

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930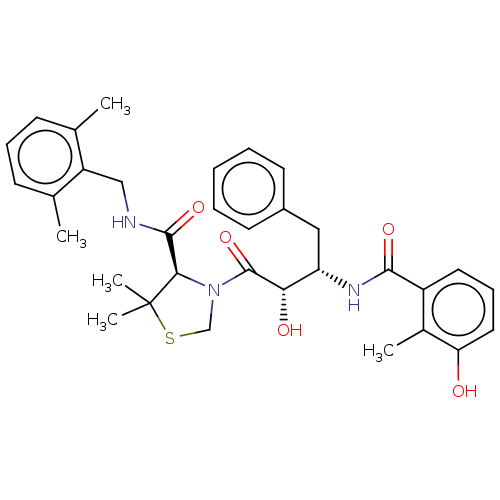 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM719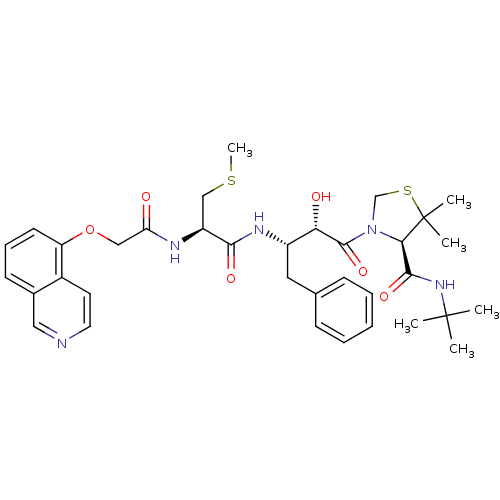 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0880 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323469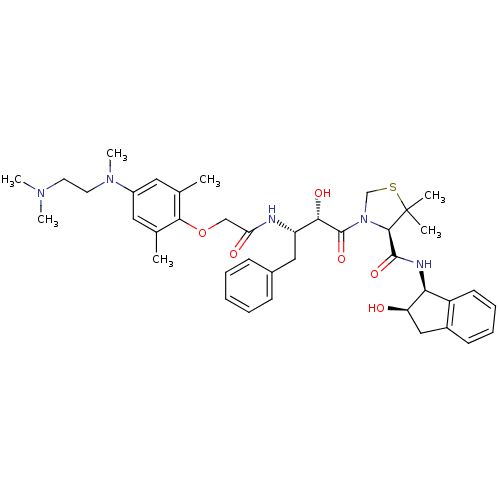 ((R)-3-((2S,3S)-3-(2-(4-((2-(dimethylamino)ethyl)(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323472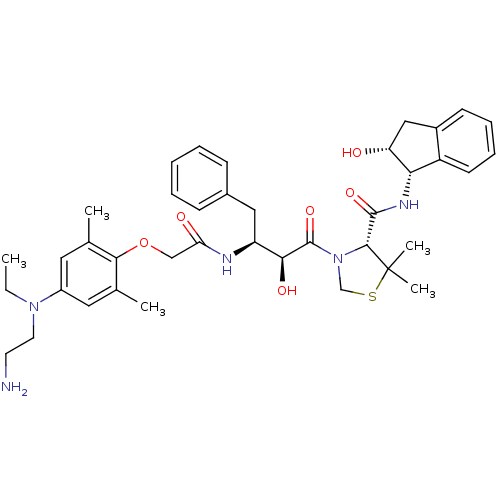 ((R)-3-((2S,3S)-3-(2-(4-((2-aminoethyl)(ethyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM718 ((4R)-3-[(2S,3S)-3-[(2-ethyl-3-hydroxyphenyl)formam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -58.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121729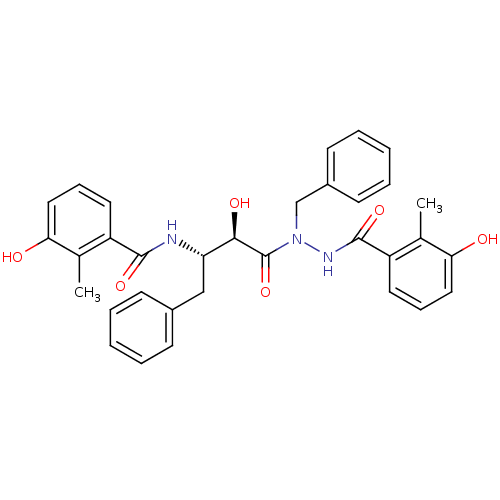 (CHEMBL368169 | KNI-1167 | N-[(S)-3-[N-Benzyl-N'-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480931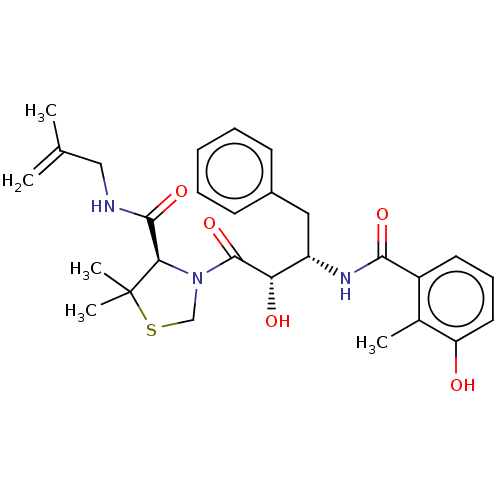 (CHEMBL575512 | KNI-1614) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209553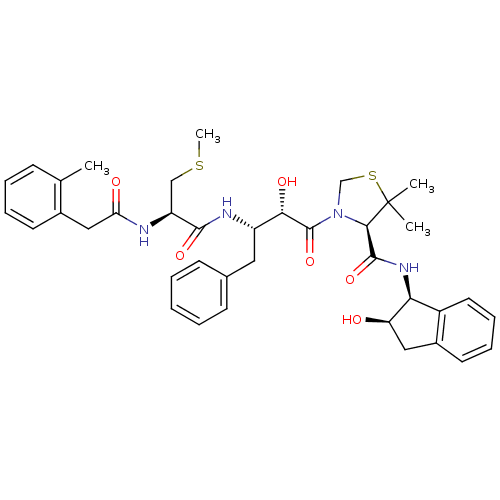 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121730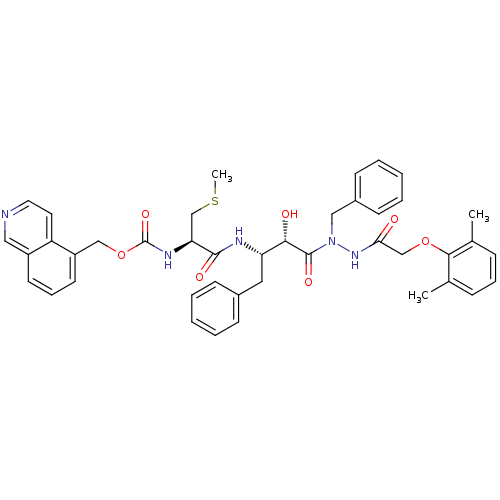 (CHEMBL366433 | {1-[(S)-3-{N-Benzyl-N'-[2-(2,6-dime...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM717 ((4R)-N-[(2-chlorophenyl)methyl]-3-[(2S,3S)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.330 | -56.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121732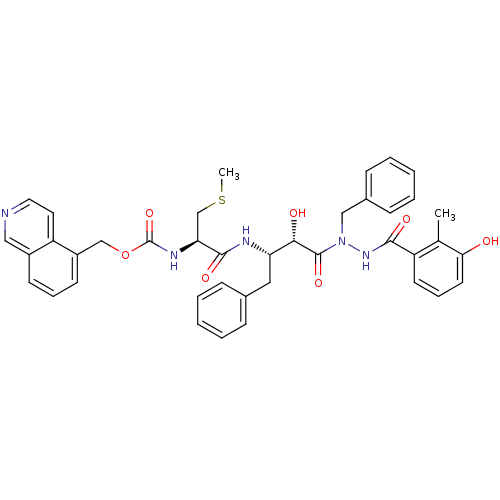 (CHEMBL172850 | {1-[(S)-3-[N-Benzyl-N'-(3-hydroxy-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559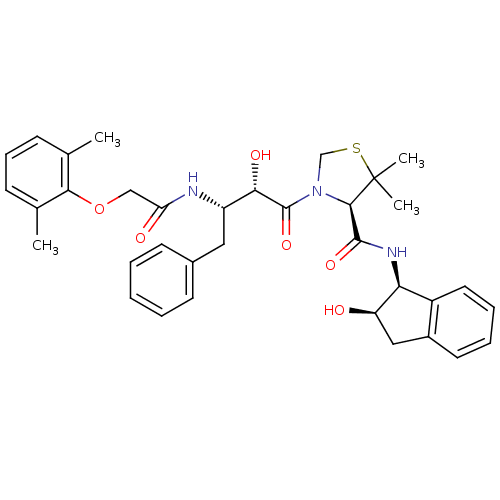 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem 16: 10049-60 (2008) Article DOI: 10.1016/j.bmc.2008.10.011 BindingDB Entry DOI: 