

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein phosphatase 1D (Homo sapiens (Human)) | BDBM50118477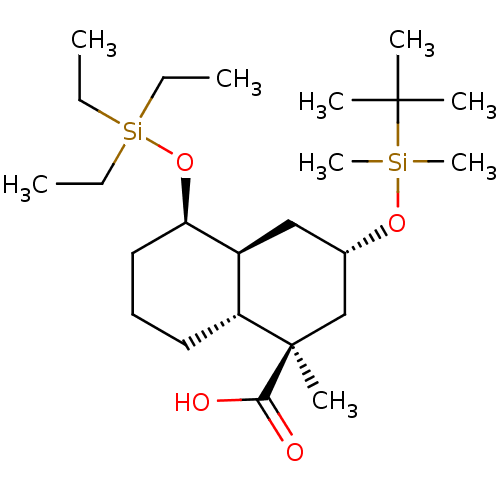 (CHEMBL3613748) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Non-competitive inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEP... | Bioorg Med Chem 23: 6246-9 (2015) Article DOI: 10.1016/j.bmc.2015.08.042 BindingDB Entry DOI: 10.7270/Q26975C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030575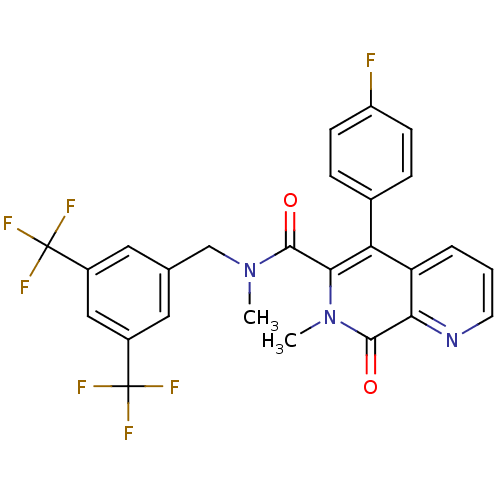 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030573 (7-Methyl-8-oxo-5-phenyl-7,8-dihydro-[1,7]naphthyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030576 (4-(4-Fluoro-phenyl)-2-methyl-1-oxo-1,2-dihydro-iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426652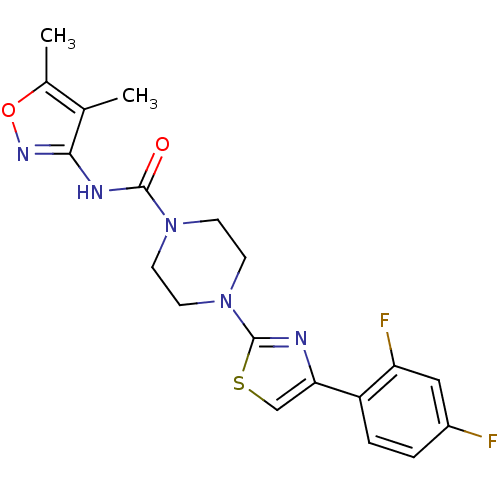 (CHEMBL2326178) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426652 (CHEMBL2326178) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426651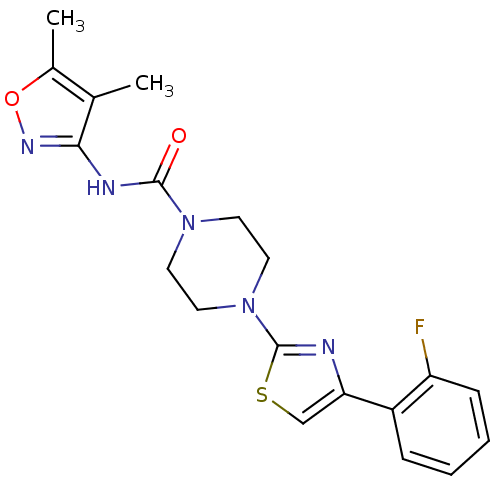 (CHEMBL2326194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426650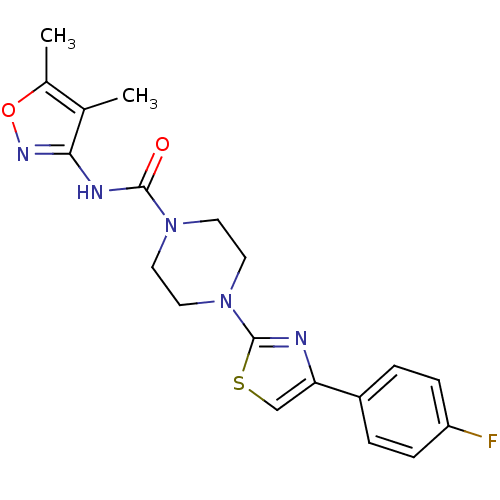 (CHEMBL2326177) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426651 (CHEMBL2326194) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426648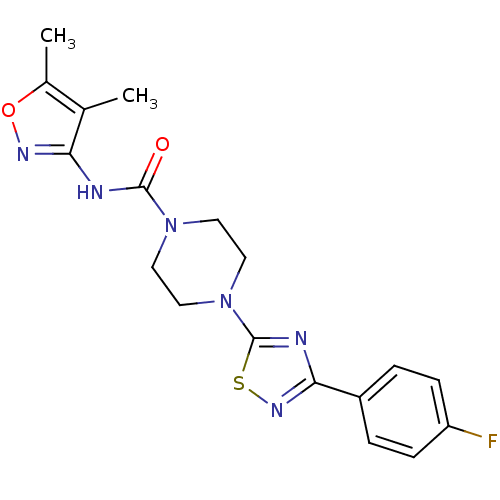 (CHEMBL2326197) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426650 (CHEMBL2326177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426649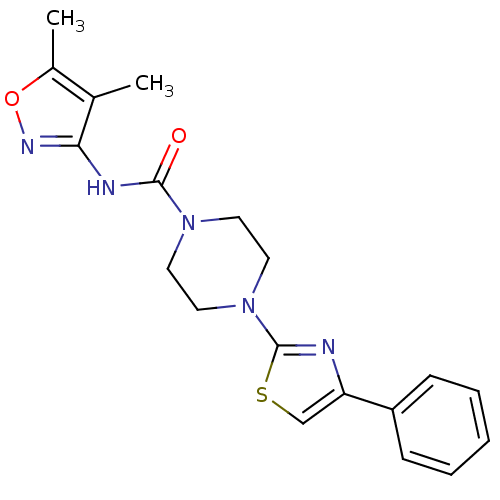 (CHEMBL2326192) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426649 (CHEMBL2326192) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030584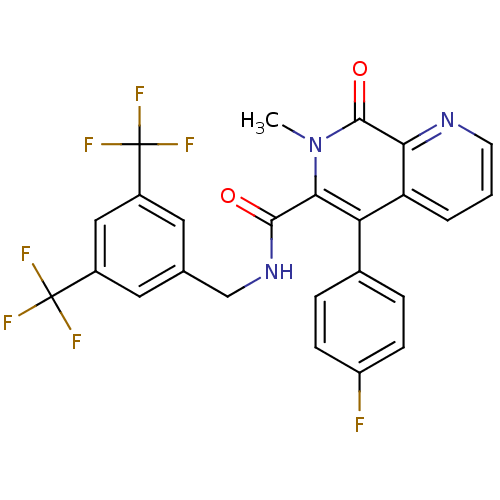 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426647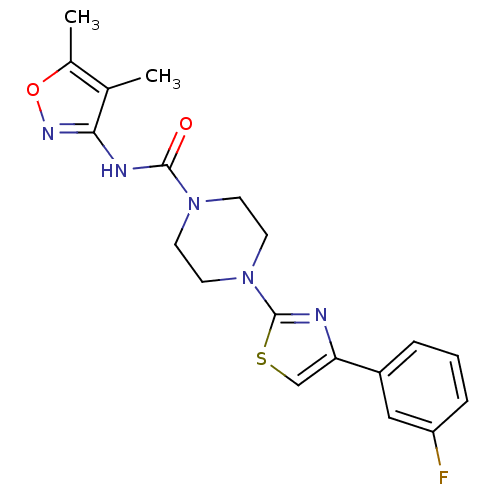 (CHEMBL2326196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030581 (2-Methyl-1-oxo-4-phenyl-1,2-dihydro-isoquinoline-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426645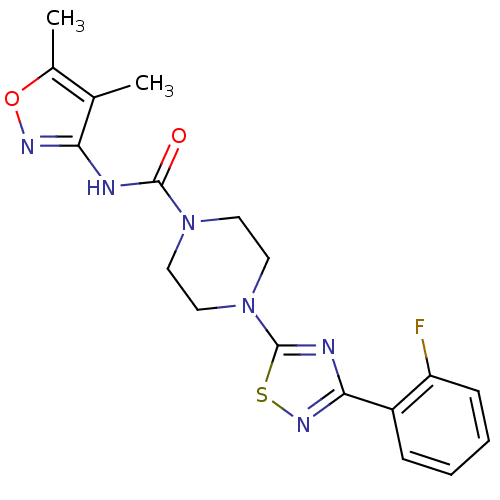 (CHEMBL2326193) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030572 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426648 (CHEMBL2326197) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426646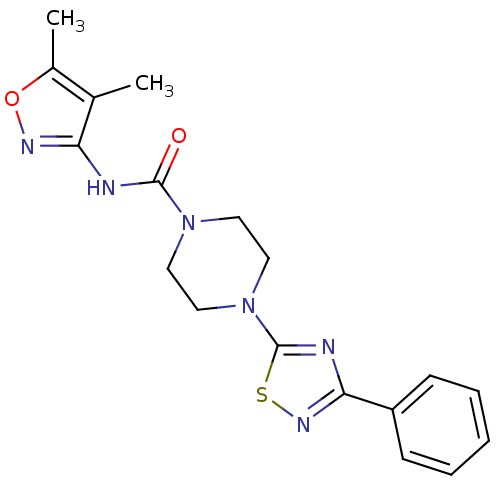 (CHEMBL2326189) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426647 (CHEMBL2326196) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426646 (CHEMBL2326189) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426644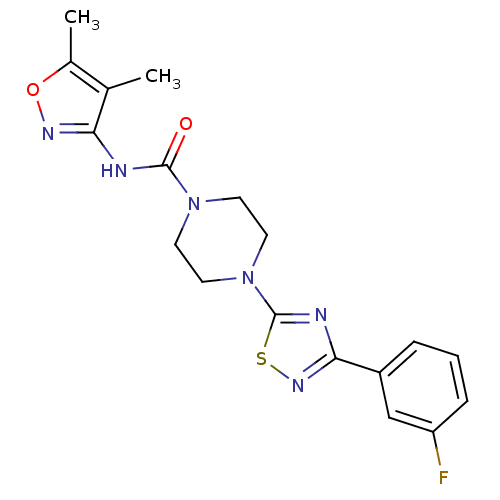 (CHEMBL2326195) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426645 (CHEMBL2326193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50244993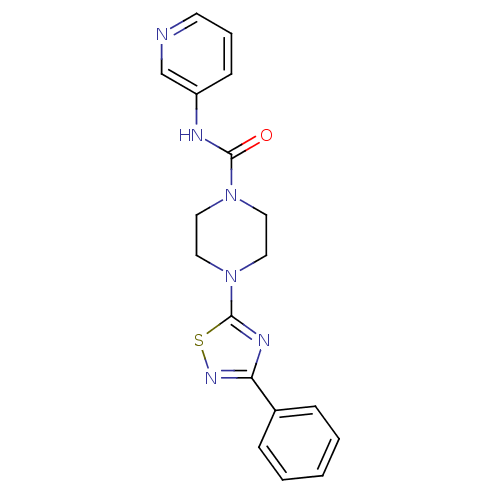 (4-(3-phenyl-1,2,4-thiadiazol-5-yl)-N-(pyridin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030607 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030611 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426643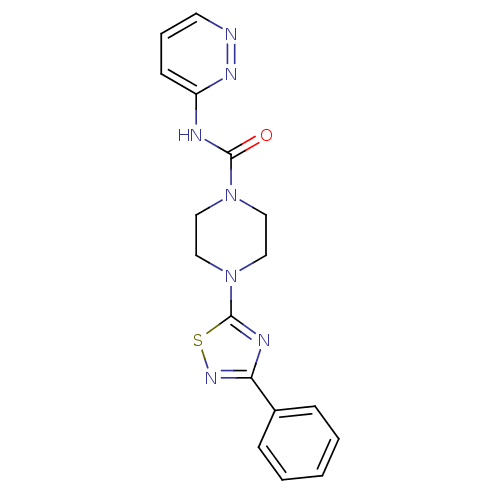 (CHEMBL2326190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426644 (CHEMBL2326195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426643 (CHEMBL2326190) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030574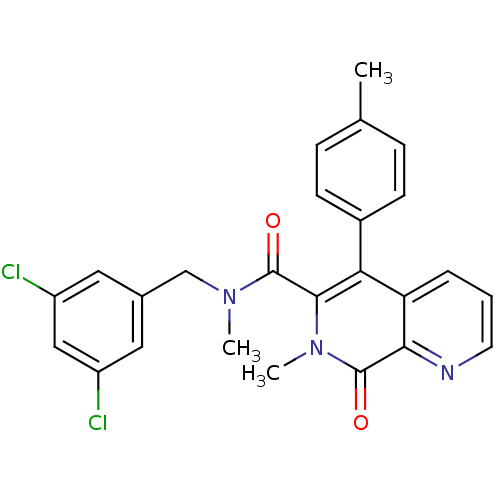 (7-Methyl-8-oxo-5-p-tolyl-7,8-dihydro-[1,7]naphthyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030591 (6-Chloro-2-methyl-1-oxo-4-phenyl-1,2-dihydro-isoqu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426641 (CHEMBL2326181) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50244993 (4-(3-phenyl-1,2,4-thiadiazol-5-yl)-N-(pyridin-3-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of FAAH (unknown origin) | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030569 (1-(2-Chloro-benzyl)-1-methyl-3-(2,6,7-trimethyl-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030582 (7-Methyl-8-oxo-5-p-tolyl-7,8-dihydro-[1,7]naphthyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426642 (CHEMBL459152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030585 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426642 (CHEMBL459152) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426641 (CHEMBL2326181) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030594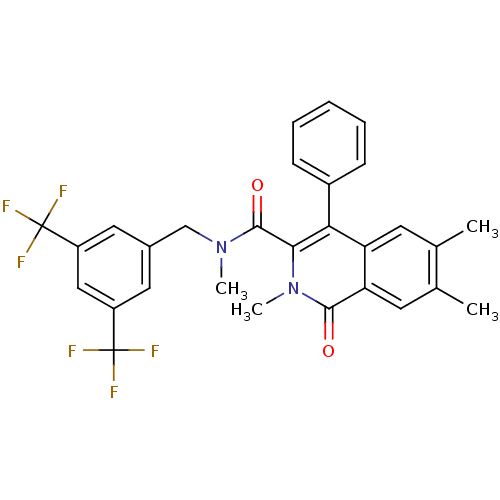 (2,6,7-Trimethyl-1-oxo-4-phenyl-1,2-dihydro-isoquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50244718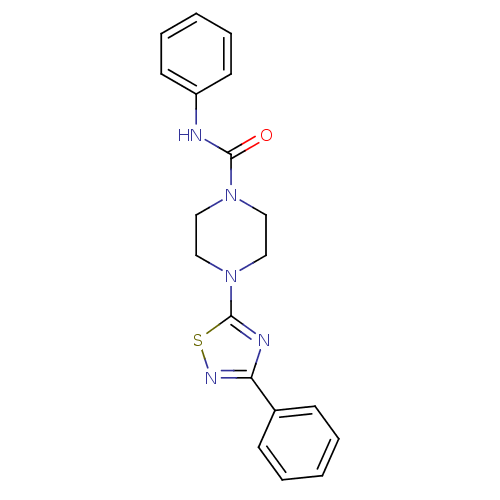 (CHEMBL460273 | JNJ-1661010 | N-phenyl-4-(3-phenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26736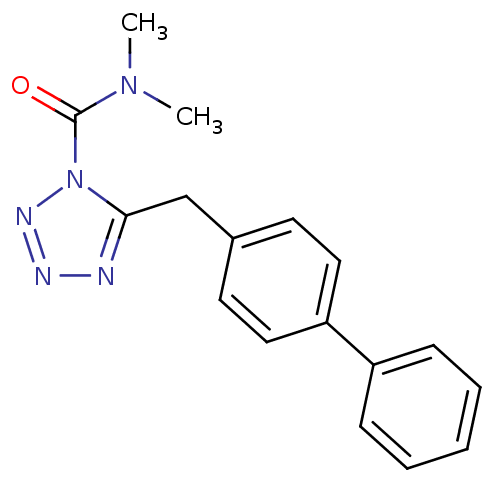 (CHEMBL509860 | LY2183240 | N,N-dimethyl-5-[(4-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of FAAH (unknown origin) | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030604 (3-[(2-Methoxy-benzylamino)-methyl]-2,6,7-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030583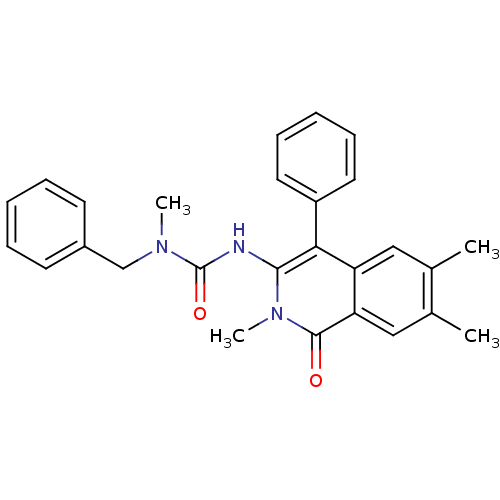 (1-Benzyl-1-methyl-3-(2,6,7-trimethyl-1-oxo-4-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030571 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |