(CC2)C2CC2)ccc1Oc1ccc(SC)cc1 Show InChI InChI=1S/C26H37N3OS/c1-27-20-22-19-21(6-13-26(22)30-24-9-11-25(31-2)12-10-24)5-3-4-14-28-15-17-29(18-16-28)23-7-8-23/h6,9-13,19,23,27H,3-5,7-8,14-18,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209802
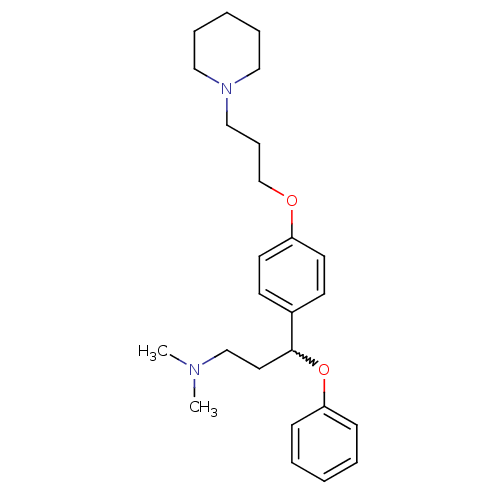
(CHEMBL438490 | N,N-dimethyl-3-phenoxy-3-(4-(3-(pip...)Show SMILES CN(C)CCC(Oc1ccccc1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.5| Show InChI InChI=1S/C25H36N2O2/c1-26(2)20-16-25(29-24-10-5-3-6-11-24)22-12-14-23(15-13-22)28-21-9-19-27-17-7-4-8-18-27/h3,5-6,10-15,25H,4,7-9,16-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217579

((5-(4-(4-fluoropiperidin-1-yl)but-1-ynyl)-2-(4-(me...)Show InChI InChI=1S/C24H29FN2OS/c1-26-18-20-17-19(5-3-4-14-27-15-12-21(25)13-16-27)6-11-24(20)28-22-7-9-23(29-2)10-8-22/h6-11,17,21,26H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217589
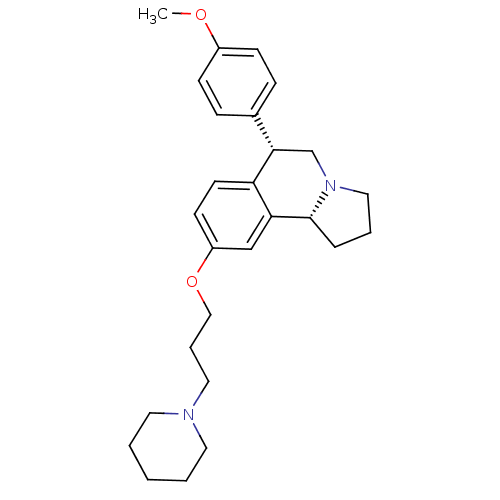
((6S,10bR)-6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)[C@@H]1CN2CCC[C@@H]2c2cc(OCCCN3CCCCC3)ccc12 Show InChI InChI=1S/C27H36N2O2/c1-30-22-10-8-21(9-11-22)26-20-29-17-5-7-27(29)25-19-23(12-13-24(25)26)31-18-6-16-28-14-3-2-4-15-28/h8-13,19,26-27H,2-7,14-18,20H2,1H3/t26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217572
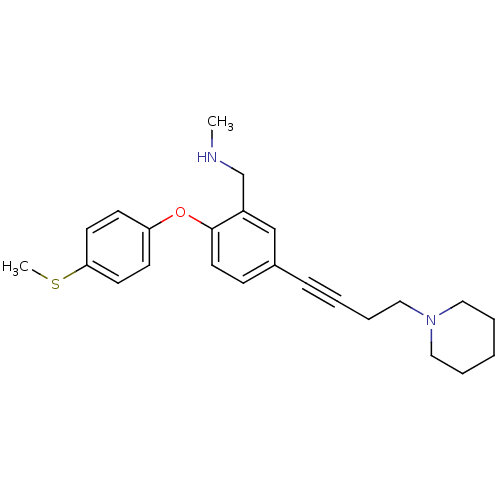
(CHEMBL393036 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C24H30N2OS/c1-25-19-21-18-20(8-4-7-17-26-15-5-3-6-16-26)9-14-24(21)27-22-10-12-23(28-2)13-11-22/h9-14,18,25H,3,5-7,15-17,19H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217565
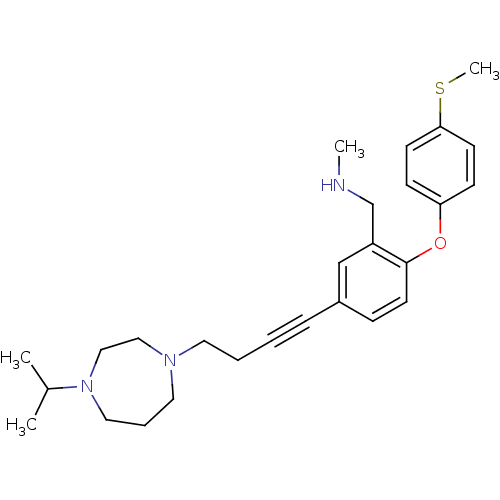
((5-(4-(4-isopropyl-1,4-diazepan-1-yl)but-1-ynyl)-2...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCCN(CC1)C(C)C Show InChI InChI=1S/C27H37N3OS/c1-22(2)30-17-7-16-29(18-19-30)15-6-5-8-23-9-14-27(24(20-23)21-28-3)31-25-10-12-26(32-4)13-11-25/h9-14,20,22,28H,6-7,15-19,21H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217572

