

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50045686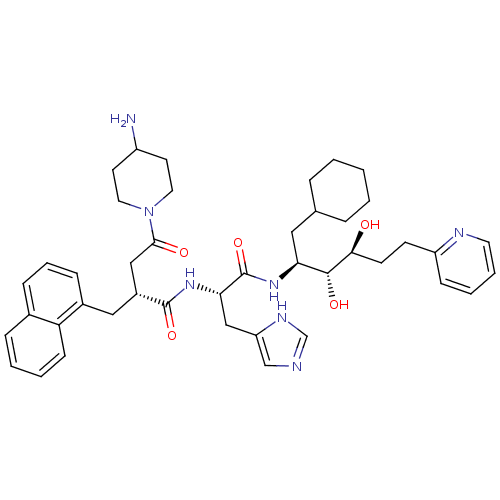 (4-(4-Amino-piperidin-1-yl)-N-[1-(1-cyclohexylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045691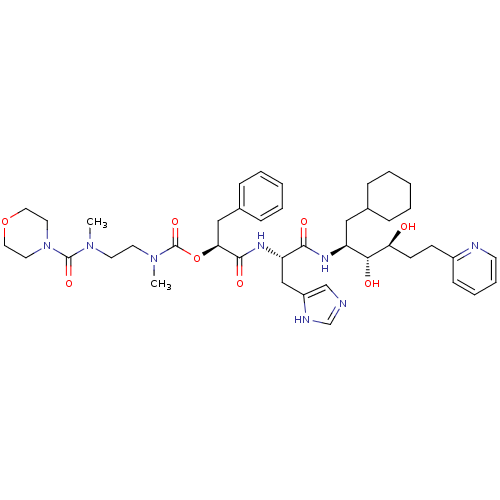 (CHEMBL94827 | Methyl-{2-[methyl-(morpholine-4-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045691 (CHEMBL94827 | Methyl-{2-[methyl-(morpholine-4-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045687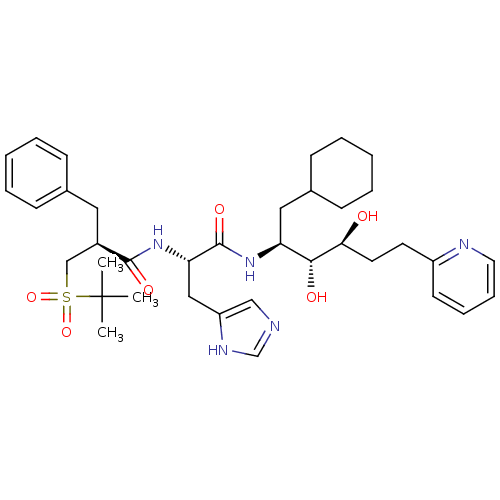 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045683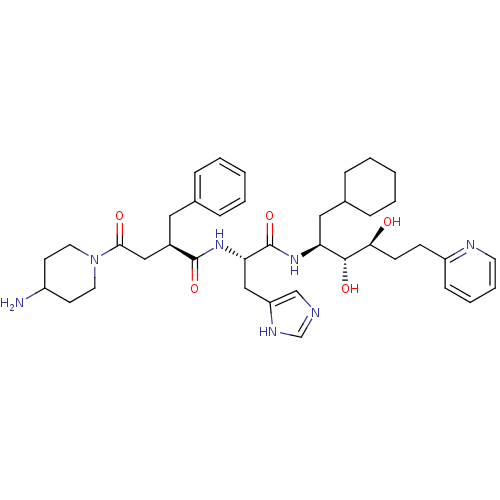 (4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045685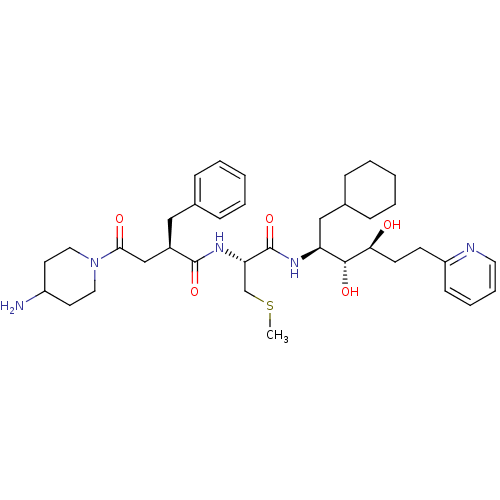 (4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045685 (4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045692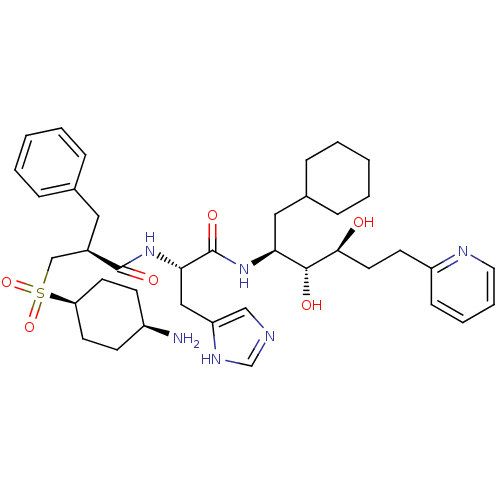 (3-(4-Amino-cyclohexanesulfonyl)-2-benzyl-N-[1-(1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045692 (3-(4-Amino-cyclohexanesulfonyl)-2-benzyl-N-[1-(1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045693 (4-Amino-piperidine-1-carboxylic acid 1-[1-(1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045693 (4-Amino-piperidine-1-carboxylic acid 1-[1-(1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045683 (4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045683 (4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045687 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045681 (4-Amino-cyclohexanecarboxylic acid {1-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM269143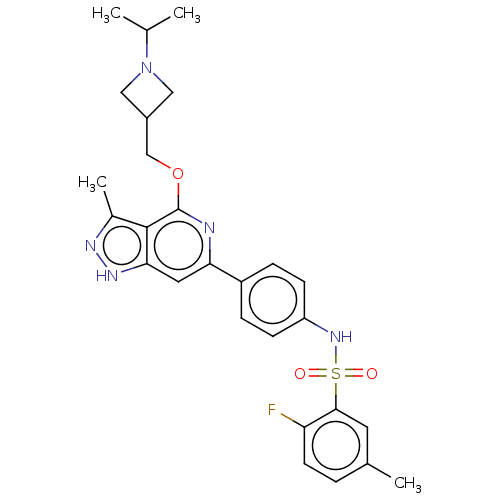 (US9718825, Example 589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM269150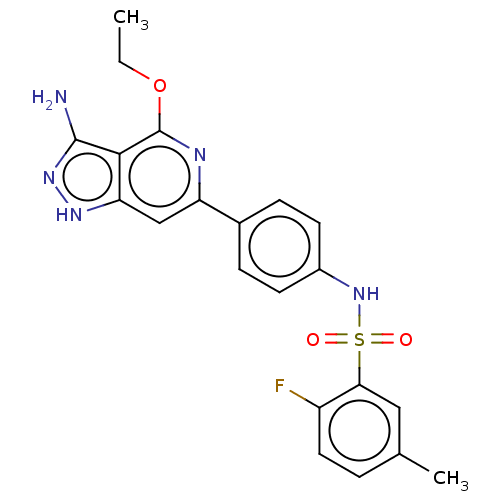 (US9718825, Example 596) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045681 (4-Amino-cyclohexanecarboxylic acid {1-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM269083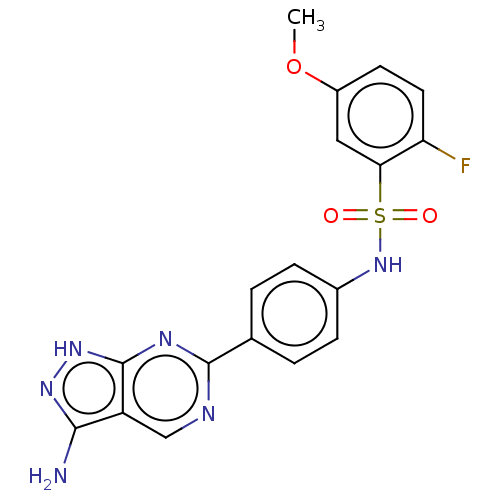 (US9718825, Example 527) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.510 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268829 (US9718825, Example 234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.510 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045684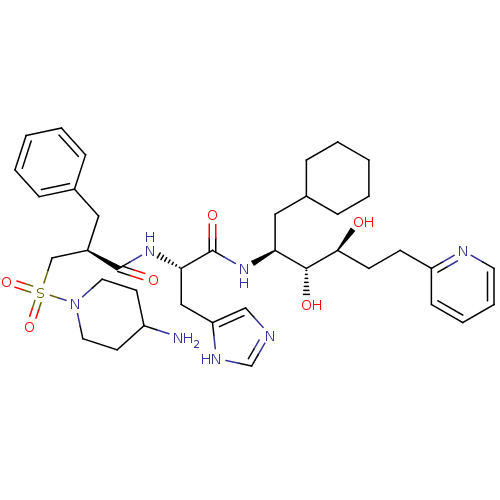 (3-(4-Amino-piperidine-1-sulfonyl)-2-benzyl-N-[1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM269008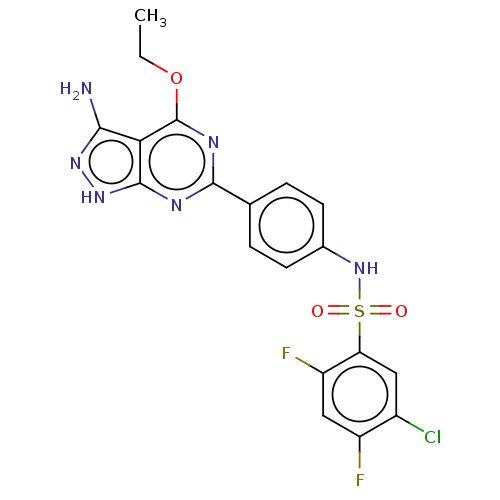 (US9718825, Example 451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268860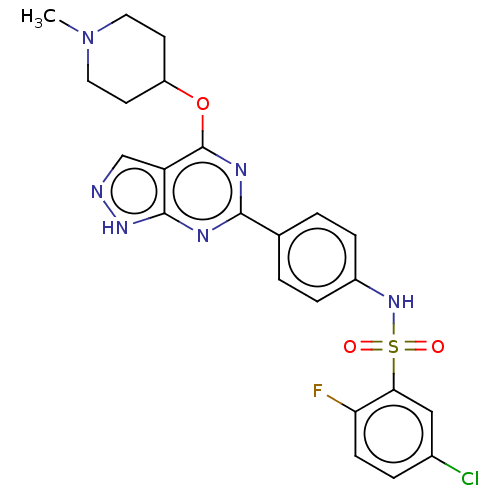 (US9718825, Example 265) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045682 (2-[4-(4-Amino-piperidin-1-yl)-2-benzyl-4-oxo-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045688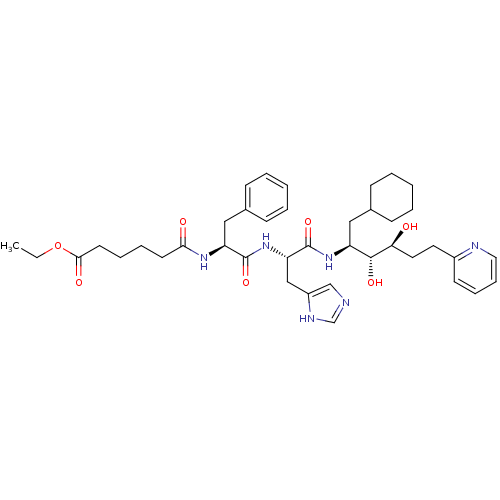 (5-{1-[1-(1-Cyclohexylmethyl-2,3-dihydroxy-5-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045684 (3-(4-Amino-piperidine-1-sulfonyl)-2-benzyl-N-[1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045688 (5-{1-[1-(1-Cyclohexylmethyl-2,3-dihydroxy-5-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Selectivity against rhesus monkey plasma renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268850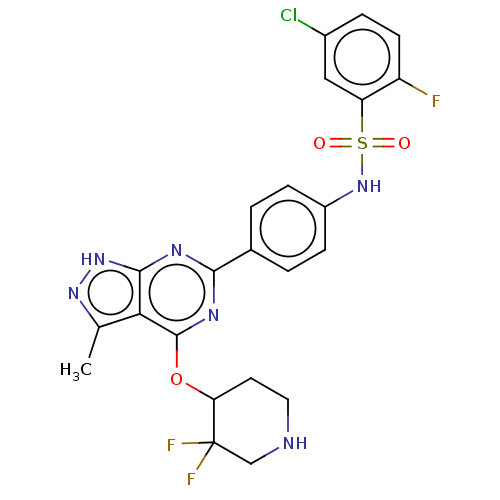 (US9718825, Example 255 | US9718825, Example 261) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045686 (4-(4-Amino-piperidin-1-yl)-N-[1-(1-cyclohexylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268828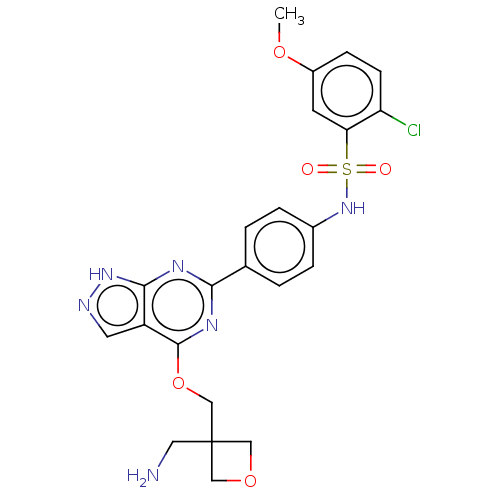 (US9718825, Example 