10.7270/Q2G160PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323470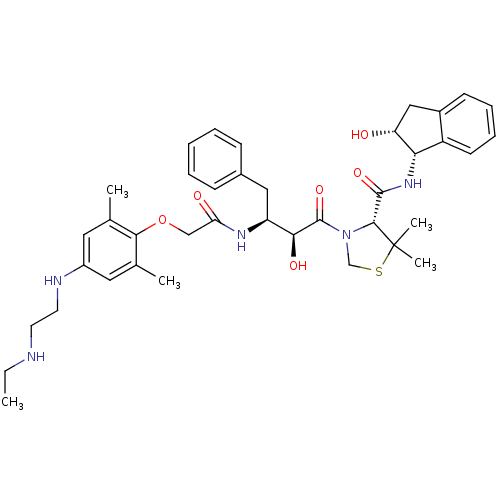 ((R)-3-((2S,3S)-3-(2-(4-(2-(ethylamino)ethylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM579 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.740 | -54.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480929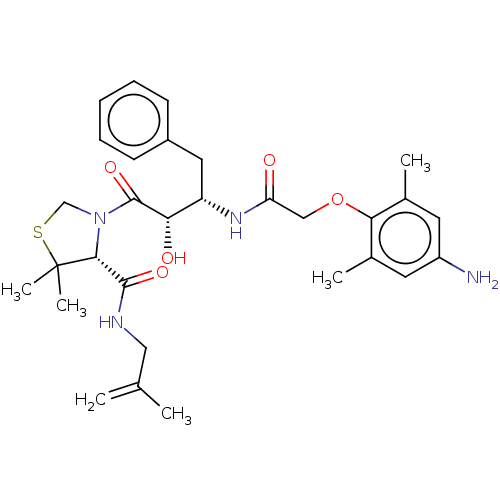 (CHEMBL573975 | KNI-1689) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001073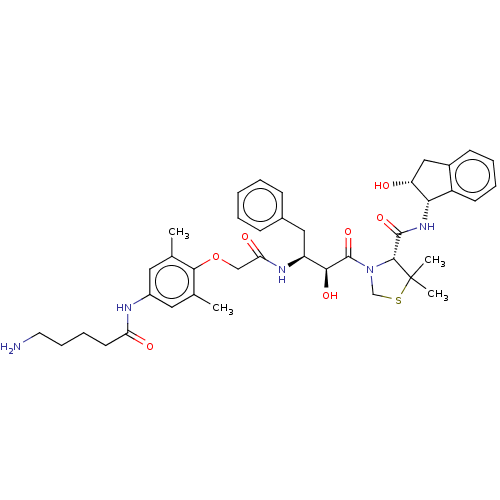 (CHEMBL3236067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001072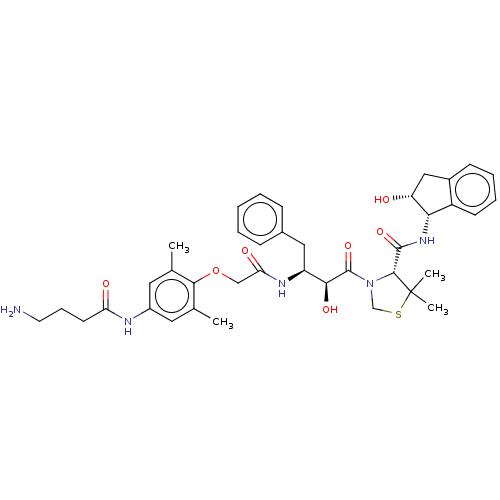 (CHEMBL3236066) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001070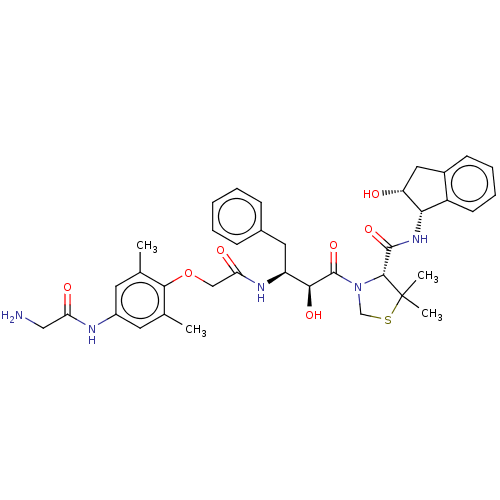 (CHEMBL3236064) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323468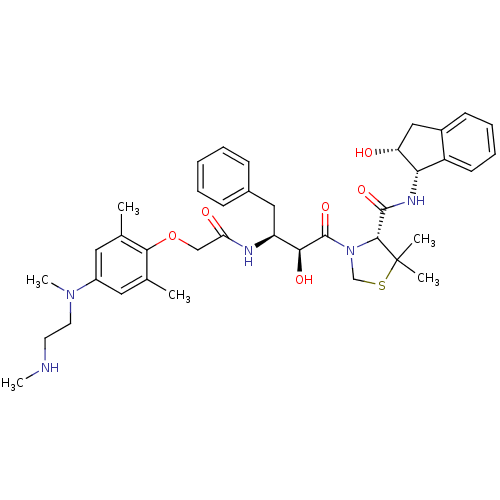 ((R)-3-((2S,3S)-3-(2-(2,6-dimethyl-4-(methyl(2-(met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM712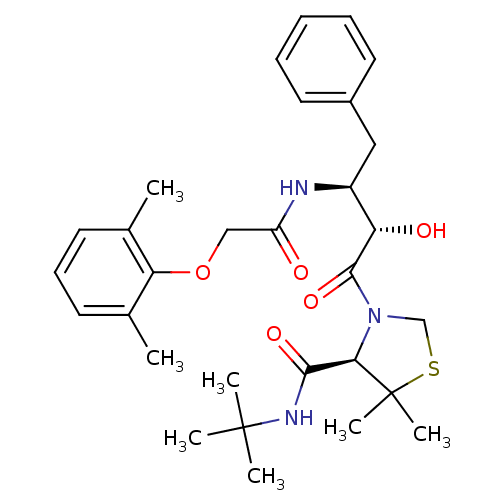 