(CHEMBL393036 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C24H30N2OS/c1-25-19-21-18-20(8-4-7-17-26-15-5-3-6-16-26)9-14-24(21)27-22-10-12-23(28-2)13-11-22/h9-14,18,25H,3,5-7,15-17,19H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217589

((6S,10bR)-6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)[C@@H]1CN2CCC[C@@H]2c2cc(OCCCN3CCCCC3)ccc12 Show InChI InChI=1S/C27H36N2O2/c1-30-22-10-8-21(9-11-22)26-20-29-17-5-7-27(29)25-19-23(12-13-24(25)26)31-18-6-16-28-14-3-2-4-15-28/h8-13,19,26-27H,2-7,14-18,20H2,1H3/t26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371294
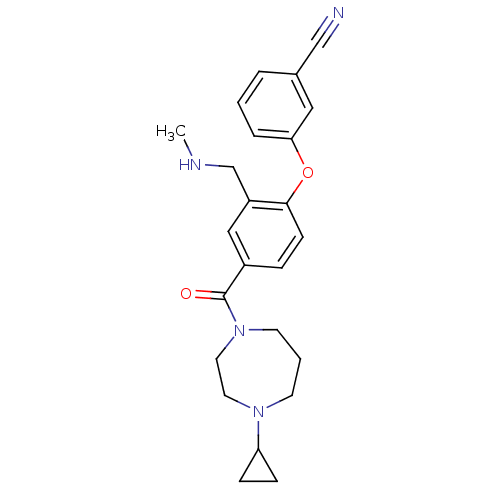
(CHEMBL257208)Show SMILES CNCc1cc(ccc1Oc1cccc(c1)C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-19(6-9-23(20)30-22-5-2-4-18(14-22)16-25)24(29)28-11-3-10-27(12-13-28)21-7-8-21/h2,4-6,9,14-15,21,26H,3,7-8,10-13,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209815
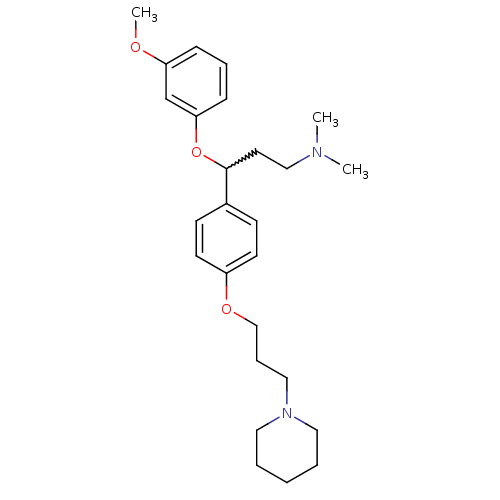
(3-(3-methoxyphenoxy)-N,N-dimethyl-3-(4-(3-(piperid...)Show SMILES COc1cccc(OC(CCN(C)C)c2ccc(OCCCN3CCCCC3)cc2)c1 |w:8.8| Show InChI InChI=1S/C26H38N2O3/c1-27(2)19-15-26(31-25-10-7-9-24(21-25)29-3)22-11-13-23(14-12-22)30-20-8-18-28-16-5-4-6-17-28/h7,9-14,21,26H,4-6,8,15-20H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209806