233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM269092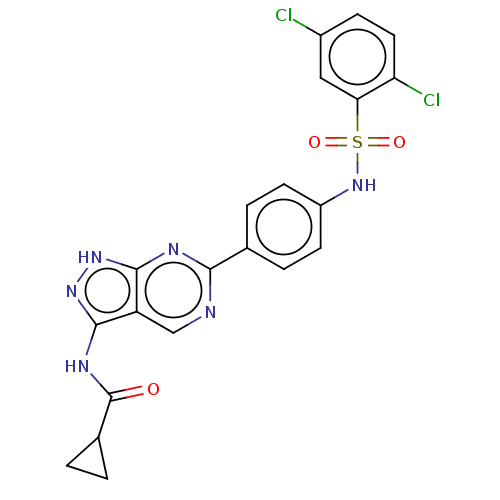 (US9718825, Example 537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045690 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268847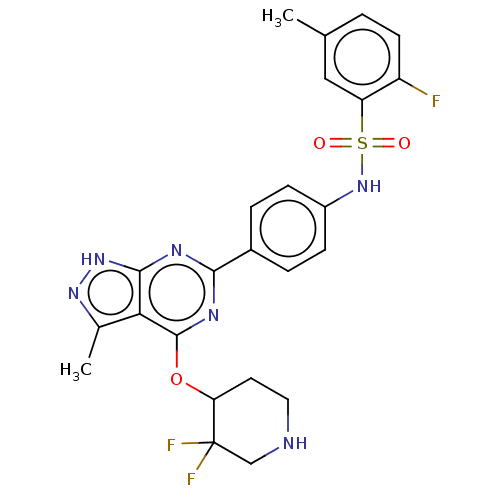 (US9718825, Example 252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268698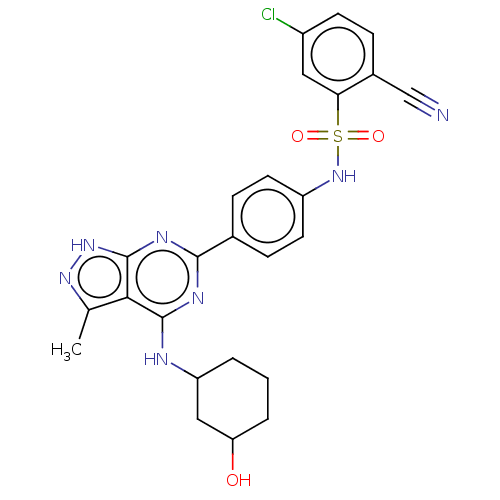 (US9718825, Example 100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045690 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Selectivity against guinea pig plasma renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268875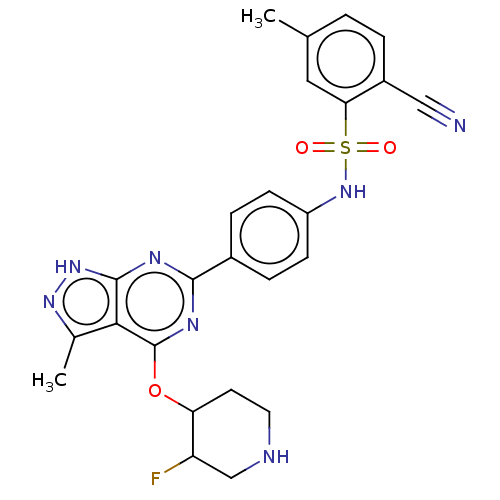 (US9718825, Example 300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268644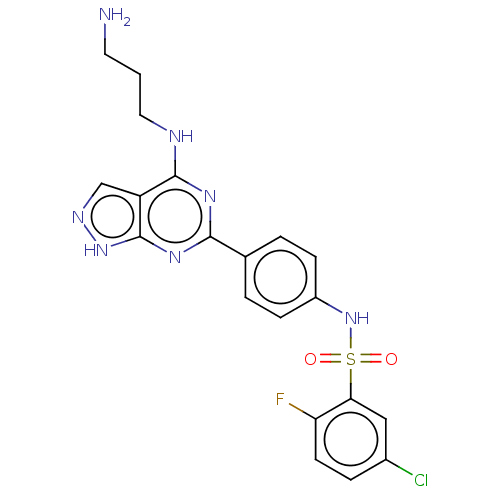 (US9718825, Example 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268648 (US9718825, Example 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268649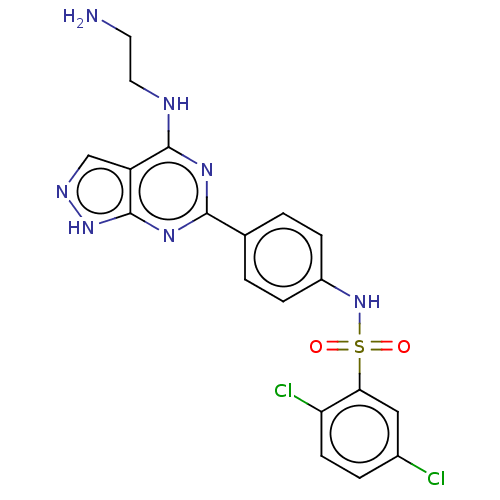 (US9718825, Example 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268650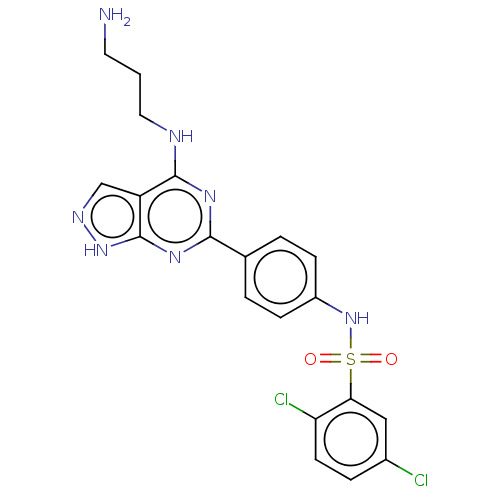 (US9718825, Example 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268659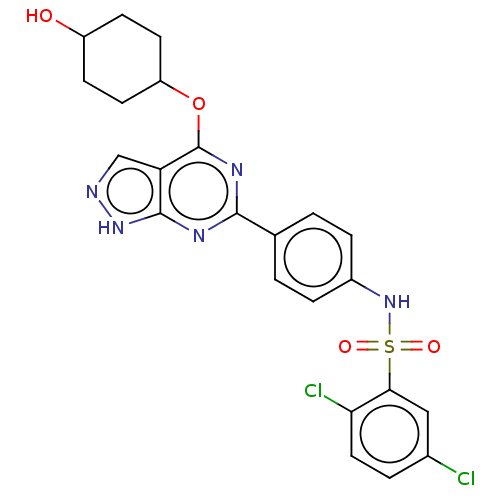 (US9718825, Example 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268660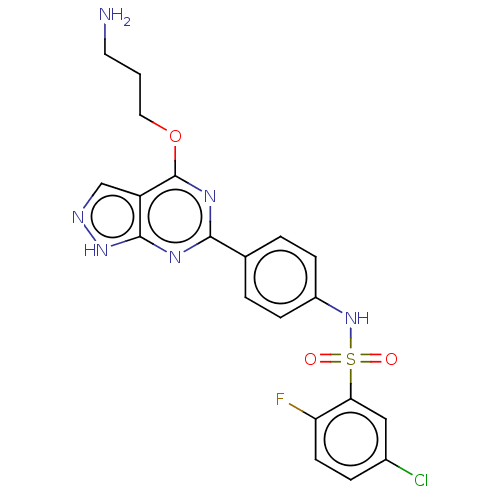 (US9718825, Example 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM50043018 (CHEMBL3355028 | US9718825, Example 63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM269021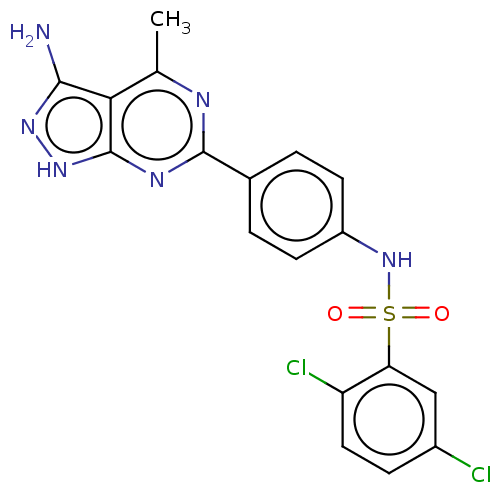 (US9718825, Example 464) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268619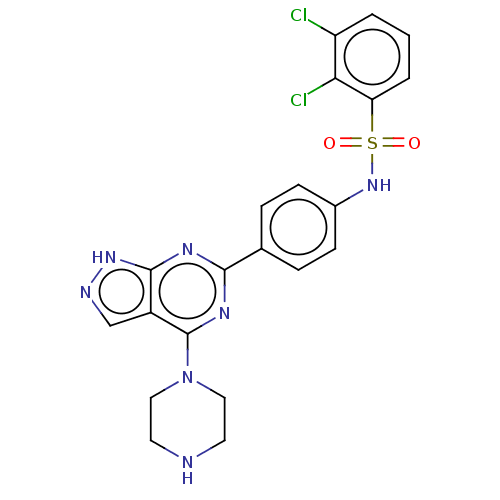 (US9718825, Example 