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[2-(2,6-dimethylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001069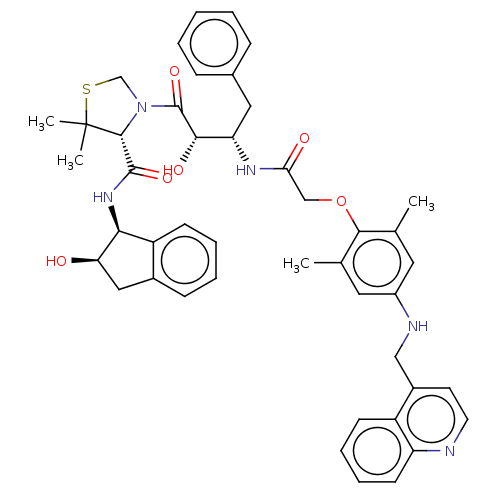 (CHEMBL3236063) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM715 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[(2-ethyl-3-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.24 | -51.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001071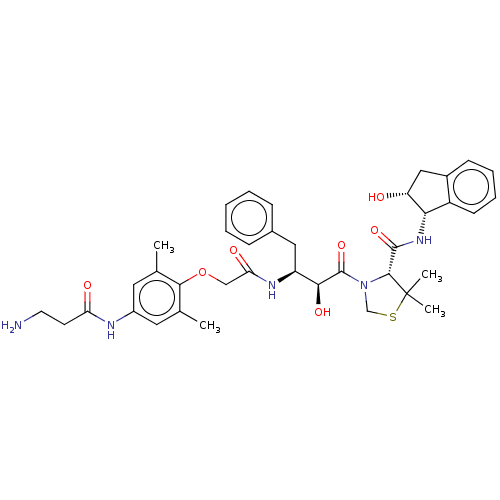 (CHEMBL3236065) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209552 ((4R)-N-[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121735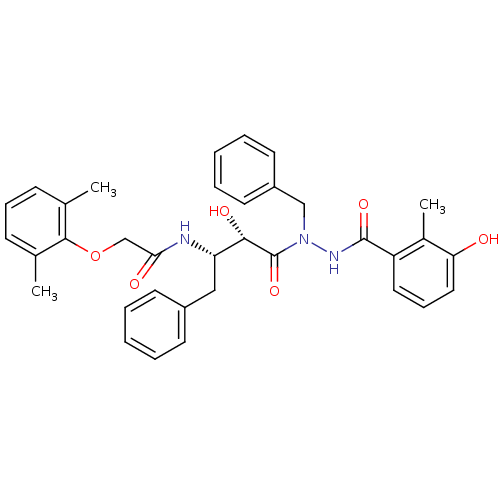 (CHEMBL367679 | KNI-1277 | N-[(S)-3-[N-Benzyl-N'-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209554 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50273723 ((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem 16: 10049-60 (2008) Article DOI: 10.1016/j.bmc.2008.10.011 BindingDB Entry DOI: 10.7270/Q2G160PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209563 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209565 ((R)-3-((2S,3S)-3-((R)-2-(2-(2-aminophenyl)acetamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323473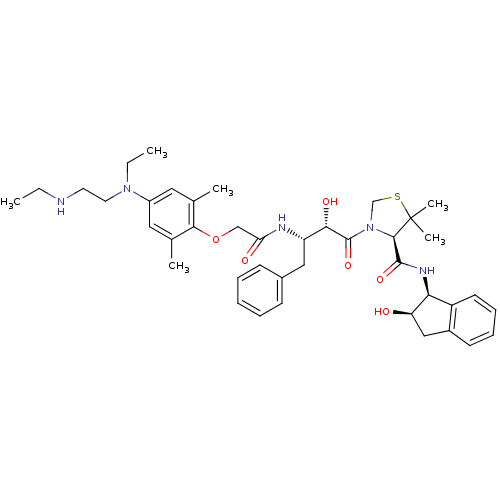 ((R)-3-((2S,3S)-3-(2-(4-(ethyl(2-(ethylamino)ethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001075 (CHEMBL3236069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001067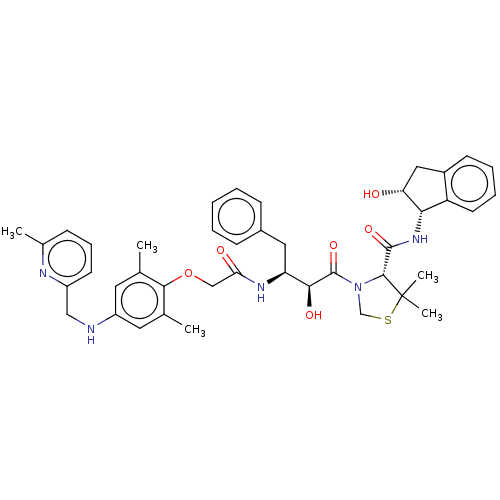 (CHEMBL3236061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001068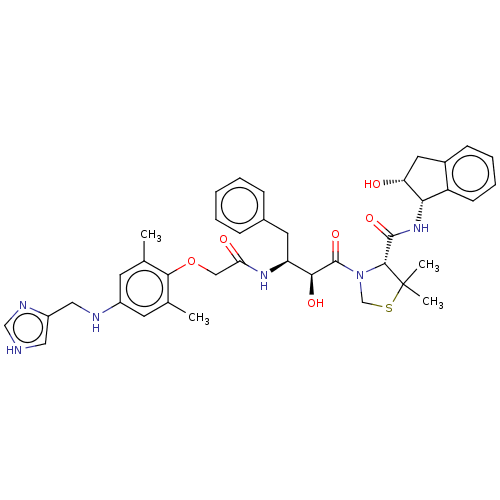 (CHEMBL3236062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50273742 ((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem 16: 10049-60 (2008) Article DOI: 10.1016/j.bmc.2008.10.011 