(CHEMBL245307 | N,N-dimethyl-3-phenyl-3-(3-(3-(pipe...)Show SMILES CN(C)CCC(Oc1cccc(OCCCN2CCCCC2)c1)c1ccccc1 |w:5.4| Show InChI InChI=1S/C25H36N2O2/c1-26(2)19-15-25(22-11-5-3-6-12-22)29-24-14-9-13-23(21-24)28-20-10-18-27-16-7-4-8-17-27/h3,5-6,9,11-14,21,25H,4,7-8,10,15-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209810
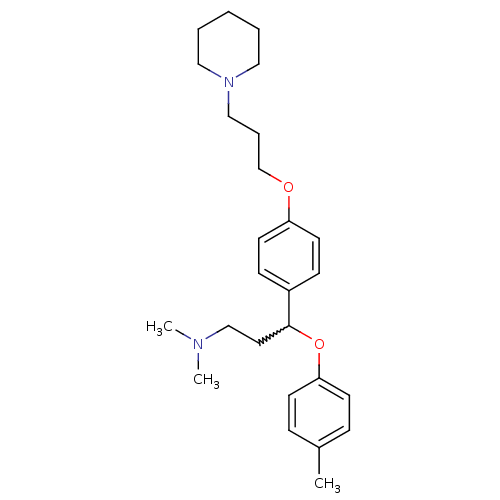
(CHEMBL245516 | N,N-dimethyl-3-(4-(3-(piperidin-1-y...)Show SMILES CN(C)CCC(Oc1ccc(C)cc1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C26H38N2O2/c1-22-8-12-25(13-9-22)30-26(16-20-27(2)3)23-10-14-24(15-11-23)29-21-7-19-28-17-5-4-6-18-28/h8-15,26H,4-7,16-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217576

(CHEMBL236046 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2O2S/c1-24-18-20-17-19(5-3-4-12-25-13-15-26-16-14-25)6-11-23(20)27-21-7-9-22(28-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371290
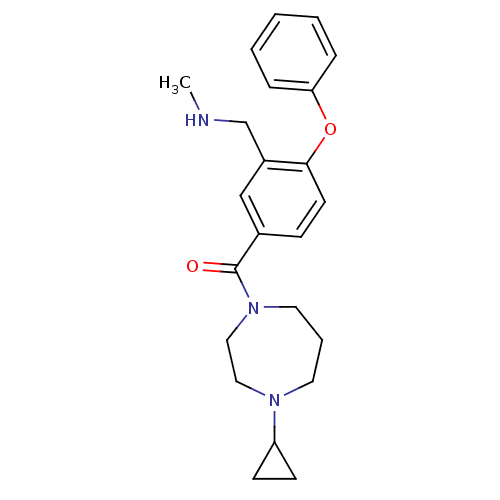
(CHEMBL401683)Show SMILES CNCc1cc(ccc1Oc1ccccc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H29N3O2/c1-24-17-19-16-18(8-11-22(19)28-21-6-3-2-4-7-21)23(27)26-13-5-12-25(14-15-26)20-9-10-20/h2-4,6-8,11,16,20,24H,5,9-10,12-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371289
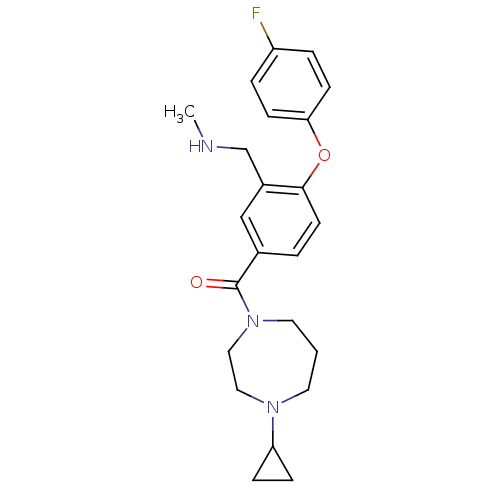
(CHEMBL258349)Show SMILES CNCc1cc(ccc1Oc1ccc(F)cc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-15-17(3-10-22(18)29-21-8-4-19(24)5-9-21)23(28)27-12-2-11-26(13-14-27)20-6-7-20/h3-5,8-10,15,20,25H,2,6-7,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217576