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268620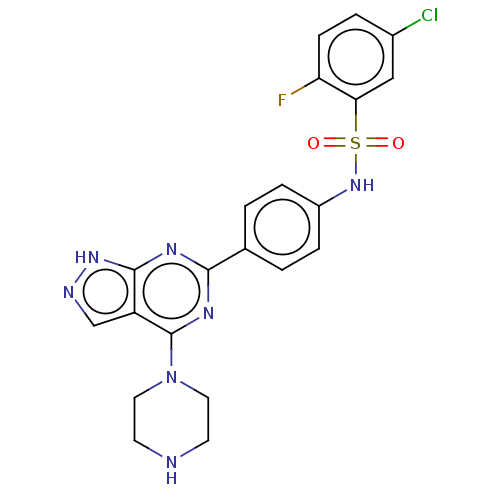 (US9718825, Example 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268621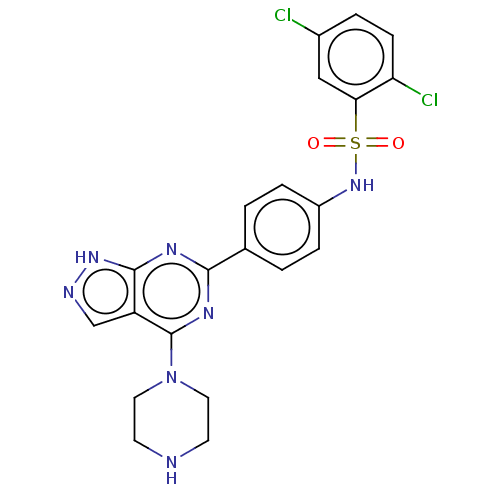 (US9718825, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268626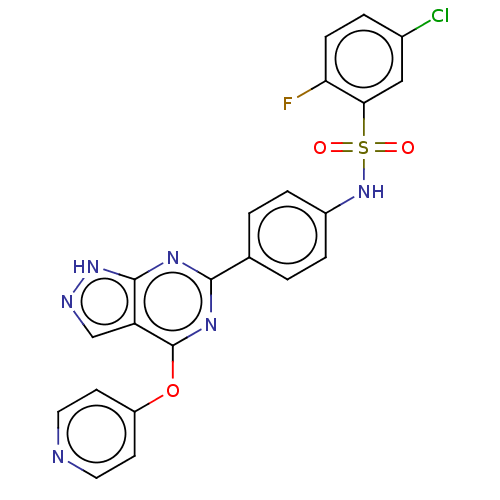 (US9718825, Example 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268627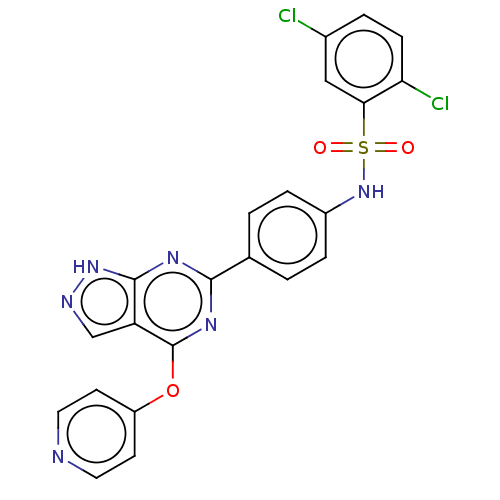 (US9718825, Example 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM268628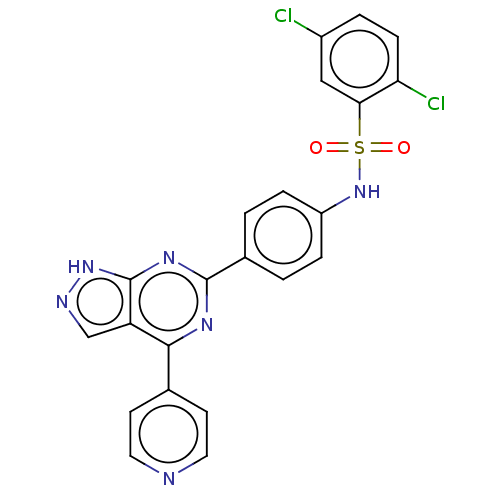 (US9718825, Example 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SANOFI US Patent | Assay Description The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... | US Patent US9718825 (2017) BindingDB Entry DOI: 10.7270/Q2VM4F82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 762 total ) | Next | Last >> |