BindingDB Entry DOI: 10.7270/Q2G160PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209555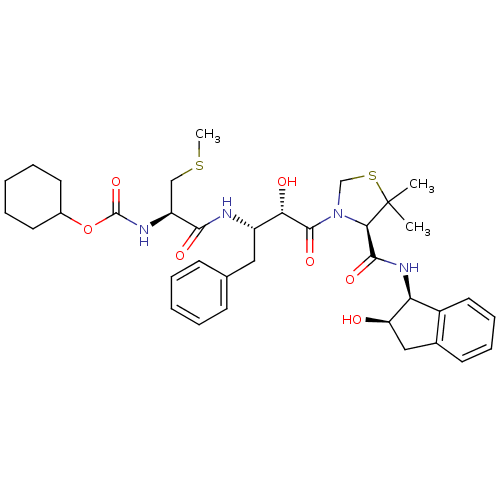 (CHEMBL394358 | cyclohexyl (R)-1-((2S,3S)-4-((R)-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121733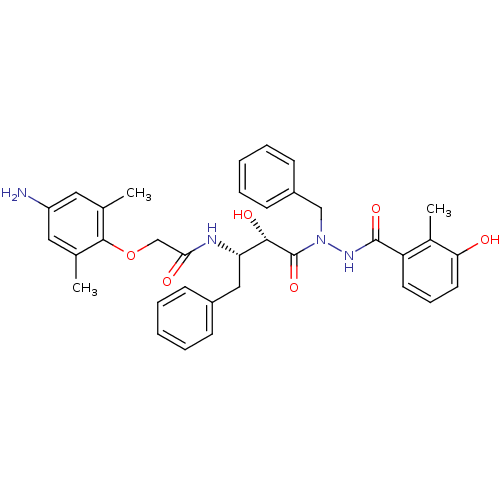 (2-(4-Amino-2,6-dimethyl-phenoxy)-N-[(S)-3-[N-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209562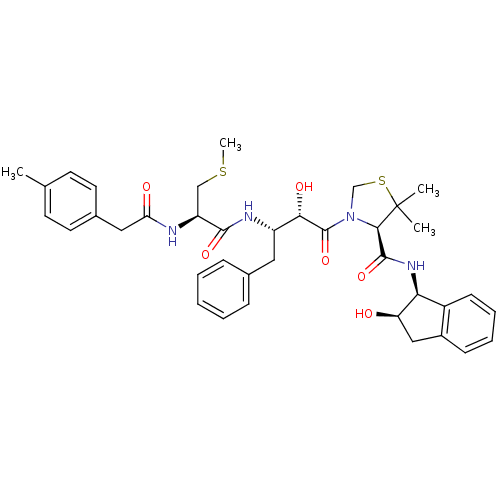 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323471 ((R)-3-((2S,3S)-3-(2-(4-(2-(diethylamino)ethylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM714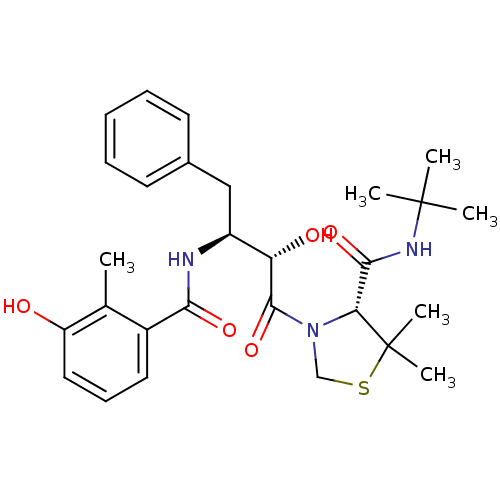 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(3-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.14 | -49.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001064 (CHEMBL3236058) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001074 (CHEMBL3236068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121728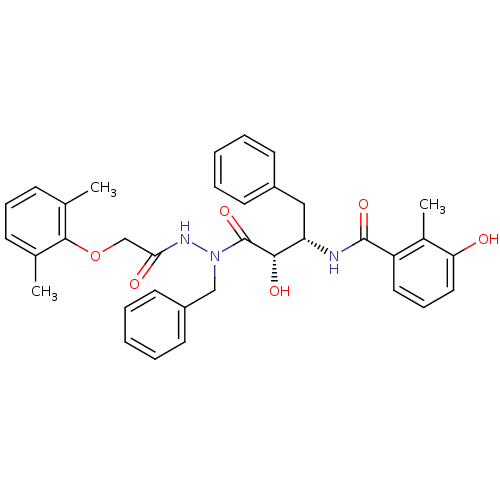 (CHEMBL172012 | KNI-1279 | N-[(S)-3-{N-Benzyl-N'-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50273751 ((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem 16: 10049-60 (2008) Article DOI: 10.1016/j.bmc.2008.10.011 BindingDB Entry DOI: 10.7270/Q2G160PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323464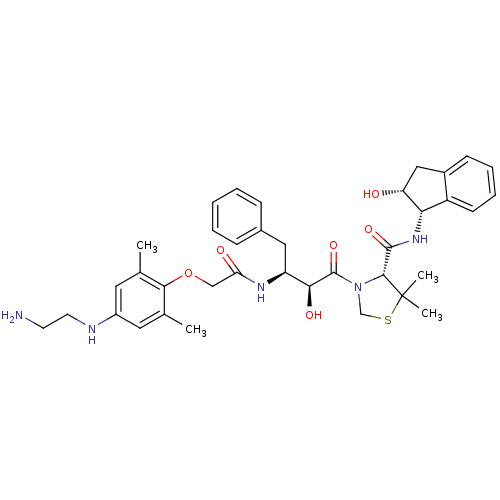 ((R)-3-((2S,3S)-3-(2-(4-(2-aminoethylamino)-2,6-dim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50273746 ((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem 16: 10049-60 (2008) Article DOI: 10.1016/j.bmc.2008.10.011 BindingDB Entry DOI: 10.7270/Q2G160PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323467 ((R)-3-((2S,3S)-3-(2-(4-((2-aminoethyl)(methyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1279 total ) | Next | Last >> |