(CHEMBL236046 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2O2S/c1-24-18-20-17-19(5-3-4-12-25-13-15-26-16-14-25)6-11-23(20)27-21-7-9-22(28-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human SERT |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209816
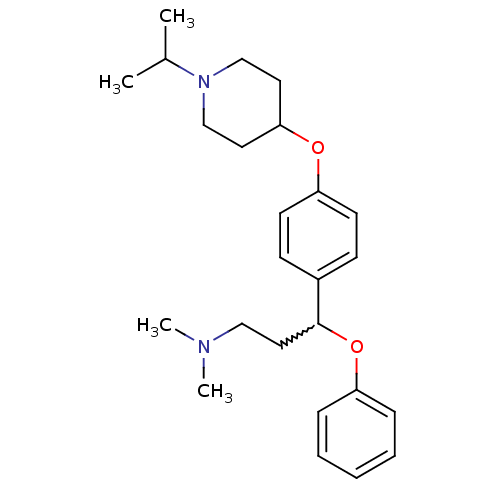
(3-(4-(1-isopropylpiperidin-4-yloxy)phenyl)-N,N-dim...)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(cc1)C(CCN(C)C)Oc1ccccc1 |w:16.18| Show InChI InChI=1S/C25H36N2O2/c1-20(2)27-18-14-24(15-19-27)28-23-12-10-21(11-13-23)25(16-17-26(3)4)29-22-8-6-5-7-9-22/h5-13,20,24-25H,14-19H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209812

((4-(3-(dimethylamino)-1-phenoxypropyl)phenyl)(4-is...)Show SMILES CC(C)N1CCN(CC1)C(=O)c1ccc(cc1)C(CCN(C)C)Oc1ccccc1 |w:17.19| Show InChI InChI=1S/C25H35N3O2/c1-20(2)27-16-18-28(19-17-27)25(29)22-12-10-21(11-13-22)24(14-15-26(3)4)30-23-8-6-5-7-9-23/h5-13,20,24H,14-19H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217577
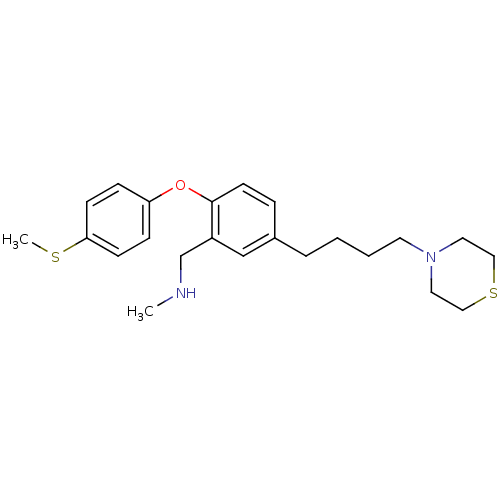
(CHEMBL236010 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H32N2OS2/c1-24-18-20-17-19(5-3-4-12-25-13-15-28-16-14-25)6-11-23(20)26-21-7-9-22(27-2)10-8-21/h6-11,17,24H,3-5,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217576

(CHEMBL236046 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2O2S/c1-24-18-20-17-19(5-3-4-12-25-13-15-26-16-14-25)6-11-23(20)27-21-7-9-22(28-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371287
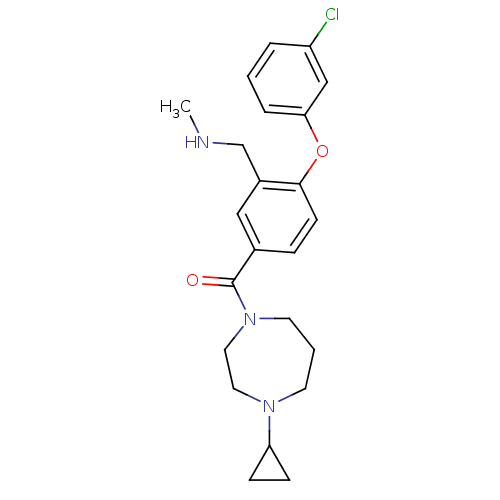
(CHEMBL272699)Show SMILES CNCc1cc(ccc1Oc1cccc(Cl)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28ClN3O2/c1-25-16-18-14-17(6-9-22(18)29-21-5-2-4-19(24)15-21)23(28)27-11-3-10-26(12-13-27)20-7-8-20/h2,4-6,9,14-15,20,25H,3,7-8,10-13,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209799
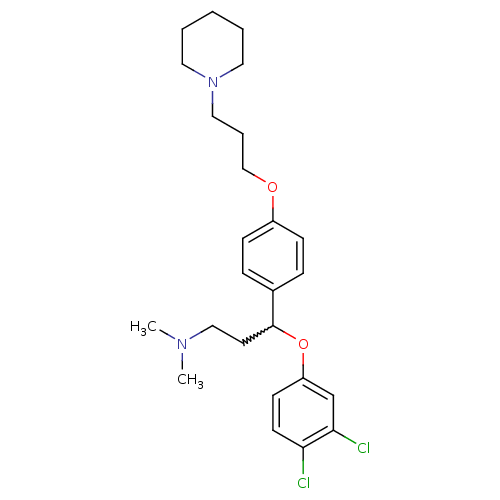
(3-(3,4-dichlorophenoxy)-N,N-dimethyl-3-(4-(3-(pipe...)Show SMILES CN(C)CCC(Oc1ccc(Cl)c(Cl)c1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C25H34Cl2N2O2/c1-28(2)17-13-25(31-22-11-12-23(26)24(27)19-22)20-7-9-21(10-8-20)30-18-6-16-29-14-4-3-5-15-29/h7-12,19,25H,3-6,13-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217595

(CHEMBL237317 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2OS2/c1-24-18-20-17-19(5-3-4-12-25-13-15-28-16-14-25)6-11-23(20)26-21-7-9-22(27-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209813
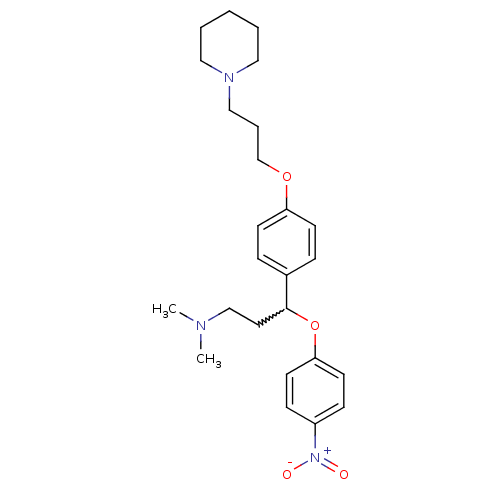
(CHEMBL246125 | N,N-dimethyl-3-(4-nitrophenoxy)-3-(...)Show SMILES CN(C)CCC(Oc1ccc(cc1)[N+]([O-])=O)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C25H35N3O4/c1-26(2)19-15-25(32-24-13-9-22(10-14-24)28(29)30)21-7-11-23(12-8-21)31-20-6-18-27-16-4-3-5-17-27/h7-14,25H,3-6,15-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217579

((5-(4-(4-fluoropiperidin-1-yl)but-1-ynyl)-2-(4-(me...)Show InChI InChI=1S/C24H29FN2OS/c1-26-18-20-17-19(5-3-4-14-27-15-12-21(25)13-16-27)6-11-24(20)28-22-7-9-23(29-2)10-8-22/h6-11,17,21,26H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209805

(3-(3-chlorophenoxy)-N,N-dimethyl-3-(4-(3-(piperidi...)Show SMILES CN(C)CCC(Oc1cccc(Cl)c1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C25H35ClN2O2/c1-27(2)18-14-25(30-24-9-6-8-22(26)20-24)21-10-12-23(13-11-21)29-19-7-17-28-15-4-3-5-16-28/h6,8-13,20,25H,3-5,7,14-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209800
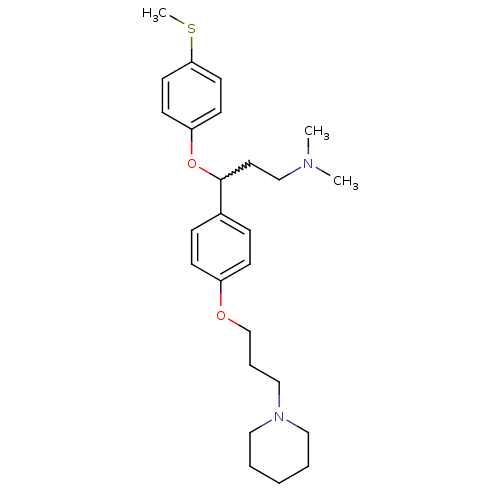
(CHEMBL394321 | N,N-dimethyl-3-(4-(methylthio)pheno...)Show SMILES CSc1ccc(OC(CCN(C)C)c2ccc(OCCCN3CCCCC3)cc2)cc1 |w:7.7| Show InChI InChI=1S/C26H38N2O2S/c1-27(2)20-16-26(30-24-12-14-25(31-3)15-13-24)22-8-10-23(11-9-22)29-21-7-19-28-17-5-4-6-18-28/h8-15,26H,4-7,16-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209799

(3-(3,4-dichlorophenoxy)-N,N-dimethyl-3-(4-(3-(pipe...)Show SMILES CN(C)CCC(Oc1ccc(Cl)c(Cl)c1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C25H34Cl2N2O2/c1-28(2)17-13-25(31-22-11-12-23(26)24(27)19-22)20-7-9-21(10-8-20)30-18-6-16-29-14-4-3-5-15-29/h7-12,19,25H,3-6,13-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209799

(3-(3,4-dichlorophenoxy)-N,N-dimethyl-3-(4-(3-(pipe...)Show SMILES CN(C)CCC(Oc1ccc(Cl)c(Cl)c1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C25H34Cl2N2O2/c1-28(2)17-13-25(31-22-11-12-23(26)24(27)19-22)20-7-9-21(10-8-20)30-18-6-16-29-14-4-3-5-15-29/h7-12,19,25H,3-6,13-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371291

(CHEMBL257009)Show SMILES CNCc1cc(ccc1Oc1ccccc1C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-18(7-10-23(20)30-22-6-3-2-5-19(22)16-25)24(29)28-12-4-11-27(13-14-28)21-8-9-21/h2-3,5-7,10,15,21,26H,4,8-9,11-14,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209803
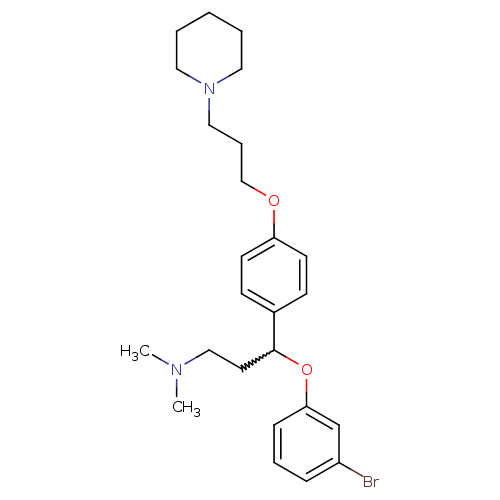
(3-(3-bromophenoxy)-N,N-dimethyl-3-(4-(3-(piperidin...)Show SMILES CN(C)CCC(Oc1cccc(Br)c1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C25H35BrN2O2/c1-27(2)18-14-25(30-24-9-6-8-22(26)20-24)21-10-12-23(13-11-21)29-19-7-17-28-15-4-3-5-16-28/h6,8-13,20,25H,3-5,7,14-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50371281
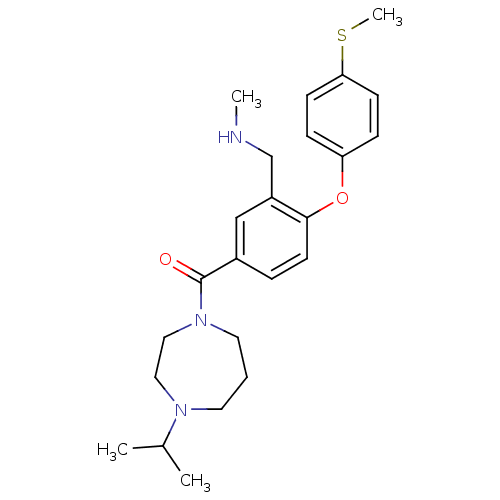
(CHEMBL267267)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C24H33N3O2S/c1-18(2)26-12-5-13-27(15-14-26)24(28)19-6-11-23(20(16-19)17-25-3)29-21-7-9-22(30-4)10-8-21/h6-11,16,18,25H,5,12-15,17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of rat SERT |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371286
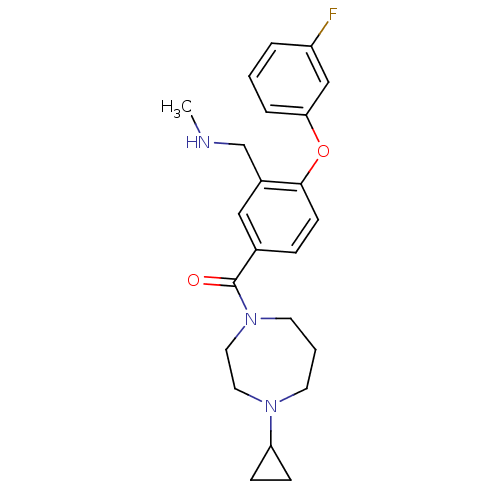
(CHEMBL401867)Show SMILES CNCc1cc(ccc1Oc1cccc(F)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-14-17(6-9-22(18)29-21-5-2-4-19(24)15-21)23(28)27-11-3-10-26(12-13-27)20-7-8-20/h2,4-6,9,14-15,20,25H,3,7-8,10-13,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217568
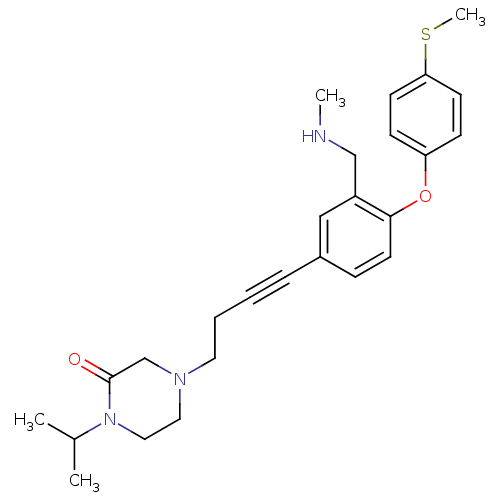
(1-isopropyl-4-(4-(3-((methylamino)methyl)-4-(4-(me...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCN(C(C)C)C(=O)C1 Show InChI InChI=1S/C26H33N3O2S/c1-20(2)29-16-15-28(19-26(29)30)14-6-5-7-21-8-13-25(22(17-21)18-27-3)31-23-9-11-24(32-4)12-10-23/h8-13,17,20,27H,6,14-16,18-19H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371280
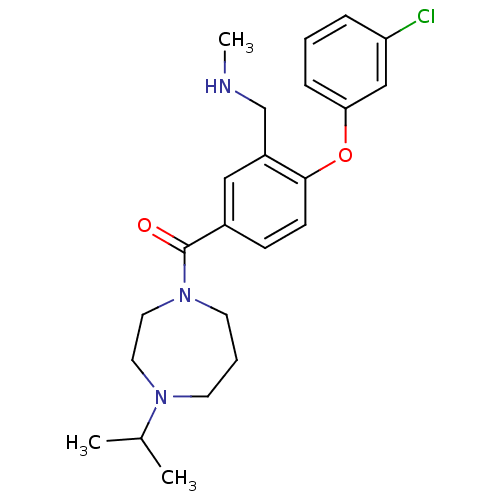
(CHEMBL272289)Show SMILES CNCc1cc(ccc1Oc1cccc(Cl)c1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-10-5-11-27(13-12-26)23(28)18-8-9-22(19(14-18)16-25-3)29-21-7-4-6-20(24)15-21/h4,6-9,14-15,17,25H,5,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50209802

(CHEMBL438490 | N,N-dimethyl-3-phenoxy-3-(4-(3-(pip...)Show SMILES CN(C)CCC(Oc1ccccc1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.5| Show InChI InChI=1S/C25H36N2O2/c1-26(2)20-16-25(29-24-10-5-3-6-11-24)22-12-14-23(15-13-22)28-21-9-19-27-17-7-4-8-18-27/h3,5-6,10-15,25H,4,7-9,16-21H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at rat SERT |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371315

(CHEMBL273136)Show SMILES CNCc1cc(ccc1Oc1cccc(OC)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H31N3O3/c1-25-17-19-15-18(7-10-23(19)30-22-6-3-5-21(16-22)29-2)24(28)27-12-4-11-26(13-14-27)20-8-9-20/h3,5-7,10,15-16,20,25H,4,8-9,11-14,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371314

(CHEMBL402949)Show SMILES CNCc1cc(ccc1Oc1ccccc1F)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-15-17(7-10-21(18)29-22-6-3-2-5-20(22)24)23(28)27-12-4-11-26(13-14-27)19-8-9-19/h2-3,5-7,10,15,19,25H,4,8-9,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data